Abstract
Introduction
A change from the supine to prone position causes hemodynamic alterations. We aimed to evaluate the effect of fluid preloading in the supine position, the subsequent hemodynamic changes in the prone position and postoperative outcomes.
Patients and methods
This prospective, assessor-blind, randomized controlled trial was conducted between March and June 2023. Adults scheduled for elective orthopaedic lumbar surgery under general anaesthesia were enrolled. In total, 80 participants were randomly assigned to fluid maintenance (M) or loading (L) groups. Both groups were administered intravenous fluid at a rate of 2 ml/kg/h until surgical incision; Group L was loaded with an additional 5 ml/kg intravenous fluid for 10 min after anaesthesia induction. The primary outcome was incidence of hypotension before surgical incision. Secondary outcomes included differences in the mean blood pressure (mBP), heart rate, pleth variability index (PVi), stroke volume variation (SVV), pulse pressure variation (PPV), stroke volume index and cardiac index before surgical incision between the two groups. Additionally, postoperative complications until postoperative day 2 and postoperative hospital length of stay were investigated.
Results
Hypotension was prevalent in Group M before surgical incision and could be predicted by a baseline PVi >16. The mBP was significantly higher in Group L immediately after fluid loading. The PVi, SVV and PPV were lower in Group L after fluid loading, with continued differences at 2–3 time points for SVV and PPV. Other outcomes did not differ between the two groups.
Conclusion
Fluid loading after inducing general anaesthesia could reduce the occurrence of hypotension until surgical incision in patients scheduled for surgery in the prone position. Additionally, hypotension could be predicted in patients with a baseline PVi >16. Therefore, intravenous fluid loading is strongly recommended in patients with high baseline PVi to prevent hypotension after anaesthesia induction and in the prone position.
Trial number
KCT0008294 (date of registration: 16 March 2023)
KEY MESSAGES
Fluid preloading could reduce the occurrence of hypotension in the prone position. Hypotension could be predicted in patients with a baseline PVi >16. Intravenous fluid preloading is strongly recommended in patients with high baseline PVi to prevent hypotension after anaesthesia induction and in the prone position.
1. Introduction
Lumbar surgery is performed to treat degenerative diseases, spinal stenosis and disc herniation [Citation1]. During lumbar surgery, the prone position is essential to secure the field of view and perform the surgery [Citation2]. However, the prone position causes hemodynamic alterations when changed from the supine position [Citation3–5]. More specifically, the prone position decreases cardiac output due to a drop in venous return caused by a decrease in effective circulatory volume, and patients with a high body mass index (BMI) experience more severe hemodynamic changes [Citation6–9]. If serious hemodynamic changes occur in the prone position, the surgical procedure is delayed; the surgery itself may also be cancelled if changes are severe and unresponsive to management.
To prevent and treat these hemodynamic deteriorations, studies related to prone positioners [Citation4,Citation10,Citation11] or the adjustment of fluid preloading to compensate for the decrease in relative circulatory volume [Citation8,Citation12,Citation13] have been conducted. In studies related to fluid administration, parameters such as stroke volume variation (SVV) and pulse pressure variation (PPV) are mainly used to determine fluid responsiveness [Citation14–16].
The pleth variability index (PVi) is a recently developed parameter that confirms fluid responsiveness; a device is non-invasively attached to a patient’s finger to monitor the fluctuation of the pulse oximeter plethysmographic waveform, and displays it as a numeric value (Rainbow SET, Masimo, Irvine, CA, USA) [Citation17–19]. While the SVV and PPV can only be assessed through arterial cannulation, the PVi can be used to monitor non-invasive fluid responsiveness.
However, to the best of our knowledge, no study has analysed hemodynamic values using the PVi after fluid preloading in the supine position, and the subsequent changes after repositioning to prone. Furthermore, we aimed to analyse whether fluid preloading is limited to the position change stage and affects postoperative outcomes.
2. Patients and methods
2.1. Study design, ethics and trial registration
This trial was designed to investigate hemodynamic changes in the prone position according to fluid loading after anaesthesia induction in patients undergoing lumbar spine surgery. This randomized, assessor-blind, prospective study was approved by the Institutional Review Board of the Kyung Hee University Hospital (KHUH 2023-02-017) on 6 March 2023 and written informed consent was obtained from all subjects participating in the trial. This study was conducted in accordance with the Declaration of Helsinki, and the trial was registered prior to patient enrolment at the Clinical Research Information Service (KCT0008294, Principal investigator: Jeong-Hyun Choi, Date of registration: 16 March 2023). The trial protocol is available from the Clinical Research Information Service. This study complies with the Consolidated Standards of Reporting Trials Checklist.
2.2. Participants
This study included adults aged 18–100 years who were scheduled to undergo elective orthopaedic lumbar surgery under general anaesthesia. The exclusion criteria were the use of medication for hypertension; avoiding fluid load due to pulmonary edoema or heart failure; preoperative use of vasopressors to maintain blood pressure (BP); use of mechanical circulatory support, such as an intra-aortic balloon pump; BMI >35 kg/m2; and American Society of Anesthesiologists physical status class ≥ IV. Enrolment began in March 2023 and ended in July 2023.
2.3. Randomization and masking
A random assignment sequence was generated using Excel with a random block size and 1:1 allocation. Study participants were randomly assigned to either a control (fluid maintenance, Group M) or test (fluid loading, Group L) group using a sealed, opaque envelope on the morning of surgery. To blind the assessor, one researcher who was blinded to the group allocation completed the case report form.
2.4. Procedures
After the patients entered the operating room, several measurements were taken. The heart rate (HR) and rhythm were recorded with a 3-lead electrocardiogram, BP was taken using a non-invasive arm cuff, and peripheral oxygen saturation and PVi were obtained by attaching an RD rainbow SET® (Masimo, Irvine, CA, USA) to the finger. Both groups were administered intravenous fluid at a rate of 2 ml/kg/h until the incision, and patients in Group L were loaded with an additional 5 ml/kg intravenous fluid for 10 min after anaesthesia induction, according to random allocation. For anaesthesia induction, remimazolam (0.1–0.2 mg/kg), rocuronium (0.8 mg/kg) and remifentanil (0.5–1 µg/kg) were administered, while sevoflurane and remifentanil were used to maintain anaesthesia in both groups. Sevoflurane was administered at 0.8–1.2 of the minimum alveolar concentration with the goal of maintaining a bispectral index of 30–60, and remifentanil was administered at a rate of 0.05–0.1 µg/kg/min to maintain within 20% of the initial HR and BP. Arterial cannulation was performed for arterial BP monitoring in the radial artery of a site that did not monitor the PVi and was connected to the FloTrac/EV1000 system (Edwards Lifesciences, Irvine, CA, USA) to measure the stroke volume index (SVI), cardiac index (CI) and SVV. Subsequently, patients were placed in the prone position, and hemodynamic data were recorded before the incision.
2.5. Outcomes and data collection
The primary outcome was incidence of hypotension before surgical incision.
The secondary outcomes included differences in BP, HR, PVi, SVV, PPV, SVI and CI before surgical incision, total amount of fluid administered and blood transfused intraoperatively, estimated blood loss, urine output, operation time, and postoperative discharge to the intensive care unit between the two groups. Additionally, based on medical records, the occurrence of postoperative pulmonary complications (PPC), postoperative nausea and vomiting (PONV), fever, delirium and acute kidney injury (AKI) until postoperative day (POD) 2 as postoperative complications and postoperative hospital length of stay (HLOS) were investigated.
Hypotension was defined as a decrease in systolic BP below 20% of the baseline value; 1 µg/kg of phenylephrine or 5 mg of ephedrine were administered as a vasopressor if hypotension occurred before surgical incision. PPC were defined as newly developed respiratory infections, respiratory failure, pulmonary edoema and atelectasis compared with preoperative status [Citation20]. Fever was defined as tympanic temperature ≥38.3 °C [Citation21]. AKI was defined as a change in postoperative serum creatinine level ≥0.3 mg/dL compared with the preoperative level until POD 2, according to the Kidney Disease: Improving Global Outcomes criteria [Citation22].
Hemodynamic data – including BP, HR, PVi, PPV and SVV – were recorded after the following events: entering the operating room, anaesthesia induction, fluid loading, change to prone position and surgical incision. Intraoperative data were expected to vary depending on the level and type of surgery; therefore, only hemodynamic data were recorded before the surgical incision.
2.6. Statistical analysis and sample size calculation
Data are expressed as median [interquartile range] or number (%). Fisher’s exact test or the chi-square test was used to analyse categorical variables. The normality of continuous variables was evaluated using the Shapiro–Wilk test. The independent variable t-test or Wilcoxon rank-sum test was used to analyse continuous variables. Univariate logistic regression analysis was performed to identify factors associated with the occurrence of hypotension before surgical incision. Among these factors, multivariate logistic regression analysis including factors with a p value <0.2 (or previously known to be clinically important) was performed. Receiver operating characteristic curve analysis was performed to identify factors that could predict the occurrence of hypotension before surgical incision, and the cut-off value was obtained using the maximum Youden index (sensitivity + specificity − 100). Statistical significance was set at p < 0.05. Statistical analyses were performed using SPSS software (version 22.0; SPSS Inc., Chicago, IL, USA).
For the sample size calculation, the mean blood pressure (mBP) was 80.6 ± 11.8 mmHg and 71.8 ± 14.3 mmHg in the loading and control groups, respectively; this corresponded with a previous study that examined fluid loading before anaesthesia induction among patients undergoing cervical spine surgery [Citation13]. Based on these data and the results of a G*Power analysis, a sample size of 36 patients per group was calculated (t-test, two-tailed, α error = 0.05, power = 0.8). Considering a dropout rate of 10%, the total number of participants was calculated to be 80, with 40 participants in each group.
3. Results
3.1. Study population
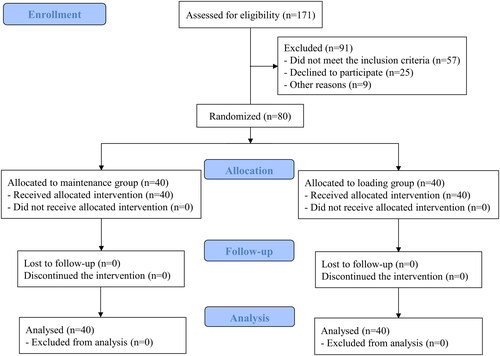
A total of 171 patients were recruited of which 91 were excluded and 80 were randomly assigned into two groups of equal size. Finally, 40 patients in each group were analysed without any loss to follow-up (). There were no significant differences between the groups regarding demographic data, medical history, preoperative laboratory test results or the number of operated levels (). There were no serious complications related to anaesthesia or surgery during the study.
Table 1. Demographic data of the study cohort.
3.2. Primary outcome
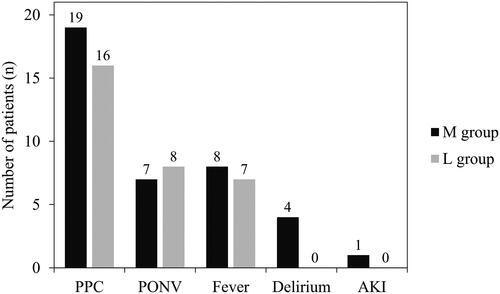
The incidence of hypotension before surgical incision was significantly higher in Group M than in Group L (). Moreover, a high baseline PVi was associated with hypotension in the logistic regression analysis of hypotension, which was less likely to occur in Group L (; ). In the receiver operating characteristic curve analysis of the baseline PVi and occurrence of hypotension before surgical incision, the cut-off value for PVi was 16 ().
Figure 2. Forest plot of the odds ratios for hypotension before surgical incision.
BP: blood pressure; PVi: pleth variability index.
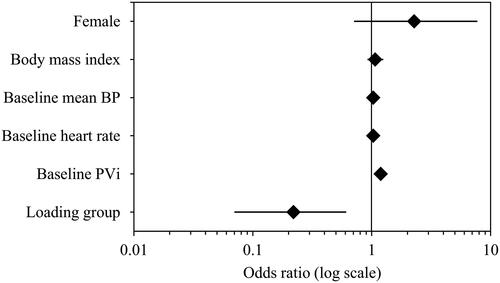
Figure 3. Receiver operating characteristic curve analysis of pleth variability index according to hypotension before surgical incision.
PVi: pleth variability index; AUC: area under curve; Sens: sensitivity; Spec: specificity; PPV: positive predictive value; NPV: negative predictive value.
Table 2. Perioperative data of the study cohort.
Table 3. Univariable and multivariable logistic regression analysis of factors associated with hypotension before surgical incision.
3.3. Secondary outcomes
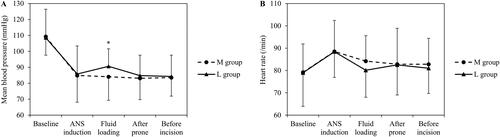
The mBP was significantly higher in Group L immediately after fluid loading, while the other parameters were similar in both groups (). There was no significant difference in HR between the two groups before surgical incision (; Supplementary Table 1).
Figure 4. Mean blood pressure and heart rate before surgical incision.
M: maintenance; L: loading; ANS: anaesthesia. *Significant differences.
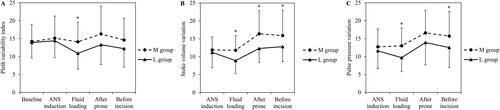
The PVi was significantly lower in Group L immediately after fluid loading; there were no significant differences between the two groups at other time points (). The SVV measured after arterial cannulation was significantly lower in Group L after fluid loading until surgical incision (), and the PPV was significantly lower in Group L immediately after fluid loading and before surgical incision (; Supplementary Table 2). The SVI and CI were not significantly different between the two groups before surgical incision (Supplementary Table 3).
Figure 5. Pleth variability index, stroke volume variation and pulse pressure variation before surgical incision.
M: maintenance; L: loading; ANS: anaesthesia. *Significant differences.
The dose of phenylephrine administered before skin incision was significantly higher in Group M. In each patient who received ephedrine, 5 mg was administered concomitantly with phenylephrine rather than alone (two patients in Group M and one patient in Group L); no significant differences were observed between the two groups. The amounts of intraoperative fluid administration and blood transfusion were higher in Group M; however, there was no significant difference between the groups. The estimated blood loss, urine output, operation time, discharge to the intensive care unit and postoperative HLOS were also comparable between the two groups ().
The incidences of composite PPC, pulmonary edoema and delirium as postoperative complications were not significantly different between the two groups (; Supplementary Table 4). The incidences of PONV, fever and AKI were also comparable between the two groups ().
4. Discussion
Our results indicate that fluid loading before a postural change can reduce the occurrence of hypotension before surgical incision in patients scheduled for lumbar surgery in the prone position. A higher baseline PVi was associated with the development of hypotension before surgical incision, with a cut-off value of 16. All three indices of fluid responsiveness decreased after fluid loading, with lower SVVs and PPVs, at several time points prior to incision. The incidence of postoperative complications was not significantly different; therefore, fluid loading before the postural change to the prone position did not increase the occurrence of postoperative complications or reduce the occurrence of hypotension before the surgical incision. Patients with a high baseline PVi should be prepared for hypotension, and fluid loading after anaesthesia induction is strongly recommended.
This study once again confirmed the usability of the PVi for predicting fluid responsiveness. Additionally, fluid preloading was demonstrated to be a safe method that did not increase postoperative complications. Throughout the study, we could not observe significant decreases in the incidence of PPC, amount of intraoperative fluid administered or amount of blood transfused in Group L owing to the relatively small sample size. However, we expect to determine whether these differences can be confirmed in further large-scale studies.
In this study, the PVi, SVV and PPV were evaluated as parameters of fluid responsiveness. All three parameters differed between the two groups immediately after fluid loading; however, only the SVV showed a significant difference between the two groups before surgical incision after fluid loading. The PVi did not show a significant difference between the two groups after posture change to the prone position. Monnet et al. [Citation23] reported that the PVi is less reliable than the PPV and SVV for predicting fluid responsiveness. Lee et al. [Citation24] also reported that the predictive power of PVi for fluid responsiveness was the lowest among the three parameters. In the present study, the PVi showed the smallest difference among the three parameters between the two groups after fluid loading, which may be a limitation of non-invasive monitoring.
Nevertheless, before the induction of anaesthesia, only the non-invasive PVi was measured at baseline. Still, unlike the SVV and PPV measured after the administration of anaesthetics, the baseline PVi could predict the development of hypotension before surgical incision. As such, the SVV and PPV measured in a hemodynamically altered state due to the administration of anaesthetics may not be useful for predicting hypotension. This demonstrates the value of non-invasive PVi in evaluating patients’ preanesthetic states. In previous studies, the cut-off value of the PVi for predicting fluid responsiveness or hypotension was approximately 12–16 in various situations [Citation25–28], matching the cut-off value of this study. In patients scheduled for lumbar surgery in the prone position, hypotension can be predicted by a baseline PVi >16 before the induction of anaesthesia, and fluid loading to prevent hypotension is highly recommended. The advantage of being able to non-invasively measure the PVi before the induction of anaesthesia is that the PVi showed patterns similar to those of the SVV and PPV; therefore, PVi was sufficient to use in clinical practice.
Additionally, there was a concern at the study design stage as to whether the incidence of PPC, such as pulmonary edoema, would increase due to a fluid load of 5 ml/kg in Group L. However, a fluid load of 5 ml/kg is the dose commonly administered when circulating plasma volume is insufficient in general clinical situations [Citation29]. There was no significant difference between the two groups regarding postoperative complications. The occurrence of PPC and delirium tended to be lower in Group L, without statistical significance; therefore, a fluid load of 5 ml/kg does not increase postoperative complications, and timely fluid administration can prevent hypotension. Further large-scale studies of fluid loading and postoperative complications are planned.
4.1. Limitations
This study had several limitations. First, the number of operating levels was not unified; however, there was no significant difference between the two groups in the randomized assignment, and we attempted to generalize the results to patients undergoing surgery in the prone position. Due to differences in the surgical incision range and surgical procedure, hemodynamic data during surgery were excluded from the analysis. Further research is required to unify the operative level and analyse the intraoperative data. Second, we could not measure the baseline SVV and PPV before anaesthesia induction. At this medical centre, we do not attempt arterial cannulation while awake unless the patient requires particularly strict BP control. This is to avoid patients’ complaints and complications such as vessel injury and unintentional movement. For research purposes alone, no additional painful procedures could be performed. The baseline PVi was measured to determine the patients’ basal fluid responsiveness, and significant results were obtained. Third, the PVi is a tool designed to evaluate fluid responsiveness in patients under mechanical ventilation. The accuracy of the baseline PVi in the present study may be unreliable, owing to its measurement in patients receiving spontaneous ventilation. However, the results for PVi in spontaneous ventilation were clinically significant; if these results are repeated in further research, we believe that the scope for the clinical use of the PVi may expand in the future, and we believe that the results of this study can serve as a good reference for further studies. Fourth, the cardiopulmonary function of individual participants could not be measured. Pulmonary function or 6-min walking tests – which require additional time and cost – may be considered; however, additional tests were difficult to conduct for research purposes alone. Still, there was no difference in age, sex and BMI between the two groups, and we attempted to minimize bias by excluding underlying diseases that could affect cardiopulmonary function. Finally, the BP of both groups was maintained without significant differences at the remaining time points by administering a vasopressor to patients with hypotension, except immediately after fluid loading. This may have led to an underestimation of the effect on fluid load, such as postoperative outcomes. Additionally, the effects of phenylephrine and ephedrine on HR cannot be excluded. The HR may have been higher in Group M owing to a lack of effective circulatory volume; however, the HR decreased owing to the administration of more phenylephrine, which may have offset the difference in HR between the two groups. Nonetheless, ethically, hypotension must be corrected, even if the effect of fluid loading cannot be accurately assessed. Further studies should be conducted to analyse the effects of intravenous fluid loading or vasopressor administration in cases of hypotension.
5. Conclusions
In patients scheduled for lumbar surgery in the prone position, fluid loading after anaesthesia induction could reduce the occurrence of hypotension until the surgical incision. Additionally, hypotension could be predicted in patients with a baseline PVi >16. Therefore, in patients with a high baseline PVi, intravenous fluid loading is strongly recommended to prevent hypotension after anaesthesia induction and in the prone position. Furthermore, large-scale prospective studies on the fluid load and postoperative outcomes should be conducted.
Author contributions
Sangho Lee: conceptualization, formal analysis, validation and writing – original draft. Doh Yoon Kim: investigation and writing – review and editing. Jihoon Han: writing – review and editing. Kyungmi Kim: data curation, formal analysis and writing – original draft. Ann Hee You: methodology, visualization and writing – original draft. Hee Yong Kang: supervision and writing – review and editing. Sung Wook Park: conceptualization, supervision and writing – review and editing. Mi Kyeong Kim: validation, supervision and writing – review and editing. Jung Eun Kim: formal analysis, supervision and writing – review and editing. Jeong-Hyun Choi: conceptualization, validation, supervision and writing – review and editing.
Supplemental Material
Download Zip (63.2 KB)Acknowledgements
The authors gratefully acknowledge the statistical support provided by the Department of Clinical Epidemiology and Biostatistics at the Asan Medical Center, University of Ulsan College of Medicine.
Disclosure statement
The authors report no conflict of interest.
Data availability statement
The datasets used and analysed in the current study are available from the corresponding author upon reasonable request. The data are not publicly available because of privacy and ethical restrictions.
References
- Schnake KJ, Rappert D, Storzer B, et al. Lumbar fusion – indications and techniques. Orthopade. 2019;48(1):50–58.
- Lee JH, Lee JH, Yoon KS, et al. Effect of intraoperative position used in posterior lumbar interbody fusion on the maintenance of lumbar lordosis. J Neurosurg Spine. 2008;8(3):263–270. doi: 10.3171/SPI/2008/8/3/263.
- Edgcombe H, Carter K, Yarrow S. Anaesthesia in the prone position. Br J Anaesth. 2008;100(2):165–183. doi: 10.1093/bja/aem380.
- Dharmavaram S, Jellish WS, Nockels RP, et al. Effect of prone positioning systems on hemodynamic and cardiac function during lumbar spine surgery: an echocardiographic study. Spine (Phila Pa 1976). 2006;31(12):1388–1393; discussion 1394. doi: 10.1097/01.brs.0000218485.96713.44.
- Jozwiak M, Monnet X, Teboul JL. Optimizing the circulation in the prone patient. Curr Opin Crit Care. 2016;22(3):239–245. doi: 10.1097/MCC.0000000000000308.
- Hao D, Low S, Di Fenza R, et al. Prone positioning of intubated patients with an elevated body-mass index. N Engl J Med. 2022;386(14):e34. doi: 10.1056/NEJMvcm2108494.
- Ni L, Fan Y, Bian J, et al. Effect of body mass on oxygenation and intra-abdominal pressure when using a Jackson surgical table in the prone position during lumbar surgery. Spine (Phila Pa 1976). 2018;43(14):965–970. doi: 10.1097/BRS.0000000000002505.
- Ali A, Dorman Y, Abdullah T, et al. Ability of mini-fluid challenge to predict fluid responsiveness in obese patients undergoing surgery in the prone position. Minerva Anestesiol. 2019;85(9):981–988. doi: 10.23736/S0375-9393.19.13276-2.
- Lai C, Adda I, Teboul JL, et al. Effects of prone positioning on venous return in patients with acute respiratory distress syndrome. Crit Care Med. 2021;49(5):781–789. doi: 10.1097/CCM.0000000000004849.
- Jin SJ, Park YS, Kim SH, et al. Effect of prone positional apparatus on the occurrence of acute kidney injury after spine surgery. World Neurosurg. 2019;128:e597–e602. doi: 10.1016/j.wneu.2019.04.216.
- Kim E, Kim HC, Lim YJ, et al. Comparison of intra-abdominal pressure among 3 prone positional apparatuses after changing from the supine to the prone position and applying positive end-expiratory pressure in healthy euvolemic patients: a prospective observational study. J Neurosurg Anesthesiol. 2017;29(1):14–20. doi: 10.1097/ANA.0000000000000257.
- Lee CT, Lee TS, Chiu CT, et al. Mini-fluid challenge test predicts stroke volume and arterial pressure fluid responsiveness during spine surgery in prone position: a STARD-compliant diagnostic accuracy study. Medicine. 2020;99(6):e19031. doi: 10.1097/MD.0000000000019031.
- Paul A, Sriganesh K, Chakrabarti D, et al. Effect of preanesthetic fluid loading on postinduction hypotension and advanced cardiac parameters in patients with chronic compressive cervical myelopathy: a randomized controlled trial. J Neurosci Rural Pract. 2022;13(3):462–470. doi: 10.1055/s-0042-1749459.
- Biais M, Bernard O, Ha JC, et al. Abilities of pulse pressure variations and stroke volume variations to predict fluid responsiveness in prone position during scoliosis surgery. Br J Anaesth. 2010;104(4):407–413. doi: 10.1093/bja/aeq031.
- Messina A, Montagnini C, Cammarota G, et al. Assessment of fluid responsiveness in prone neurosurgical patients undergoing protective ventilation: role of dynamic indices, tidal volume challenge, and end-expiratory occlusion test. Anesth Analg. 2020;130(3):752–761. doi: 10.1213/ANE.0000000000004494.
- Berger K, Francony G, Bouzat P, et al. Prone position affects stroke volume variation performance in predicting fluid responsiveness in neurosurgical patients. Minerva Anestesiol. 2015;81(6):628–635.
- Fischer MO, Lemoine S, Tavernier B, et al. Individualized fluid management using the pleth variability index: a randomized clinical trial. Anesthesiology. 2020;133(1):31–40. doi: 10.1097/ALN.0000000000003260.
- Cannesson M, Desebbe O, Rosamel P, et al. Pleth variability index to monitor the respiratory variations in the pulse oximeter plethysmographic waveform amplitude and predict fluid responsiveness in the operating theatre. Br J Anaesth. 2008;101(2):200–206. doi: 10.1093/bja/aen133.
- Forget P, Lois F, de Kock M. Goal-directed fluid management based on the pulse oximeter-derived pleth variability index reduces lactate levels and improves fluid management. Anesth Analg. 2010;111(4):910–914. doi: 10.1213/ANE.0b013e3181eb624f.
- Jammer I, Wickboldt N, Sander M, et al. Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: European Perioperative Clinical Outcome (EPCO) definitions: a statement from the ESA-ESICM joint taskforce on perioperative outcome measures. Eur J Anaesthesiol. 2015;32(2):88–105. doi: 10.1097/EJA.0000000000000118.
- Niven DJ, Léger C, Stelfox HT, et al. Fever in the critically ill: a review of epidemiology, immunology, and management. J Intensive Care Med. 2012;27(5):290–297. doi: 10.1177/0885066611402463.
- Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–c184. doi: 10.1159/000339789.
- Monnet X, Guérin L, Jozwiak M, et al. Pleth variability index is a weak predictor of fluid responsiveness in patients receiving norepinephrine. Br J Anaesth. 2013;110(2):207–213. doi: 10.1093/bja/aes373.
- Lee SH, Chun YM, Oh YJ, et al. Prediction of fluid responsiveness in the beach chair position using dynamic preload indices. J Clin Monit Comput. 2016;30(6):995–1002. doi: 10.1007/s10877-015-9821-5.
- Liu YF, Song LL, Ma W, et al. Dynamic variables to predict fluid responsiveness in young children. Pediatr Int. 2023;65(1):e15477. doi: 10.1111/ped.15477.
- Lee HC, Tsai YF, Tsai HI, et al. Pulse oximeter-derived pleth variability index is a reliable indicator of cardiac preload in patients undergoing liver transplantation. Transplant Proc. 2016;48(4):1055–1058. doi: 10.1016/j.transproceed.2015.12.106.
- Thirunelli RK, Nanjundaswamy NH. A prospective observational study of plethysmograph variability index and perfusion index in predicting hypotension with propofol induction in noncardiac surgeries. Anesth Essays Res. 2021;15(2):167–173.
- Yüksek A. Utility of the pleth variability index in predicting anesthesia-induced hypotension in geriatric patients. Turk J Med Sci. 2021;51(1):134–139. doi: 10.3906/sag-1912-132.
- Rijs K, Mercier FJ, Lucas DN, et al. Fluid loading therapy to prevent spinal hypotension in women undergoing elective caesarean section: network meta-analysis, trial sequential analysis and meta-regression. Eur J Anaesthesiol. 2020;37(12):1126–1142. doi: 10.1097/EJA.0000000000001371.