Abstract
Background
Swift identification and diagnosis of gastrointestinal infections are crucial for prompt treatment, prevention of complications, and reduction of the risk of hospital transmission. The radiological appearance on computed tomography could potentially provide important clues to the etiology of gastrointestinal infections. We aimed to describe features based on computed tomography of patients diagnosed with Campylobacter, Salmonella or Shigella infections in South Sweden.
Methods
This was a retrospective observational population-based cohort study conducted between 2019 and 2022 in Skåne, southern Sweden, a region populated by 1.4 million people. Using data from the Department of Clinical Microbiology combined with data from the Department of Radiology, we identified all patients who underwent computed tomography of the abdomen CTA two days before and up to seven days after sampling due to the suspicion of Campylobacter, Salmonella or Shigella during the study period.
Results
A total of 215 CTAs scans performed on 213 patients during the study period were included in the study. The median age of included patients was 45 years (range 11–86 years), and 54% (114/213) of the patients were women. Of the 215 CTAs, 80% (n = 172) had been performed due to Campylobacter and 20% (n = 43) due to Salmonella enteritis. CTA was not performed for any individual diagnosed with Shigella during the study period. There were no statistically significant differences in the radiological presentation of Campylobacter and Salmonella infections.
Conclusion
The most common location of Campylobacter and Salmonella infections was the cecum, followed by the ascending colon. Enteric wall edema, contrast loading of the affected mucosa, and enteric fat stranding are typical features of both infections. The CTA characteristics of Campylobacter and Salmonella are similar, and cannot be used to reliably differentiate between different infectious etiologies.
Introduction
The global burden of gastrointestinal infections is significant, with an estimated 1.7 billion cases of childhood diarrheal disease every year according to the World Health Organization (WHO) [Citation1]. Each year, foodborne illnesses caused by Campylobacter, non-typhoidal Salmonella and Shigella are estimated to cause 96, 78, and 51 million episodes, respectively [Citation2]. These infections are linked to contaminated water and food sources, insufficient hygiene practices, and poor sanitation and are more prevalent in developing countries. Campylobacter, non-typhoidal Salmonella and Shigella have a wide spectrum of clinical presentations ranging from mild self-limiting gastroenteritis to severe invasive disease and death. In 2019, non-typhoidal Salmonella caused approximately 215,000 deaths globally [Citation3]. For patients with severe disease requiring hospitalization, computed tomography is sometimes performed during the work-up, as the clinical presentation of bacterial gastroenteritis can mimic other conditions, such as inflammatory bowel disease, drug-induced colitis, and ischemic colitis.
Previously, only a few reports on CT findings of gastrointestinal infections have been published, including only a few patients [Citation4–6]. CT features have been suggested as indicative of specific bacteria [Citation7]. Swift identification and diagnosis of gastrointestinal infections are crucial for prompt treatment, prevention of complications, and reduction in the risk of hospital transmission. In situations where microbiological results are prolonged or absent, the radiological appearance on computed tomography could potentially provide important clues to the etiology of gastrointestinal infection.
In this study, we aimed to describe features based on computed tomography of patients diagnosed with Campylobacter, Salmonella or Shigella infections in South Sweden. Additionally, we wanted to investigate if there were statistically significant differences in the radiological appearances between the different etiologies.
Methods
Study design and setting
This was a retrospective observational population-based cohort study conducted between 2019 and 2022 in Skåne, southern Sweden, a region populated by 1.4 million people. The Clinical Microbiology Laboratory in Lund is responsible for all the microbiological diagnostics in this region. Data were retrieved from stool cultures of all patients diagnosed with Campylobacter, Salmonella and Shigella in the region during the study period.
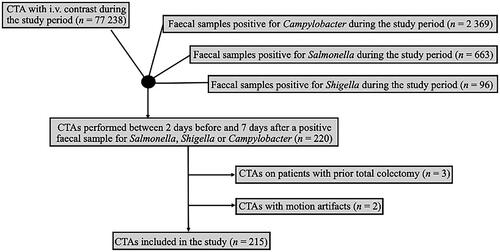
Data were retrieved from all patients who underwent computed tomography of the abdomen (CTA) with intravenous contrast during the study period from PACS IDS7 (Sectra, Linköping, Sweden). Using both registers, we identified all patients who underwent CTA two days before and up to seven days after being diagnosed with Campylobacter, Salmonella or Shigella during the study period. Patients could be included multiple times in the study if they presented multiple times with the same or different aetiology.
The intravenous contrast used in the study was Omnipaque 350 mg I/mL, dosed according to patients’ weight, with reduced dose in patients with impaired kidney function (estimated glomerular filtration rate <45 mL/min/1.73 m2) and/or older age (>70 years). Therefore, CTAs without intravenous contrast was not included. CTAs in which oral contrast was administered, in addition to intravenous contrast, were also included.
Patients who had previously undergone total colectomy were excluded, as were those with CTAs that could not be assessed due to severe motion artifacts. The medical records of all patients were reviewed using Melior software (Melior, Siemens Healthcare Service, Upplands Väsby, Sweden).
Ethics
This study was approved by the Swedish Ethical Review Authority (DNR-2021-04866) as well as an institutional approval. This national authority waived the need for patient consent.
Outcome
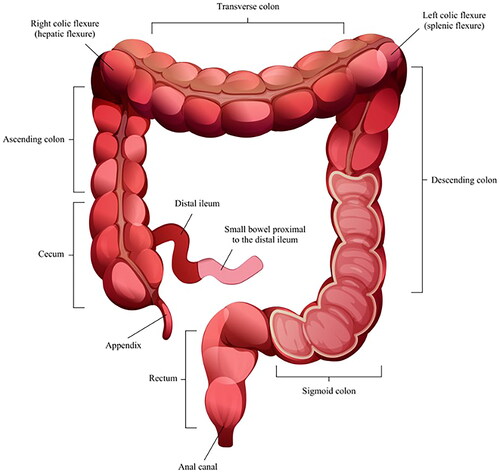
Each CTA was examined using a predefined study protocol with twenty different variables of interest. The distal small bowel and colon were divided into 11 different segments (), and the presence of increased mesenteric vascular appearance (comb sign) and contrast loading of the affected mucosa and serosa were noted (yes or no). Additionally, the thickness of the most affected enteric wall was measured in millimeters, using the mean value of two random measurements. If edema was present or not (yes/no), any enlarged mesenteric lymph nodes were noted (no = <1 cm, yes ≥1 cm). Also, the presence or absence of ascites, enteric fat stranding and the presence or absence of skipped lesions was recorded. Pancolitis was defined as colitis affecting the cecum and rectum. The presence of a water halo sign and an empty colon sign, which has been previously described as a radiological appearance, was noted [Citation8–10]. In addition, the presence of gas intramurally in the mesenterial vessels or abdomen was noted. Short-axis measurements and attenuation according to the Hounsfield unit were recorded for the largest lymph nodes.
The second author (SP) examined all CTAs blindly, with no information on the microbiological etiology of the included patients. In addition, C-reactive protein (CRP), peripheral total leukocyte counts, and temperature ± one day of CTA were extracted from the medical records.
Microbiology
Gastrointestinal pathogens were detected in fecal samples using a culture-based method until February 2020 and with a PCR-directed culture method using Amplidiag (Mobidiag, Ltd.). To identify Campylobacter before the introduction of Amplidiag PCR, selective Campylobacter-agar (Neogen) was used, and CampyGen (Thermo Scientific) was used to achieve a microaerophilic environment. After the introduction of Amplidiag PCR, Campylobacter was only cultured from fecal samples upon specific requests. When the primary culture was used and when a PCR-directed culture was the main method, Salmonella and Shigella were cultured when detected. The culture was based on xylose-lysine-deoxycholate (XLD) agar, Salmonella CHROMAgar (CHROMAgar), and Rappaport broth used for enrichment of Salmonella. In addition to MALDI-TOF MS, agglutination using antisera (SSI Diagnostica for Salmonella and SIFIN Antisera for Shigella, earlier from Reagensia for both) was used for Salmonella and Shigella typing.
Statistical analysis
Numerical data are presented as medians, means, and ranges, and qualitative data are presented as proportions (%). The chi-square test was used for categorical data, and the Student’s t-test and Mann–Whitney test were used for continuous variables. Pearson’s correlation test was used for correlation analysis. Statistical significance was set at p < 0.05. Statistical analyses were performed using Prism, version 7 (GraphPad Software).
Results
Patient characteristics
In total, 215 CTAs performed on 213 patients with Salmonella and Campylobacter during the study period were included in the study (). No patient with Shigella was included. The median age of included patients was 45 years (range 11–86) and 54% (114/213) of the patients were women. In total, 23 patients (11%) had previous gastric bypass surgery: 22 patients (13%) in the Campylobacter group, compared to one (2%) in the Salmonella group. In the Campylobacter group, five (3%) and one (1%) patients suffered from ulcerative colitis and Mb Crohn, respectively. In the Salmonella group, one patient (2%) was previously diagnosed with Mb Crohn (). Out Of the 215 CTAs, 80% (n = 172) had been performed due to Campylobacter and 20% (n = 43) to enteric Salmonella enteritis. Also, 19% of these (8/43) also had Salmonella growth in blood cultures, in addition to a Salmonella-positive fecal sample.
Table 1. Baseline characteristics.
Out of the 172 CTAs performed due to Campylobacter, 76 (44%) were performed with oral contrast, in addition to intravenous contrast. For Salmonella, this number was 10 (23%).
None of the patients in whom CTA was performed due to Campylobacter had a bloodstream infection due to Campylobacter. Two CTAs were performed on one individual diagnosed with both Campylobacter and Salmonella during the study period, one year apart. One patient was subjected to two CTAs, five weeks apart due to Campylobacter infection.
CTA was not performed for any individual diagnosed with Shigella during the study period. No CTA was performed with intraperitoneal gas, ileus, or toxic megacolon during the study period. For both infections due to Campylobacter and Salmonella, several enteric segments were mostly involved ().
Campylobacter and characteristics of CTA
In total, 170 (99%) of CTAs were pathological, according to a predefined study protocol. For enteric inflammation, the most common pathological location was the cecum (n = 151, 88%), followed by the ascending colon (n = 144, 84%), and the right colic flexure (n = 134, 78%) (). Increased mesenteric vascular appearance was seen in a minority of CTAs (n = 74, 43%), and whereas contrast loading of the affected mucosa was typical (n = 138, 80%), contrast loading of the affected serosa was less common (n = 116, 67%). Enteric wall edema was typical (n = 115, 67%) and the median thickness of the enteric wall was 4 mm (range 1.5–10 mm). Ascites and lymph nodes enlarged more than 1 cm were observed in less than half of the patients (n = 64, 38%; and n = 76, 44%, respectively).
Table 2. Enteric segment involved.
Enteric fat stranding was frequent (n = 121, 70%), but the presence of skipped lesions (n = 16, 9%) were rare. For each patient, a median of seven enteric segments (range 0–11) was pathological in Campylobacter etiology.
Salmonella and characteristics of CTA
In total, 42 (98%) of CTAs were pathological, according to the study protocol. For enteric inflammation, the most common pathological location was the cecum (n = 34, 74%), followed by the ascending colon (n = 31, 72%), and the distal ileum (n = 29, 67%) (). Increased mesenteric vascular appearance was frequent (n = 24, 56%), contrast loading of the affected mucosa was typical (n = 36, 84%), as well as contrast loading of the affected serosa (n = 29, 67%). The majority showed enteric wall edema (n = 29, 67%), and the median thickness of the enteric wall was 4 mm (range 1–8 mm). The presence of ascites and enlarged lymph nodes was observed in more than one third of the patients (n = 16, 37% and n = 15, 35%, respectively). Enteric fat stranding was commonly observed (n = 30, 70%), but skipped lesions were uncommon (n = 2, 4%). In total, a median of five enteric segments (range 0–10) were pathological in Salmonella CTAs. There was no statistical difference between the number of segments involved in CTAs in patients with Salmonella growth in blood cultures (median 7 segments), in addition to a Salmonella-positive fecal sample, compared to patients with Salmonella-positive fecal samples only (median 4 segments, p = 0.07).
Comparison of Campylobacter and Salmonella aetiology
For the predefined study protocol variables, no statistically significant differences were observed in the radiological presentations of Campylobacter and Salmonella ().
Table 3. Comparison of CTA findings between Campylobacter and Salmonella.
For Campylobacter, there was a statistically significant correlation between the number of segments involved and crp (r = 0.2, p = 0.009), but not with leukocyte count (r = 0.02, p = 0.8) or temperature (r = 0.1, p = 0.2) (). For Salmonella, there was no statistically significant correlation between the number of segments involved and crp (r = −0.1, p = 0.5), leukocyte count (r = −0.2, p = 0.3), or temperature (r = 0.1, p = 0.5).
Table 4. Further comparisons between Campylobacter and Salmonella.
Discussion
This population-based study in South Sweden aimed to describe features on computed tomography of patients diagnosed with enteric Campylobacter, Salmonella and Shigella infections in South Sweden and to investigate possible differences in the radiological appearances between the different etiologies. Since there were no patients with microbiological evidence of Shigella, only CTAs with symptoms of Campylobacter and Salmonella were included in the study. We found that 99% of the CTAs performed displayed pathological features, and the most common location of pathological features for both Campylobacter and Salmonella was the cecum, followed by the ascending colon. Contrast loading of the affected mucosa and serosa was commonly observed in both the infections. Enteric wall edema, enteric fat stranding, and water halo signs were typical for both infections. The radiological appearances of both Campylobacter and Salmonella infections.
To our knowledge, our study is the most comprehensive account of the features of Campylobacter and Salmonella enteric infections on computed tomography, including a sizable cohort of patients compared to previous case reports and reviews. Previous reports have included between one hundred and seventeen cases [Citation7–13].
Fat stranding on CT was common in both Campylobacter and Salmonella etiologies. Although fat stranding can occur in a variety of disorders within the abdomen, some studies indicate that fat stranding that is disproportionally more severe than the degree of wall thickening could suggest an inflammatory condition such as diverticulitis [Citation14,Citation15].
Our study is in agreement with previous studies (one case report, one review) locating Campylobacter and Salmonella enteric infections on the right side of the colon [Citation4,Citation13]. The rate of pancolitis was higher in our study (42% for Campylobacter, 30% for Salmonella) compared 26% in a previous study, but this study included colitis of any etiology [Citation16].
Enlarged lymph nodes of both Campylobacter and Salmonella etiologies were observed in a minority of CTAs in our study, of both Campylobacter and Salmonella etiologies. Skipped lesions are uncommon manifestations of Campylobacter and Salmonella infections. Skip lesions are often observed in Crohn’s disease, a disease characterized by transmural inflammation that affects the entire gastrointestinal tract [Citation17]. The radiological appearance of enteric infections can mimic the appearance of inflammatory bowel disease, and the clinical presentation can be similar. A ‘comb’ sign, together with small bowel involvement and enlarged lymph nodes could suggest inflammatory colitis, rather than infectious [Citation8]. The same article found that an ‘empty colon’ sign, with a continuous distribution of inflammation and absence of enlarged lymph nodes, suggests infectious colitis. Our aim was not to compare radiological appearances between inflammatory bowel disease and Campylobacter/Salmonella, but our findings could be used to compare radiological characteristics of infectious colitis in future review articles.
In our study, ascites was a common manifestation of infection, which has been previously described in patients with blood cultures positive for S. enterica subsp. enterica ser. typhi, and S. enterica subsp. enterica ser. Paratyphi [Citation6]: We did not include Salmonella typhi or Salmonella paratyphi in our study.
The characteristics of CTA of Campylobacter and Salmonella are similar and cannot reliably be used to differentiate between different infectious etiologies. We believe that fecal samples for the detection of gastrointestinal pathogens are essential to differentiate inflammatory bowel disease from gastrointestinal infections.
No patient underwent CTA due to Shigella enteric infection during the study period, we are therefore unable to report on CTA features of Shigella. To our knowledge, there are few previous studies addressing this.
The rate of CTAs performed in patients suffering from Campylobacter and Salmonella was 1:5, which is consistent with the numbers from the Public Health Agency of Sweden, which revealed that Campylobacter is five times more common than Salmonella [Citation18].
For Campylobacter, we found a statistically significant correlation between C-reactive protein level and the spread of inflammation, but not with leukocyte count or temperature. It seems reasonable that enlarged inflammation is correlated with higher CRP; however, no such correlation was observed for CTAs performed due to Salmonella.
The strengths of our study include the population-based study design, covering three full years using two different registries for case finding, and the fact that the radiologist examined all CTAs blindly. The limitations of our study include the fact that CTAs were reviewed by only one radiologist. Ideally, two or more radiologists should have examined all CTAs blindly, with reported inter-rater reliability and agreement. A few variables were not explored when CTAs were examined, such as spleen enlargement. We could not analyze the serotypes of the pathogens to investigate any statistical associations between different serotypes and CT features, nor did we investigate patient demographics with respect to CT features.
A minority of the CTAs in our study were performed on patients with IBD (4%) and previous colectomy (2%), and 40% of CTAs were performed using oral contrast. If this is the case, our results are unlikely, but cannot be ruled out.
Conclusion
The most common site of infection for both Campylobacter and Salmonella was the cecum, followed by the ascending colon. Enteric wall edema, contrast loading of the affected mucosa, and enteric fat stranding are typical features of both infections. The characteristics of CTA of Campylobacter and Salmonella are similar and cannot reliably be used to differentiate between different infectious etiologies.
Author contributions
OL, AB, and TS conceived the study and performed data acquisition together with SP. The project administration was provided by OL and TS. The methodology study design was finalized by AB, TS, SP, and OL. Data curation, analysis, and visualization were performed using SP and OL. The manuscript was initially drafted by OL and was critically revised by AB, SP, and TS. All the authors approved the final version of the manuscript.
Acknowledgements
We thank Erik Thimansson for input on the selected variables.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The participants of this study did not give written consent for their data to be shared publicly, so due to the sensitive nature of the research supporting data is not available.
Additional information
Funding
References
- World H Organization. Diarrhoeal disease; 2023 [cited 2023 Aug 24]. Available from: https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease.
- Kirk MD, Pires SM, Black RE, et al. World health organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: a data synthesis. PLoS Med. 2015;12(12):e1001921. doi: 10.1371/journal.pmed.1001921.
- Collaborators AR. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629–655.
- Hokama A, Kawata A, Kishimoto K, et al. Images of interest. Gastrointestinal: campylobacter enterocolitis. J Gastroenterol Hepatol. 2005;20(11):1789–1789. doi: 10.1111/j.1440-1746.2005.04160.x.
- Balthazar EJ, Charles HW, Megibow AJ. Salmonella- and Shigella-induced ileitis: CT findings in four patients. J Comput Assist Tomogr. 1996;20(3):375–378. doi: 10.1097/00004728-199605000-00008.
- Hennedige T, Bindl DS, Bhasin A, et al. Computed tomography features in enteric fever. Ann Acad Med Singap. 2012;41(7):281–286.
- Horton KM, Corl FM, Fishman EK. CT evaluation of the colon: inflammatory disease. Radiographics. 2000;20(2):399–418. doi: 10.1148/radiographics.20.2.g00mc15399.
- Plastaras L, Vuitton L, Badet N, et al. Acute colitis: differential diagnosis using multidetector CT. Clin Radiol. 2015;70(3):262–269. doi: 10.1016/j.crad.2014.11.008.
- Wessling J. Radiological imaging of acute infectious and non-infectious enterocolitis. Radiologe. 2018;58(4):302–311. doi: 10.1007/s00117-018-0379-3.
- Duffin C, Mirpour S, Catanzano T, et al. Radiologic imaging of bowel infections. Semin Ultrasound CT MR. 2020;41(1):33–45. doi: 10.1053/j.sult.2019.10.004.
- Puylaert JB, Van der Zant FM, Mutsaers JA. Infectious ileocecitis caused by yersinia, Campylobacter, and Salmonella: clinical, radiological and US findings. Eur Radiol. 1997;7(1):3–9. doi: 10.1007/s003300050098.
- Brodey PA, Fertig S, Aron JM. Campylobacter enterocolitis: radiographic features. AJR Am J Roentgenol. 1982;139(6):1199–1201. doi: 10.2214/ajr.139.6.1199.
- Hennedige T, Bindl DS, Bhasin A, et al. Spectrum of imaging findings in Salmonella infections. AJR Am J Roentgenol. 2012;198(6):W534–9. doi: 10.2214/AJR.11.7621.
- Fernandes T, Oliveira MI, Castro R, et al. Bowel wall thickening at CT: simplifying the diagnosis. Insights Imaging. 2014;5(2):195–208. doi: 10.1007/s13244-013-0308-y.
- Pereira JM, Sirlin CB, Pinto PS, et al. Disproportionate fat stranding: a helpful CT sign in patients with acute abdominal pain. Radiographics. 2004;24(3):703–715. doi: 10.1148/rg.243035084.
- Meyer J, Schrenzel J, Balaphas A, et al. Mapping of etiologies of computed tomography-proven acute colitis: a prospective cohort study. Sci Rep. 2022;12(1):9730. doi: 10.1038/s41598-022-13868-w.
- Feuerstein JD, Cheifetz AS. Crohn disease: epidemiology, diagnosis, and management. Mayo Clin Proc. 2017;92(7):1088–1103. doi: 10.1016/j.mayocp.2017.04.010.
- Sweden TPHAo. Statistik om smittsamma sjukdomar A-Ö. Available from: https://www.folkhalsomyndigheten.se/folkhalsorapportering-statistik/statistik-a-o/.
Appendix A.
Table A1. Number of enteric segments involved.