Abstract
Objective
Tension-type headache is the most common type of primary headache and results in a huge socioeconomic burden. This network meta-analysis (NMA) aimed to compare the efficacy and safety of simple analgesics for the treatment of episodic tension-type headache (ETTH) in adults.
Methods
We searched the Cochrane Library, PubMed, Web of Science, Embase, Chinese BioMedical Literature database and International Clinical Trials Registry Platform databases for eligible randomized clinical trials reporting the efficacy and/or safety of simple analgesics. A Bayesian NMA was performed to compare relative efficacy and safety. The surface under the cumulative ranking curve (SUCRA) was calculated to rank interventions. PROSPERO registration number: CRD42018090554.
Results
We highlighted six studies including 3507 patients. For the 2 h pain-free rate, the SUCRA ranking was ibuprofen > diclofenac-K > ketoprofen > acetaminophen > naproxen > placebo. All drugs except naproxen reported a higher 2 h pain-free rate than placebo, with a risk ratio (RR) of 2.86 (95% credible interval, CrI: 1.62–5.42) for ibuprofen and 2.61 (1.53–4.88) for diclofenac-K. For adverse events rate, the SUCRA ranking was: metamizol > diclofenac-K > ibuprofen > lumiracoxib > placebo > aspirin > acetaminophen > naproxen > ketoprofen. The adverse event rates of all analgesics were no higher than those of placebo, except for ketoprofen. Moreover, all drugs were superior to placebo in the global assessment of efficacy. In particular, the RR of lumiracoxib was 2.47 (1.57–4.57). Global heterogeneity I2 between the studies was low.
Conclusions
Simple analgesics are considered more effective and safe as a placebo for ETTH in adults. Our results suggest that ibuprofen and diclofenac-K may be the two best treatment options for patients with ETTH from a comprehensive point of view (both high-quality evidence).
KEY MESSAGES
To our knowledge, this is the first network meta-analysis comparing the available data on adult patients with episodic tension-type headache (ETTH) treated with different simple analgesics recommended by the current guidelines.
Ibuprofen (400 mg) and diclofenac-K (12.5 mg, 25 mg) are potentially the most effective and safe treatment options, supported by high-quality evidence.
Introduction
Tension-type headache (TTH) is the most common type of primary headache, affecting an estimated 2.3 billion cases worldwide [Citation1,Citation2]. It is also one of the most common reasons why over-the-counter analgesics are purchased. TTH typically manifests as mild-to-moderate pain in the head, which is usually described as a feeling of tight bands around the head. TTH may be associated with disability, lower work efficiency, absenteeism or decreased learning ability, leading to deteriorated quality of life [Citation2].
Since 1995, TTH has been classified as chronic tension-type headache (CTTH) and episodic tension-type headache (ETTH) by the International Headache Society (IHS) in the first edition of the International Classification of Headache Disorders (ICHD). The latter type is further classified into frequent and infrequent subtypes in ICHD-II [Citation3,Citation4]. Among them, peripheral pain mechanism plays a dominant role in the pathophysiology of ETTH, while CTTH is induced by a central pain mechanism [Citation5]. Pharmacotherapy is recommended for a TTH patient if non-pharmaceutical therapy shows limited effect [Citation6]. Drugs for symptomatic relief are routinely prescribed to ETTH patients, while prophylactics should be considered for CTTH patients [Citation4,Citation7].
Clinical practice guidelines (CPGs) from different countries recommend drug therapies, among which simple analgesic agent monotherapies are prioritized for acute treatment of ETTH in adults. Other acute interventions could be considered when the preferred drugs are unresponsive, such as combination agents or muscle relaxants. However, the recommendation levels and strengths of simple analgesics are inconsistent among CPGs for ETTH [Citation7–16]. Taking ibuprofen and ketoprofen as an example, they are recommended as level A in the EFNS guideline, while the Italian guideline lists them as level II [Citation7,Citation15]. Other confusion in CPGs is that multiple simple analgesics are listed at the same level without a preferential order. Such inconsistency in recommendation and absence of preferential orders in CPGs brings clinicians and patients who treat with over-the-counter into bewilderment in their choice of simple analgesics, leading to greater discrepancies in clinical practice. In some cases, these discrepancies may lead to an increased risk of adverse events, drug abuse or drug dependence, especially when these medications are not used rationally.
This inconsistency can be attributed to the lack of direct evidence. Few published studies have reported head-to-head comparisons of medications for acute episodes of TTH. Thus, a pairwise meta-analysis was not feasible because of insufficient direct evidence. Previous studies have shown that combining direct evidence with indirect evidence may improve the accuracy of evaluation of therapeutic measures [Citation17]. To compare all recommended therapies by synthesizing currently available direct and indirect clinical evidence, it is necessary to perform a network meta-analysis (NMA) [Citation18]. Hence, we performed an NMA to provide a ranking of simple analgesics for ETTH in adults based on their efficacy and safety.
Methods
Search strategy and selection criteria
This NMA is reported in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement [Citation19]. The study protocol has been registered on PROSPERO (http://www.crd.york.ac.uk/PROSPERO; registration number CRD42018090554) and published on BMJ Open [Citation20]. The PRISMA NMA checklist is provided in Supplementary Table S1. We conducted a structured search of the Cochrane Library, PubMed, Web of Science, Embase, Chinese BioMedical Literature database and International Clinical Trials Registry Platform (ICTRP) database from their inception to 30 June 2023. With free text words and medical subject heading, we searched for the following simple analgesic agents: aspirin/acetylsalicylic acid, acetaminophen/paracetamol, lumiracoxib, ibuprofen, ketoprofen, naproxen, diclofenac, diclofenac-K and metamizol/dipyrone. Details of the search strategy are presented in Supplementary Table S2. We included only published randomized clinical trials (RCTs) of ETTH treatments, in which at least one simple analgesic monotherapy was compared with another, or with either a blank control or placebo. Studies involving multiple or nonpharmaceutical therapies were excluded. The patients were over 18 years old and were diagnosed with ETTH in accordance with the ICHD-II [Citation4]. There were no restrictions on sex, race and nationality of the studied patients or the dosage of drugs. Publications in either English or Chinese are acceptable. In addition, we manually screened the reference lists of the included articles for potentially eligible studies. Two authors (R.X. and Y.W.) independently searched for eligible studies and screened them by their titles and abstracts, following the inclusion and exclusion criteria specified above. Disagreements were resolved by a third referee (H.L.).
Data extraction and quality assessment
Data including characteristics of the studies, demographics of the studied patients, interventions and outcome measurements were extracted from the included studies with pre-designed spreadsheets by two authors (R.X. and Y.W.). Two reviewers evaluated the risk of bias of each study in the following areas using the Cochrane Risk of Bias tool [Citation18]: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other sources of bias. Each study was judged to have either ‘low risk bias’, ‘high risk of bias’ or ‘unclear risk of bias’ in accordance with the bias evaluation criteria. Disagreements were resolved by a third expert (H.L.). Additionally, we presented a four-step approach to rate the quality of evidence in each of the direct, indirect and NMA estimates based on methods developed by the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group. Evidence was rated as high, moderate, low and very low quality depending on their research limitations (risk of bias), indirectness, inconsistency, imprecision and publication bias [Citation21].
Outcome measures
We chose the proportion of patients being pain-free at two hours postdose (2 h pain free rate) as the primary efficacy measurement, which is recommended by the Guidelines for Controlled Trials of Drugs in Tension-Type Headache [Citation22]. The proportion of patients coming across adverse events rate (adverse events rate) during a trial was selected as the primary safety measurement because it is the most commonly reported safety indicator in trials [Citation23]. The secondary efficacy measurement was the proportion of patients reporting good, very good or excellent in global assessment of efficacy.
Data synthesis and statistical analysis
First, a summary describing the characteristics of the included studies, demographics of patients, interventions, and an assessment of the risk of bias was provided. In addition, we summarized the evidence network graphically for presentation. Subsequently, a Bayesian NMA was conducted to compare the efficacy and safety of different pharmaceutical monotherapies using their medians of posterior distribution. Heterogeneity among studies was assessed using Q test and I2 index [Citation24]. An NMA with fixed effects model within the Bayesian framework was performed on Just Another Gibbs Sampler (JAGS version 4.2.0) and on R (version 3.4.4; R Foundation for Statistical Computing, Vienna, Austria) using ‘gemtc’, ‘R2WinBUGS’, ‘lattice’ and ‘coda’ packages for further analysis [Citation25]. Pooled estimates were obtained by The Markov chains Monte Carlo (MCMC) simulation. The effect size was captured using the risk ratio (RR) and 95% credible interval (95% CrI). The numbers needed to treat and absolute risk reduction were also calculated. Four Markov chains were run simultaneously with 5000 pre-iterations and 50,000 iterations, and a step size of 1. The convergence of MCMC was evaluated using the trace plot and Brooks–Gelman–Rubin statistics [Citation26]. When a closed loop was formed, inconsistency between direct and indirect comparison of evidence was measured by the node-splitting model. Eventually, the probability of each therapy being the most efficacious treatment was estimated using the posterior probability. Surface under the cumulative ranking curve (SUCRA) was used to rank the medications by their probabilities to be the best one [Citation27]. Similarity between clinical and methodological characteristics of these studies was compared qualitatively [Citation28]. Publication bias and small-sample research effects were evaluated with funnel plots. Sensitivity analysis was used to compare the performance of different effect models (fixed and random) and effect sizes (RR and odds ratio (OR)) for different outcome measures using the deviance information criterion (DIC). The difference could be regarded as acceptable in the case of DIC < 5. We performed additional subgroup analyses for the primary efficacy measurement of interest on medications that were prescribed in multiple dosages, which were compared as subgroups after this type of medication was analysed as a single group.
Results
Database search
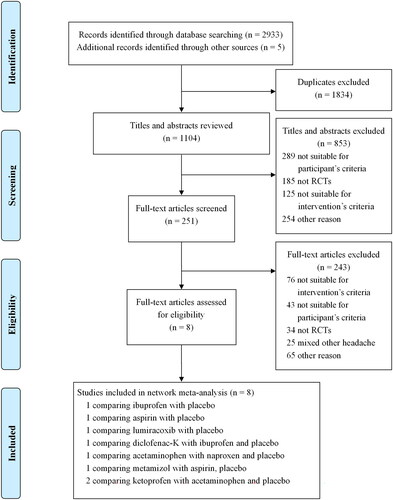
The PRISMA flow diagram of the study search process is shown in . In total, 2933 citations were identified. One thousand eight hundred and thirty-four duplicates were removed, and 251 potentially eligible articles were retrieved in full text. Eight studies were included [Citation29–36], comprising a total of 3507 patients diagnosed with ETTH, comparing nine types of simple analgesics or placebo.
Study characteristics and bias assessment (similarity assessment)
Supplementary Table S3 summarizes the main characteristics of the included studies. The included trials were conducted between 1998 and 2015, with a mean sample size of 438 (range, 150–900). The average age reported in these studies ranges from 30 to 45 years. Women accounted for the majority of the sampled patients. A total of 2526 participants were randomly assigned to the intervention group and 981 to the placebo group. The demographics and clinical characteristics of the patients were reported in all studies. The IHS/ICHD diagnostic criteria were referenced or used in the inclusion and exclusion criteria for almost every study. Fifty percent of these trials were performed in the United States, 25.0% in Germany and the rest in the UK, Spain and Brazil.
The risk of bias for each included study is presented in Supplementary Table S4. The methods of random sequence generation and allocation concealment were not clearly reported in most of the studies (62.5% and 75.0%, respectively). One study was rated as having a high risk of bias in blinding evaluation (participants and personnel, outcome assessment) [Citation31], while the other studies (87.5%) were considered low risk. As for attrition bias, the completeness of the outcome data for each study was rated as having a low risk of bias. Reporting bias was considered high in two studies (not all of the pre-specified primary outcomes of the study have been reported) [Citation35,Citation36], and six studies (75.0%) were rated as low risk. Five studies (62.5%) did not present sufficient information to assess whether another important risk of bias existed. These results indicated that the clinical and methodological characteristics of the included studies were similar and comparable.
Heterogeneity
The global I2 index for the 2 h pain-free rate and adverse events rate was 0, with I2 for global assessment of efficacy at 17.7, all of which indicated low heterogeneity.
Model convergence
The Bayesian models in this study reported satisfactory convergence based on density plots, trace plots and Brooks–Gelman–Rubin diagnostic diagrams, with a potential scale reduction factor value approaching 1. Due to low heterogeneity, a fixed effects model was used for a 2 h pain-free rate, adverse event rate and global assessment of efficacy, with DIC values of 18.02, 35.01 and 23.52, respectively.
Evidence network
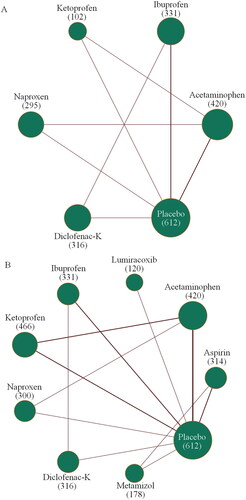
and Supplementary Figure S1 show the evidence network plots of eligible comparisons for efficacy and safety, with the sample size of each drug being over 100 participants. Four included studies reported a 2 h pain-free rate as a measure of the efficacy of acetaminophen, ibuprofen, ketoprofen, naproxen, diclofenac-K and placebo [Citation29]. In terms of safety, all eight included studies reported the adverse event rate, involving each type of simple analgesic and placebo [Citation29,Citation32,Citation33,Citation35]. As for the secondary efficacy measurement, the global assessment of efficacy, was reported in four included studies, which involved acetaminophen, lumiracoxib, ibuprofen, ketoprofen, diclofenac-K and placebo [Citation31,Citation32,Citation35,Citation36].
Figure 2. Evidence network plots of treatment comparisons for primary efficacy (A) and safety (B). The width of the lines was proportional to the number of studies comparing every pair of treatments. The size of each circle is proportional to the number of randomly assigned participants (i.e. sample size, given in parentheses).
Findings from NMA
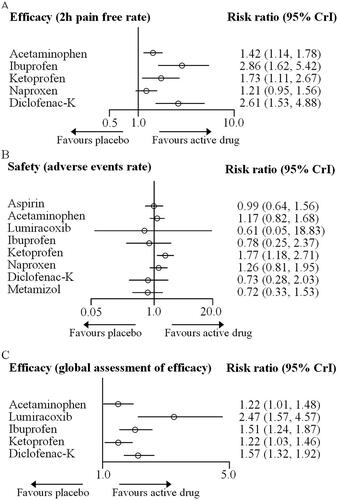
As shown in , forest plots for the efficacy and safety outcomes were generated. In terms of the 2 h pain-free rate, all simple analgesics except naproxen were more effective than the placebo. Ibuprofen (RR 2.86, 95% CrI 1.62–5.42), diclofenac-K (RR, 2.61, 1.53–4.88), ketoprofen (RR, 1.73, 1.11–2.67) and acetaminophen (RR, 1.42, 1.14–1.78) were associated with a higher 2 h pain-free rate than placebo. Regarding safety, all simple analgesics reported similar adverse event rates compared to placebo, except for ketoprofen (RR 1.77, 95% CrI 1.18–2.71). Besides, all simple analgesics reported higher global assessment of efficacy than that of placebo, with RRs ranging between 2.47 (95% CrI 1.57–4.57) for lumiracoxib and 1.22 (1.01, 1.48) for acetaminophen.
Figure 3. Forest plots for the outcomes of efficacy and safety. Simple analgesics were compared with placebo, which was the reference compound.
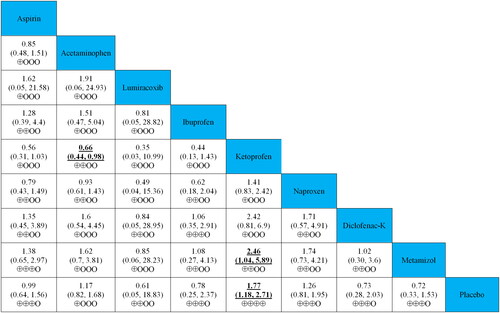
The relative effects of head-to-head comparisons to assess the differences between simple analgesics for efficacy and safety are also shown in and and Supplementary Figure S2. The 2 h pain free rate of ibuprofen and diclofenac-K was more efficacious than those of acetaminophen and naproxen. Regarding safety, acetaminophen and metamizol reported lower adverse event rates than ketoprofen (RRs ranging between 0.41 and 0.66). Furthermore, the global assessment of the efficacy of lumiracoxib was better than that of acetaminophen and ketoprofen (RRs 0.49).
Figure 4. Relative effects of head-to-head comparisons for efficacy (2 h pain free rate). Drugs are reported in the alphabetical order. Data are presented as risk ratios (RRs) and 95% credible intervals in the column-defining treatment compared to the row-defining treatment. RRs higher than one favour column-defining treatments. Reciprocals should be used to obtain the RRs for comparisons in the opposite direction. The significant results are shown in bold and underlined. The certainty of the evidence (according to GRADE) has been incorporated in Supplementary Table S7. (⊕⊕⊕⊕) High-quality evidence. (⊕⊕⊕O) Moderate-quality evidence. (⊕⊕OO) Low-quality evidence. (⊕OOO) Very low-quality evidence.
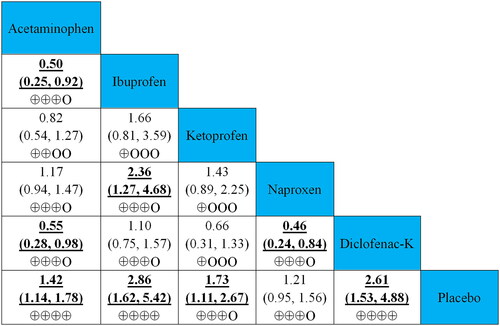
Figure 5. Relative effects of head-to-head comparisons for safety (adverse events rate). Drugs are reported in the alphabetical order. Data are presented as risk ratios (RRs) and 95% credible intervals in the column-defining treatment compared to the row-defining treatment. RRs higher than one favour column-defining treatments. Reciprocals should be used to obtain the RRs for comparisons in the opposite direction. The significant results are shown in bold and underlined. The certainty of the evidence (according to GRADE) has been incorporated in Supplementary Table S7. (⊕⊕⊕⊕) High-quality evidence. (⊕⊕⊕O) Moderate-quality evidence. (⊕⊕OO) Low-quality evidence. (⊕OOO) Very low-quality evidence.
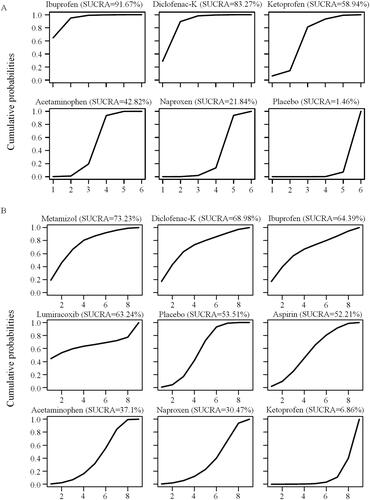
Rankings of medications
The priority of simple analgesics for ETTH was ranked based on the cumulative probability plots and SUCRA values (). Ibuprofen was found to have the highest probability (91.7%) of being the best treatment for ETTH, with a 2 h pain-free rate, whereas placebo was the worst option with a probability of 1.5%. The ranking of all medications of interest by efficacy was ibuprofen > diclofenac-K > ketoprofen > acetaminophen > naproxen > placebo. In terms of adverse event rates, metamizol was found to have the highest probability of 73.2% being the safest simple analgesic, whereas ketoprofen was the least common, with a probability of 6.9%. Safety was ranked as follows: metamizol > diclofenac-K > ibuprofen > lumiracoxib > placebo > aspirin > acetaminophen > naproxen > ketoprofen. The ranking of secondary outcomes is shown in Supplementary Figure S3.
Adverse events
As shown in , 315 (8.6%) adverse events among 3648 participants were reported in all the included studies. No serious adverse events or deaths occurred. Adverse events involving the digestive system (mainly nausea and dyspepsia) occurred in 31.1% (98 cases) of the reported events. Adverse events of the nervous system (including drowsiness, dizziness and xerostomia) totalled 85 cases (27.0%).
Table 1. Summary of adverse events reported in included studies.
Consistency
Since none of the three outcome measurements made a closed loop, we were unable to separate evidence in one loop into direct and indirect comparisons using the node-splitting model.
Sensitivity analysis and small-study effect
The sensitivity analysis results of different effect models and effect sizes between outcomes using DICs are presented in Supplementary Table S5. Among the three outcome measures of interest, all the differences in DICs between the effect models (fixed- and random-effect models) and effect sizes (RR and OR) were less than 5, which could be regarded as acceptable. We also conducted comparison-adjusted funnel plots for the outcome measures; see Supplementary Figure S4. After using the trim and fill method, the results were not reversed, which indicates that the results are robust with small sample sizes.
Subgroup analysis
As shown in Supplementary Table S6, groups of patients taking ibuprofen and diclofenac-K were further divided into subgroups according to dosage or ingredients of tablets for subgroup analysis. Patients prescribed ibuprofen were divided into two subgroups: sodium ibuprofen (ibuprofenNa) and standard ibuprofen (ibuprofenStd) by the respective ingredients. Patients in both subgroups received either a single dose of 400 mg ibuprofen ibuprofenStd or ibuprofenNa at an equivalent dose. The IbuprofenNa group reported no statistically significant difference from the ibuprofenStd group in the 2 h pain-free rate, with an RR of 1.39 (95% CrI 0.42–4.79). In addition, both ibuprofenNa and IbuprofenStd were better than placebo at a 2 h pain-free rate (RR 3.95 [95% CrI 1.08–15.27] and 2.81 [1.60–5.40], respectively). The diclofenac-K group of patients was divided into two subgroups by dosage (single dose of either 12.5 mg or 25 mg). There was no significant difference between the two subgroups in the 2 h pain-free rate (12.5 mg vs. 25 mg, RR 0.81 [95% CrI 0.51–1.25]), both of which were more efficacious than the placebo (RR 2.30 [1.27–4.47] and 2.85 [1.60–5.46], respectively).
Quality assessment of evidence
We presented the details of the quality assessment (GRADE) in Supplementary Table S7 and incorporated the judgments in and and Supplementary Figure S2. The certainty of the evidence for the relative effects of efficacy and acceptability varies. It was graded as high- or moderate-quality evidence for most (73.3%) of the comparisons involving acetaminophen, ibuprofen and diclofenac-K at a 2 h pain-free rate. Low- or very-low-quality evidence was reported in 77.8% and 66.7% of adverse event rates and global assessment of efficacy, respectively.
Discussion
This NMA is a comprehensive analysis of the available data on adult patients with ETTH treated with different simple analgesics recommended by the current guidelines, aiming to provide evidence-based medical evidence for clinical practice decisions. Our results indicate that simple analgesics were more efficacious than placebo in terms of the 2 h pain-free rate and global assessment of efficacy, while being as safe as the latter. Among the acute medications of interest, ibuprofen (400 mg) was the most likely (91.7%) to be the optimal treatment for ETTH (high-quality evidence), with adverse event rates similar to those of other simple analgesics. In addition, a previous study reported that ibuprofen had fewer short-term side effects than other simple analgesics for ETTH [Citation37]. Hence, we recommend ibuprofen over other simple analgesics for the acute treatment of ETTH, which coincides with the recommendation of several CPGs [Citation7,Citation11]. Similarly, diclofenac-K (12.5 mg and 25 mg) was also found to be a good option (83.3% probability of being the best) for rapid and short-term ETTH relief (high quality evidence), for its efficacy and safety measurement were not statistically different from those of ibuprofen. Furthermore, dosage-wise subgroup analysis found no statistically significant difference between the two dosages when measured a 2 h pain-free rate. Considering that a smaller dosage lowers drug safety risks and eases the economic burden on patients, we recommend a low single dose (12.5 mg) for treating ETTH, unless a higher dosage is necessary when headache intensifies progressively. Although a high dosage of diclofenac-K (50–100 mg) proved efficacious for migraine, no trials hitherto have evaluated the efficacy of such a dosage on ETTH [Citation7,Citation32]. In addition, we found that ibuprofen and diclofenac-K were more effective for acute therapy than acetaminophen and naproxen (both moderate quality evidence), for a higher headache pain-free rate at 2 h.
Ibuprofen, known for its analgesic, anti-inflammatory and antipyretic properties, is rapidly and completely absorbed after oral administration, exhibiting a pharmacokinetic profile with extensive (>98%) plasma protein binding and a low apparent volume of distribution [Citation38]. It penetrates into the central nervous system and accumulates at peripheral sites, which is necessary for its therapeutic effects. The primary metabolic pathway of ibuprofen involves oxidative metabolism by CYP enzymes to inactive metabolites, with a relatively short plasma half-life, requiring frequent administration to maintain therapeutic plasma concentrations [Citation38,Citation39]. Regarding the pharmacodynamics, ibuprofen exerts its effects primarily through the non-selective reversible inhibition of the cyclooxygenase enzymes COX-1 and COX-2, leading to the suppression of prostanoid synthesis, key mediators in pain perception [Citation40]. Additionally, ibuprofen’s ability to cross the blood-brain barrier allows it to exert central analgesic effects. Furthermore, ibuprofen may activate the antinociceptive axis through binding to the cannabinoid receptors and through inhibition of fatty acid amide hydrolase, which metabolizes the endocannabinoid anandamide [Citation41]. This interaction with the endocannabinoid system, along with previous findings suggesting that ibuprofen may enhance the levels of anandamide, contributes to ibuprofen’s analgesic effects. The activation of cannabinoid receptors (CB1 and CB2) by anandamide in the central nervous system can offer an additional mechanism by which ibuprofen relieves pain, beyond its COX-inhibitory activity [Citation40]. This potential modulation of the endocannabinoid system by ibuprofen could partially explain its superior efficacy in the treatment of ETTH compared to other analgesics. Similarly, the pharmacological action of diclofenac potassium is primarily based on its inhibition of cyclooxygenase [Citation42]. Diclofenac potassium is a non-selective, reversible and competitive inhibitor of cyclooxygenase, subsequently blocking the conversion of arachidonic acid into prostaglandin precursors.
Another efficacy measurement, the global assessment of efficacy, is provided by participants after a certain course of treatment, in comparison to a 2 h pain-free rate measuring rapid response. It is often evaluated as excellent, very good, good, fair or poor by participants, reflecting the combined effect of medication efficacy, the natural prognosis of the disease, and placebo effects. Among the included studies, patients reported good or higher global assessment of efficacy in placebo groups, ranging from 20% to 52% (average 40.8%). Additionally, we found that all simple analgesics showed a greater global assessment of efficacy than placebo (RRs > 1), with lumiracoxib being the best. Although lumiracoxib was reported to be as safe as other treatments based on our NMA results, we still need to consider its potential risk to the hepatic system. It was reported by European Medicines Agency that long-term use of lumiracoxib might cause severe hepatic damage [Citation43]. They declared in December 2007 that the risk of lumiracoxib outweighed its benefit after extensive evaluation, and recommended withdrawal of this analgesic throughout the European Union.
An adverse event rate of 8.6% was estimated from the included studies, mainly affecting the digestive and nervous systems, which coincided with previous studies. Forest plots showed that the adverse event rates of simple analgesics were generally similar to those of placebo. Neither severe adverse events nor death events were reported in any of the included trials, indicating that simple analgesics were safe for clinical use. Avoiding medication overuse headache (MOH) is another important goal in ETTH treatment. No MOH cases were reported in this study. Nevertheless, the frequency of administering acute symptomatic drug use needs to be cautiously limited (i.e. no more than 14 days per month of non-steroidal anti-inflammatory drug use) [Citation44].
Regarding the risk of bias, we found that more than half of the studies did not specify how their random sequences were generated or how the allocation of patients was concealed. To reduce bias, it is recommended that details concerning the design and implementation of studies be disclosed in future reports on clinical trials. When using the results of this study, the scope of application should be noted. Special populations (e.g. pregnant participants) were not included in this study. Additionally, the participants included in our study were dominated by European and American origins. Subjects from some countries and regions (e.g. Asia and Africa, where relevant clinical studies are scarce) were not included in this study. Therefore, our findings may not be completely applicable to patients in other areas, because the prevalence of TTH varies widely among countries and regions. For example, it was reported that the prevalence of TTH in Europe was 80%, compared to 20–30% in Asia or the Americas [Citation45]. Considering such racial sensitivity, more multicentre trials will be needed in these countries and races in the future.
As with all studies, we cannot avoid several limitations. One limitation of this study was the lack of currently available evidence, especially high-quality randomized controlled trials, from which a closed loop failed to form in our NMA. As a result, the node-splitting model cannot be used to evaluate the consistency between direct and indirect comparisons of evidence. However, we could not include monotherapies of other categories into our NMA, such as antiemetic and complementary alternative medicine. However, very few trials that met our criteria were found involving these drugs, despite extensive searches (e.g. on the ICTRP database, ClinicalTrials.gov or manually screened the reference lists of included articles). With the available data of the present study, it was feasible for us only to perform NMA with simple analgesics for the acute treatment of ETTH. Meanwhile, the possibility of neglecting unpublished studies or reports could not be ruled out, which might have led to an overestimation of the efficacy and safety of these analgesics. In addition, studies of combination and traditional medicine therapy [Citation46], which may be our next research direction, were not included in this research. For example, several guidelines have recommended that combination therapies, particularly those analgesics containing caffeine, are often considered second-choice drugs for the acute treatment of TTH [Citation7,Citation9,Citation12]. In light of previous meta-analyses and emerging evidence, the findings suggest that combining caffeine 130 mg with analgesic medications, such as acetaminophen, acetylsalicylic acid and ibuprofen, significantly improves efficacy over the analgesic alone [Citation47]. It is reported that caffeine combination therapy is well tolerated by the vast majority of patients, and adverse events are predictable and almost universally mild and transient. Finally, since data at the individual patient level were not available, we only used data published in the included studies, which may affect the accuracy of the results.
While clinicians’ confusion regarding the choice of drugs for ETTH could be resolved by the findings of this study, it is important to recognize the dynamic nature of headache disorders and the ever-changing medical landscape, as exemplified by the COVID-19 pandemic [Citation48]. Headache is among the most frequent symptoms persisting or newly developing after COVID-19 as part of the so-called long COVID syndrome [Citation49]. This condition can manifest either as a worsening of a pre-existing primary headache or as a new headache (intermittent or daily). Notably, most headaches associated with COVID-19 are classified as headaches attributed to a systemic viral infection. These headaches typically exhibit bilateral and pressing qualities, and their presentation often aligns more closely with the phenotype of TTHs rather than migrainous ones. Acute COVID-19 headaches that develop into long COVID headaches are often treatment-resistant [Citation50]. Treatment should take into account headache phenotype, comorbidities and additional post-COVID-19 symptoms. This highlights the complexity and the need for a nuanced approach to treatment. Patients with long COVID headache require a multidisciplinary treatment approach, including pharmacological (acute and preventative) and non-pharmacological strategies [Citation51]. This comprehensive treatment approach is essential to address the various manifestations and the potentially resistant nature of long COVID headaches. In the context of evolving treatment strategies for headache disorders, recent advances have sparked a growing interest in exploring the potential treatment options targeting calcitonin gene-related peptide (CGRP) for TTH. These drugs, such as fremanezumab and galcanezumab [Citation52], have shown efficacy in migraine and cluster headache prevention by modulating the trigeminovascular system’s neuropeptide CGRP, which is implicated in headache pathogenesis [Citation53]. Additionally, acute treatments with CGRP receptor antagonists, such as rimegepant or ubrogepant, have demonstrated the capability to eliminate headache symptoms in a significant portion of patients [Citation54]. However, they are associated with adverse effects like nausea and dry mouth in a minority of cases. These findings suggest a promising direction for future research in the application of CGRP-targeting drugs for the management of TTH.
Conclusions
In summary, this study recommends the use of simple analgesics as an acute treatment for ETTH in adults. The recommended selection order was ibuprofen, diclofenac-K, ketoprofen, acetaminophen and naproxen. Ibuprofen (400 mg) and diclofenac-K (12.5 mg and 25 mg) may be the two best treatment options with respect to efficacy and safety performance (both high-quality evidence). Although simple analgesics have been proven as safe as placebo, clinicians still need to pay attention to potential adverse events related to the digestive or nervous systems. To avoid MOH, the frequency of administering simple analgesics as acute medication should be limited to no more than 14 days per month.
Author contributions
R.X.: study design, data acquisition, data analysis and interpretation, and manuscript writing. J.L.: data acquisition and manuscript writing. Y.J.: data acquisition and manuscript writing. J.T.: methodological guidance, interpretation of data, and critical revision of the manuscript for important intellectual content. H.L.: study concept and design, interpretation of data, and manuscript writing. Y.C.: study concept and critical revision of the manuscript for important intellectual content. Y.W.: data acquisition and critical revision of the manuscript for important intellectual content. W.C.: critical revision of the manuscript for intellectual content. F.X.: critical revision of the manuscript for important intellectual content. All authors read, critically reviewed and approved the final manuscript.
Supplemental Material
Download PDF (659.2 KB)Acknowledgements
We extend our heartfelt gratitude to all those who have contributed to the enrichment and completion of this article. Our sincere thanks go to the colleagues and peer reviewers whose invaluable insights, feedback and suggestions significantly enhanced and refined the quality of our research.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data generated or analysed in this study are included in this published article. The datasets generated and/or analysed during this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Deuschl G, Beghi E, Fazekas F, et al. The burden of neurological diseases in Europe: an analysis for the Global Burden of Disease Study 2017. Lancet Public Health. 2020;5(10):1–14. doi: 10.1016/S2468-2667(20)30190-0.
- Crystal SC, Robbins MS. Epidemiology of tension-type headache. Curr Pain Headache Rep. 2010;14(6):449–454. doi: 10.1007/s11916-010-0146-2.
- Schoenen J. Guidelines for trials of drug treatments in tension-type headache. First edition: International Headache Society Committee on clinical trials. Cephalalgia. 1995;15(3):165–179. doi: 10.1046/j.1468-2982.1995.015003165.x.
- Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders. Cephalalgia. 2004;24(Suppl. 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x.
- Bendtsen L. Central sensitization in tension-type headache—possible pathophysiological mechanisms. Cephalalgia. 2000;20(5):486–508. doi: 10.1046/j.1468-2982.2000.00070.x.
- Jensen RH. Tension-type headache – the normal and most prevalent headache. Headache. 2018;58(2):339–345. doi: 10.1111/head.13067.
- Bendtsen L, Evers S, Linde M, et al. EFNS guideline on the treatment of tension-type headache – report of an EFNS Task Force. Eur J Neurol. 2010;17(11):1318–1325. doi: 10.1111/j.1468-1331.2010.03070.x.
- British Association for the Study of Headache (BASH). Guidelines for all healthcare professionals in the diagnosis and management of migraine, tension-type, cluster and medication-overuse headache. 3rd ed., Hull, UK; 2010.
- Vuković CV, Kes VB, Serić V, et al. Report of the Croatian Society for Neurovascular Disorders, Croatian Medical Association. Evidence based guidelines for treatment of primary headaches – 2012 update. Acta Clin Croat. 2012;51(3):323–378.
- Steiner TJ, Martelletti P. Aids for management of common headache disorders in primary care. J Headache Pain. 2007;8(Suppl. 1):S2.
- Moisset X, Mawet J, Guegan-Massardier E, et al. French guidelines for the emergency management of headaches. Rev Neurol. 2016;172(6–7):350–360. doi: 10.1016/j.neurol.2016.06.005.
- Institute for Clinical Systems Improvement (ICSI). Health care guideline: diagnosis and treatment of headache. 10th ed., Bloomington, MI; 2011.
- National Institute for Health and Care Excellence. Diagnosis and management of headache in young people and adults. CG150. London: NICE; 2012.
- Scottish Intercollegiate Guidelines Network (SIGN). The diagnosis and management of headache in adults (Guideline No. 107). Edinburgh: SIGN; 2008.
- Sarchielli P, Granella F, Prudenzano MP, et al. Italian guidelines for primary headaches: 2012 revised version. J Headache Pain. 2012;13(Suppl. 2):S31–S70. doi: 10.1007/s10194-012-0437-6.
- Becker WJ, Findlay T, Moga C, et al. Guideline for primary care management of headache in adults. Can Fam Physician. 2015;61(8):670–679.
- Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated 2011 Mar]. London, UK: The Cochrane Collaboration; 2011.
- Mavridis D, Giannatsi M, Cipriani A, et al. A primer on network meta-analysis with emphasis on mental health. Evid Based Ment Health. 2015;18(2):40–46. doi: 10.1136/eb-2015-102088.
- Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–784. doi: 10.7326/M14-2385.
- Xie R, Tian J, Wang Y, et al. Efficacy and safety of different drug monotherapies for tension-type headache in adults: study protocol for a Bayesian network meta-analysis. BMJ Open. 2019;9(1):e023748. doi: 10.1136/bmjopen-2018-023748.
- Puhan MA, Schünemann HJ, Murad MH, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ. 2014;349(5):g5630. doi: 10.1136/bmj.g5630.
- Bendtsen L, Bigal ME, Cerbo R, et al. Guidelines for controlled trials of drugs in tension-type headache: second edition. Cephalalgia. 2010;30(1):1–16. doi: 10.1111/j.1468-2982.2009.01948.x.
- Moore RA, Derry S, Wiffen PJ, et al. Evidence for efficacy of acute treatment of episodic tension-type headache: methodological critique of randomised trials for oral treatments. Pain. 2014;155(11):2220–2228. doi: 10.1016/j.pain.2014.08.009.
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557.
- R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2020.
- Brooks SP, Gelman A. Alternative methods for monitoring convergence of iterative simulations. J Comput Graph Stat. 1998;7:434–445.
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64(2):163–171. doi: 10.1016/j.jclinepi.2010.03.016.
- Kim H, Gurrin L, Ademi Z, et al. Overview of methods for comparing the efficacies of drugs in the absence of head-to-head clinical trial data. Br J Clin Pharmacol. 2014;77(1):116–121. doi: 10.1111/bcp.12150.
- Packman E, Leyva R, Kellstein D. Onset of analgesia with ibuprofen sodium in tension-type headache: a randomized trial. J Pharm Health Care Sci. 2015;1(1):13. doi: 10.1186/s40780-015-0012-9.
- Gatoulis SC, Voelker M, Fisher M. Assessment of the efficacy and safety profiles of aspirin and acetaminophen with codeine: results from 2 randomized, controlled trials in individuals with tension-type headache and postoperative dental pain. Clin Ther. 2012;34(1):138–148. doi: 10.1016/j.clinthera.2011.11.018.
- Packman E, Packman B, Thurston H, et al. Lumiracoxib is effective in the treatment of episodic tension-type headache. Headache. 2005;45(9):1163–1170. doi: 10.1111/j.1526-4610.2005.00239.x.
- Kubitzek F, Ziegler G, Gold MS, et al. Low-dose diclofenac potassium in the treatment of episodic tension-type headache. Eur J Pain. 2003;7(2):155–162. doi: 10.1016/S1090-3801(02)00094-0.
- Prior MJ, Cooper KM, May LG, et al. Efficacy and safety of acetaminophen and naproxen in the treatment of tension-type headache. A randomized, double-blind, placebo-controlled trial. Cephalalgia. 2002;22(9):740–748. doi: 10.1046/j.1468-2982.2002.00419.x.
- Martínez-Martín P, Raffaelli EJr., Titus F, et al. Efficacy and safety of metamizol vs. acetylsalicylic acid in patients with moderate episodic tension-type headache: a randomized, double-blind, placebo- and active-controlled, multicentre study. Cephalalgia. 2001;21(5):604–610. doi: 10.1046/j.1468-2982.2001.00216.x.
- Steiner TJ, Lange R. Ketoprofen (25 mg) in the symptomatic treatment of episodic tension-type headache: double-blind placebo-controlled comparison with acetaminophen (1000 mg). Cephalalgia. 1998;18(1):38–43. doi: 10.1046/j.1468-2982.1998.1801038.x.
- Mehlisch DR, Weaver M, Fladung B. Ketoprofen, acetaminophen, and placebo in the treatment of tension headache. Headache. 1998;38(8):579–589. doi: 10.1046/j.1526-4610.1998.3808579.x.
- Verhagen AP, Damen L, Berger MY, et al. Behandeling van spanningshoofdpijn: paracetamol en NSAID’s werken: een systematische review [Treatment of tension type headache: paracetamol and NSAIDS work: a systematic review]. Ned Tijdschr Geneeskd. 2010;154:A1924.
- Davies NM. Clinical pharmacokinetics of ibuprofen. The first 30 years. Clin Pharmacokinet. 1998;34(2):101–154. doi: 10.2165/00003088-199834020-000023.
- Rainsford KD. Ibuprofen: pharmacology, efficacy and safety. Inflammopharmacology. 2009;17(6):275–342. doi: 10.1007/s10787-009-0016-x.
- Lucas S. The pharmacology of indomethacin. Headache. 2016;56(2):436–446. doi: 10.1111/head.12769.
- Mazaleuskaya LL, Theken KN, Gong L, et al. PharmGKB summary: ibuprofen pathways. Pharmacogenet Genomics. 2015;25(2):96–106. doi: 10.1097/FPC.0000000000000113.
- Joshi S, Rapoport AM. Diclofenac potassium for oral solution (CAMBIA®) in the acute management of a migraine attack: clinical evidence and practical experience. Ther Adv Neurol Disord. 2017;10(4):217–226. doi: 10.1177/1756285616684494.
- European Medicines Agency. Lumiracoxib; 2020 [online] [cited 2020 Sep 24]. Available from: https://www.ema.europa.eu/en/medicines/human/referrals/lumiracoxib#overview-section
- Garza I, Swanson JW. Answers to frequently asked questions about migraine. Mayo Clin Proc. 2006;81(10):1387–1391; quiz 1392. doi: 10.4065/81.10.1387.
- Stovner LJ, Hagen K, Jensen R, et al. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia. 2007;27(3):193–210. doi: 10.1111/j.1468-2982.2007.01288.x.
- Ren Y, Li H, Wang Y, et al. Report of guidelines for diagnosis and treatment of common internal diseases in Chinese medicine: headache. J Evid Based Med. 2020;13(1):70–80. doi: 10.1111/jebm.12378.
- Lipton RB, Diener HC, Robbins MS, et al. Caffeine in the management of patients with headache. J Headache Pain. 2017;18(1):107. doi: 10.1186/s10194-017-0806-2.
- Tana C, Giamberardino MA, Martelletti P. Long COVID and especially headache syndromes. Curr Opin Neurol. 2023;36(3):168–174. doi: 10.1097/WCO.0000000000001153.
- Tana C, Bentivegna E, Cho SJ, et al. Long COVID headache. J Headache Pain. 2022;23(1):93. doi: 10.1186/s10194-022-01450-8.
- Caronna E, Ballvé A, Llauradó A, et al. Headache: a striking prodromal and persistent symptom, predictive of COVID-19 clinical evolution. Cephalalgia. 2020;40(13):1410–1421. doi: 10.1177/0333102420965157.
- Aiyegbusi OL, Hughes SE, Turner G, et al. Symptoms, complications and management of long COVID: a review. J R Soc Med. 2021;114(9):428–442. doi: 10.1177/01410768211032850.
- Kashiwagi K, Katsuki M, Kawamura S, et al. Fremanezumab and non-high-dose galcanezumab for comorbid cluster headache in patients with migraine: three cases. Neurol Int. 2023;15(1):318–324. doi: 10.3390/neurolint15010020.
- Mehkri Y, Hanna C, Sriram S, et al. Calcitonin gene-related peptide and neurologic injury: an emerging target for headache management. Clin Neurol Neurosurg. 2022;220:107355. doi: 10.1016/j.clineuro.2022.107355.
- Robbins MS. Diagnosis and management of headache: a review. JAMA. 2021;325(18):1874–1885. doi: 10.1001/jama.2021.1640.