Abstract
Aim:
Polycystic ovary syndrome (PCOS) is an increasingly recognized endocrine disorder. The pathogenesis is not fully known. Polycystic ovary syndrome is still difficult to diagnose correctly, despite simple diagnostic criteria. The aim of the study is to review the current knowledge about PCOS and treatment options for patients with the disease. To explore this topic, publications were reviewed and conclusions drawn from them. The incidence of hyperandrogenism in a patient with PCOS may be as high as 60–80%. Increased androgen levels affect ovulation and menstruation, and also result in hirsutism and acne. Additionally, patients have problems with proper glucose tolerance (insulin resistance), type 2 diabetes, hypertension, cardiovascular diseases and metabolic syndrome. PCOS results in various symptoms in patients.
Methods:
The latest treatment methods were analysed. A standard review of publications in the field of diagnosis and treatment of PCOS, IR and hyperandrogenism was used.
Results:
Lifestyle, especially diet, deserves special attention due to its ease of use. Sleep quality, physical activity and stress reduction are also important. Diet should be the treatment of first choice. Only if dietary intervention does not bring results, the doctor considers pharmacotherapy. Recently, acupuncture and herbal medicine, vagus nerve stimulation have been used in the treatment of PCOS and regulation of hormone levels. Patients are given supplementation to improve the quality of functioning, but it must be remembered that inappropriate doses or too long use may result in a toxic effect opposite to the therapeutic one.
Conclusion:
Appropriate diet, physical activity - lifestyle changes are crucial in the treatment of PCOS. Supplementation and pharmaceuticals support treatment. It is mandatory to examine these environmental and lifestyle factors as they not only contribute to the occurrence of the disease but also influence its progression.
Plain Language Summary
Polycystic ovary syndrome (PCOS) is a complex metabolic and hormonal disorder that occurs in women. It manifests itself in menstrual disorders, changes in appearance related to excessive hair growth and acne. PCOS is also associated with the risk of other diseases, glucose tolerance (insulin resistance), type 2 diabetes, hypertension, cardiovascular diseases and metabolic syndrome. Polycystic ovary syndrome is still difficult to diagnose correctly, despite simple diagnostic criteria.
The symptoms and course of the disease vary, specific to each patient. Patients struggle with PCOS, not being aware that it is a significant medical problem. The patients have always had problems with menstruation, so they think it is normal.
The article reviews and describes various treatment methods: Hormone therapy, pharmacological methods, supplementation, non-pharmacological methods such as herbal medicine, acupuncture.
Introduction – view of the disease
Polycystic ovary syndrome (PCOS) also known as the Stein-Leventhal syndrome, is a prevalent endocrine disorder affecting women of reproductive age. It is estimated that this condition is diagnosed in one out of 10 women of reproductive age [Citation1–5]. Ten to fifteen percent of all women suffer from this disease. While the primary cause of the disorder is an abnormality in the ovaries, additional factors such as obesity and environmental influences contribute to the development of specific symptoms and signs of disease [Citation5,Citation6].
Women who are overweight or obese have a higher incidence of this disease compared to their lean counterparts [Citation7]. One of the consequences of PCOS is the lack of ovulation, which can lead to reduced fertility. In fact, up to 73% of fertility problems are attributed to PCOS [Citation8]. PCOS can also lead to various complications and health issues such as diabetes, obesity and metabolic syndrome. Therefore, accurate diagnosis and treatment of this condition are crucial. PCOS is a highly diverse disease, and treatment should be tailored to the individual needs of each patient.
The first mentions of PCOS appeared in 1935 when patients were observed to have menstrual irregularities, reduced fertility, obesity and hirsutism. It was described as ‘secondary amenorrhea and resulting infertility, as well as obesity and male-type hair growth’ [Citation5]. The term polycystic ovary syndrome was first used in 1960 [Citation9]. In the 1970s and 1980s, the level of luteinizing hormone (LH) was considered crucial in the diagnosis. In 1990, a conference organized by the National Institute of Health declared that the primary diagnostic criteria for PCOS are infrequent ovulation, symptoms of androgen excess, and the exclusion of similar clinical entities [Citation4,Citation5,6]. Since 2003, the Rotterdam criteria have been widely used by physicians for the diagnosis of PCOS, which will be described in the subsection ‘Diagnosis of PCOS’ [Citation4].
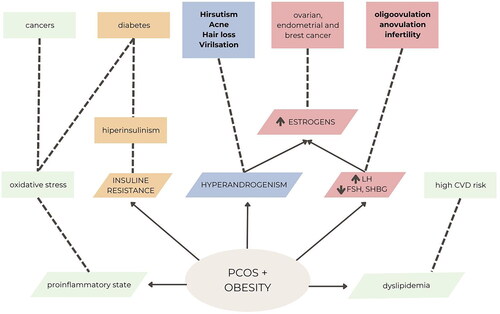
PCOS leads to various metabolic disorders, often characterized by tissue insensitivity to insulin, resulting in hyperinsulinaemia. This cascade of abnormalities leads to the overproduction of androgens by the adrenal glands and ovaries, and the elevated levels of androgens cause the typical symptoms of the disease. Hyperinsulinaemia and insulin resistance (IR) are strongly associated with an increased tendency towards obesity compared to healthy women in the general population [Citation8]. Approximately, 10% of women with PCOS will develop diabetes before the age of 40. Women with elevated androgen levels are particularly prone to IR.
Patients should monitor their health and adopt preventive behaviours to prevent the development of associated diseases. Physical activity and a proper diet are among the most effective and cost-efficient ways to manage PCOS and its accompanying conditions [Citation10].
Materials and methods
A standard review of publications in the field of diagnosis and treatment of PCOS, IR and hyperandrogenism was used.
Ethical statement
Hereby, Stańczak consciously assure that for this study, the following is fulfilled:
This material is the authors’ own original work, which has not been previously published elsewhere.
The paper is not currently being considered for publication elsewhere.
The paper reflects the authors’ own research and analysis in a truthful and complete manner.
The paper properly credits the meaningful contributions of co-authors and co-researchers.
The results are appropriately placed in the context of prior and existing research.
All sources used are properly disclosed (correct citation). Literally copying of text must be indicated as such by using quotation marks and giving proper reference.
Etiopathogenesis
The pathophysiology of PCOS is extremely complex and involves various factors such as hormonal imbalances, IR, hyperandrogenism and metabolic abnormalities. Possible known and unknown causes are multiple. There are many causes as well as a wide variety of signs, symptoms and additional health disorders that correlate with PCOS. The complexity of this issue is highlighted by the fact that there is a separate disease called polycystic ovary disease (PCO), which is a different issue from PCOS.
PCOS is primarily caused by a defect in ovarian cells, particularly theca cells. This defect leads to the excessive production of androgens, which results in the clinical and biochemical symptoms associated with the disease. Genetic factors, including ethnicity, have also been identified as contributing to the development of PCOS, with a higher frequency of the condition observed in Spanish, Native American and Mexican women [Citation11,Citation12].
The initial descriptions of the syndrome highlighted an elevated ratio of LH to follicle-stimulating hormone (FSH) as a fundamental abnormality in PCOS. It has been suggested that PCOS may arise from an increased frequency of gonadotropin-releasing hormone (GnRH) pulses, which stimulate theca cells to produce androgens. Additionally, decreased FSH levels, disrupted late luteal and early follicular phases, IR affecting adipose tissue and skeletal muscles, impaired pancreatic beta-cell function and obesity are considered underlying causes of PCOS [Citation5].
Determining the exact causality in the development of PCOS is often challenging due to the complex interactions involved. However, it is widely recognized that obesity exacerbates menstrual disorders and hyperandrogenism, while weight reduction can alleviate clinical symptoms. Insulin resistance is a significant concern in both overweight and underweight women with PCOS, with an estimated 50–70% of affected women displaying varying degrees of IR [Citation13].
The causes of PCOS are not fully known, but it is believed to be a complex interaction of genetic, hormonal and environmental factors. Many women with PCOS have IR. This means that the body cannot make good use of the insulin produced by the pancreas. Insulin builds up in the body and can cause higher androgen levels. Obesity can also increase insulin levels and worsen PCOS symptoms. The disease also has a hereditary basis. It is common for sisters or mother and daughter to suffer from it.
Chronic inflammation is also considered to be the cause of the disease. White blood cells (WBC) produce substances in response to infection or injury. This response is called low-grade inflammation. Research shows that people with PCOS have a type of long-term, low-grade inflammation that leads to androgen production by polycystic ovaries. This can lead to problems with the heart and blood vessels.
Correlations have been found between increased levels of C-reactive protein (CRP), interleukin 18 (IL-18), tumour necrosis factor (TNF-α), interleukin 6 (IL-6), WBC count, monocyte chemoattractant protein-1 (MCP-1) and macrophage inflammatory protein-1α (MIP-1α) in the PCOS women compared with age- and BMI-matched controls. Women with PCOS present also elevated levels of AGEs and increased RAGE (receptor for advanced glycation end products) expression. This chronic inflammatory state is aggravated by obesity and hyperinsulinaemia [Citation14].
Androgens
One of the key factors contributing to the symptoms of PCOS and the development of metabolic syndrome is an excess of androgen hormones. These androgens are primarily produced by the ovaries and adrenal glands. While adrenal hyperandrogenism can be present in about 20–30% of women with PCOS, it does not significantly contribute to the metabolic abnormalities associated with the condition; the frequent increase in aldosterone, the main proinflammatory hormone [Citation15].
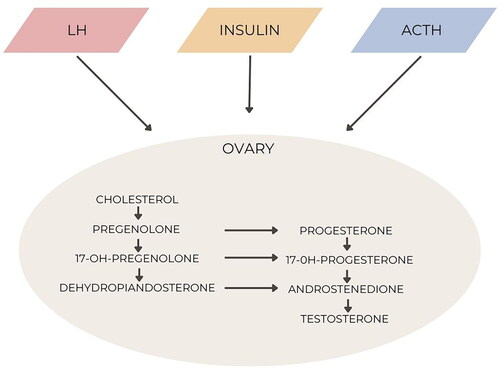
On the other hand, the excessive androgens produced by the ovaries play a crucial role in the development of PCOS. In the following sections, our focus will be on understanding the mechanisms of androgen synthesis specifically in the ovaries of women with PCOS and examining their effects at a cellular level [Citation16].
PCOS is characterized by the excessive secretion of androgens from the ovaries and/or adrenal glands. This excessive production of ovarian androgens is influenced by both intrinsic ovarian factors, such as altered steroidogenesis, and external factors like hyperinsulinaemia. Women with PCOS typically have more growing follicles compared to normal controls, but these follicles experience premature growth arrest at a size of 5–8 mm. The classic ovarian appearance in PCOS is characterized by enlarged ovaries with a string-of-pearl morphology and theca interstitial hyperplasia, which are indicative of androgen exposure. Similar ovarian morphology has been observed in women with congenital adrenal hyperplasia (CAH) and female-to-male transgender individuals [Citation17]. The dysregulation of interactions among autocrine and endocrine factors is responsible for follicular maturation and may contribute to the ovarian dysfunction seen in PCOS. Moreover, hyperandrogenism may also be related to increased androgen receptors in localized areas.
Let us briefly review the stages of follicular maturation. Primordial follicles develop during gestation and consist of oocytes arrested in meiosis, surrounded by pregranulosa cells. As a result, a woman’s ovaries are exposed to the maternal environment during gestation. Ovarian activity remains inactive until puberty begins. Limited knowledge exists about the specific morphology of follicles in prepubertal and early pubertal ovaries. Ovarian tissue obtained from prepubertal and early pubertal girls shows differences in follicle morphology and growth potential. Notably, prepubertal ovaries contain a higher proportion of abnormal nongrowing follicles that are absent in pubertal ovaries [Citation11]. The significance of this finding in terms of physiological implications remains unclear ().
Hyperinsulinaemia and insulin resistance
Hyperinsulinaemia is a condition when the body produces more insulin than it should. Insulin resistance is a condition when tissues do not respond to insulin secreted by the pancreas. When hyperinsulinaemia is combined with dysfunction in pancreatic beta cells, it poses an elevated risk for various diseases such as diabetes, hypertension, dyslipidaemia, endothelial dysfunction, atherosclerosis and cardiovascular diseases. Additionally, insulin stimulates theca cells in the ovary to produce excessive testosterone, leading to clinical symptoms of hyperandrogenism, including acne, hirsutism and alopecia [Citation18].
The phenotype observed in female patients with mutations in the insulin receptor gene includes IR, compensatory hyperinsulinaemia and hyperandrogenism [Citation19]. However, mutations in the insulin receptor gene are extremely rare in the population. On the other hand, hyperinsulinaemia and IR often affect PCOS patients [Citation20].
Women with PCOS exhibit intrinsic IR regardless of their level of obesity or androgen concentrations [Citation13]. Even lean women with PCOS show signs of IR, and an increase in body mass index (BMI) exacerbates the condition. Normal-weight adolescent girls with PCOS display peripheral IR, elevated liver fat and impaired muscle mitochondrial function compared to their counterparts without PCOS.
Insulin plays a primary role in glucose homeostasis and lipogenesis. Additionally, it has effects on carbohydrate, fat and protein metabolism and acts as a mitogenic hormone. Insulin exerts its actions through insulin receptors found in various tissues of the hypothalamic-pituitary-ovarian (HPO) axis. In steroidogenic tissues like the ovary and adrenal cortex, insulin enhances the effects of trophic hormones, promoting steroidogenesis. Compensatory hyperinsulinaemia associated with IR leads to excessive androgen secretion by the ovaries and adrenal glands while also reducing hepatic sex hormone-binding globulin (SHBG) synthesis, resulting in increased circulating testosterone levels. This creates a paradoxical situation in PCOS, where tissues like the liver, skeletal muscle and adipose tissue exhibit IR, while steroid-producing tissues and the pituitary gland retain insulin sensitivity. This paradox is evident in the differential response to insulin in granulosa-lutein cells obtained from women with PCOS: insulin-stimulated glucose uptake is impaired, but insulin-stimulated progesterone production remains intact.
The significant role of compensatory hyperinsulinaemia is supported by improved clinical outcomes with insulin-sensitizing medications and weight loss. The transient IR and hyperinsulinaemia seen during early puberty may contribute to the factors associated with the development of PCOS. The prevalence of metabolic syndrome, characterized by obesity, hypertension, dyslipidaemia and hyperglycaemia, is approximately three times higher in women with PCOS. Although there is no consensus definition of metabolic syndrome in adolescents, published criteria for paediatric populations are based on adult guidelines and include a combination of elevated triglyceride (TG) levels, low high-density lipoprotein (HDL) cholesterol levels, fasting blood glucose levels ≥110 mg/dL, increased waist circumference and hypertension based on age [Citation21]. A meta-analysis suggests that while IR serves as a common factor linking the metabolic and reproductive features of PCOS, these features develop through independent mechanisms [Citation22]. However, it is consistently observed that obesity exacerbates the symptoms of PCOS, particularly the risk of developing type 2 diabetes and metabolic syndrome.
Primary hyperinsulinaemia can precede the development of peripheral tissue IR. The debate surrounding primary IR versus primary hyperinsulinaemia is beyond the scope of this review. Importantly, various genetic and epigenetic factors, prenatal and postnatal environmental influences, and adaptations to excess nutrient intake contribute to the development of IR and hyperinsulinaemia [Citation23].
Preclinical models provide evidence of β-cell dysfunction associated with hyperinsulinaemia in monkeys and sheep prenatally exposed to androgens.
Metabolic syndrome
Hyperandrogenism, hyperinsulinaemia and IR are not the only metabolic disturbances affecting patients with PCOS. There is a familiarity for both IR and metabolic syndrome. The diagnosis of metabolic syndrome occurs when a patient meets three of the following criteria:
Abdominal obesity (waist circumference greater than 80 cm in women and 94 cm in men);
Elevated TG levels in the blood (TG level higher than 1.35 g/L in women and 1.6 g/L in men);
Reduced levels of HDL cholesterol, or treatment for this disorder (HDL level lower than 50 mg/dL in women and 40 mg/dL in men);
High blood pressure (above 140/90 mmHg);
Abnormal fasting glucose or previously diagnosed diabetes (fasting glucose level above 126 mg/dL or random glucose level above 200 mg/dL or glucose level two hours after a meal ≥200 mg/dL) (link with both IR).
Patients diagnosed with PCOS could have abdominal obesity and carbohydrate metabolism problems. They are prone to prediabetic states. It is crucial to ensure that the treatment process does not exacerbate existing conditions. Prevention of additional complications is also necessary to avoid the development of metabolic syndrome [Citation24–26].
Unfortunately, untreated or undiagnosed patients are at risk of developing additional health problems. Patients often experience tachycardia, hypertension and dyslipidaemia, which can eventually lead to the development of cardiovascular disease. Moreover, patients may exhibit chronic low-grade inflammation, which affects the endothelial lining of blood vessels and predisposes them to a higher risk of cardiovascular disease. It is worth noting that chronic smoking further increases cardiovascular risk in women, as highlighted in a recent study conducted by Morotti et al. [Citation27]. Typically, patients have elevated TG levels (in 35% of patients), total cholesterol and LDL cholesterol levels, and decreased HDL cholesterol levels (in 68% of patients). A rise in blood pressure is observed in 45% of patients. Low HDL cholesterol levels are a risk factor for cardiovascular disease since HDL plays a cytoprotective role in the endothelium of blood vessels [Citation1,Citation26].
Even in patients without IR and normal glycaemia, there is an elevated risk of developing cardiovascular disease, impaired glucose tolerance and non-insulin-dependent diabetes. In a six-year study conducted by Norman et al., where all PCOS patients initially had normal glucose levels, it was found that by the end of the study, 13% of patients had impaired glucose tolerance, and 16% of patients developed diabetes [Citation25,Citation28]. It is essential to pay particular attention to the diagnosis and prevention of carbohydrate metabolism disorders to prevent these abnormalities and the progression to diabetes.
Obesity
Obesity is frequently observed in individuals with PCOS, with a prevalence ranging from 33% to 88%. This excess weight can have a significant impact on fertility and can lead to various reproductive complications, including menstrual disorders, anovulation, reduced fertility and miscarriages. Therefore, it is crucial to address weight management early on in PCOS to improve fertility potential and overall quality of life.
Hyperandrogenism, characterized by elevated levels of androgens (male hormones), plays a critical role in the development of abdominal obesity in women with PCOS, spanning adolescence, adulthood and menopause [Citation29,Citation30]. While some studies have reported a negative association between plasma androgen levels and obesity, most of the existing literature suggests that the hyperandrogenic state in PCOS contributes to weight gain. Excessive androgens can induce various cellular activities, such as apoptosis, autophagy, mitochondrial dysfunction and endoplasmic reticulum stress, in granulosa cells and oocytes, thereby promoting the development of PCOS.
It is important to note that the effects of androgens can differ between genders. In males, androgen signalling stimulates the commitment of pluripotent mesenchymal stem cells to myogenic lineage but suppresses the adaptogenic lineage. Knockdown of androgen receptors in male mice can lead to late-onset visceral obesity and increased lipogenesis in adipose tissue and liver [Citation16]. However, the mechanism through which androgens affect fat cells in women is not well understood. Studies suggest that excessive androgens can directly increase proliferation in visceral preadipocytes and promote lipid droplet accumulation. Androgens may also inhibit the differentiation of subcutaneous mesenchymal stem cells and influence macrophage polarization [Citation31]. Additionally, androgen and adipose tissue can interact in a vicious cycle, with adipose tissue acting as a hormone supplier and modulator. Local activation of androgens in adipose tissue may play a more significant role in mediating fat mass expansion compared to increased circulating androgens [Citation7,Citation32].
Overall, excessive androgens, along with adipose tissue, play critical roles in the pathogenesis of obesity, particularly abdominal obesity, in individuals with PCOS. Additionally, the impact of androgens extends beyond the ovaries, contributing to various metabolic disorders such as cardiovascular disease, type 2 diabetes mellitus, kidney disease and obesity-related complications [Citation16,Citation31,Citation33].
Diagnose
There are different diagnostic criteria used to identify PCOS, their comparison, and evaluation ().
Table 1. Diagnostic criteria.
The Rotterdam criteria, established in 2003, are the most widely utilized and relevant criteria for diagnosing PCOS. Diagnosis of the disorder requires the fulfilment of two out of the three specified conditions:
Hyperandrogenism, detected through clinical and/or biochemical assessments.
Ovulation abnormalities.
The presence of 12 or more cysts on one ovary or an ovarian volume exceeding 10 mL.
According to the Rotterdam criteria, PCOS can be categorized into four phenotypes:
Classic, characterized by hyperandrogenism, ovulation disorders and polycystic ovaries detected through ultrasound (HOP);
With hyperandrogenism and ovulation disorders, but a normal ovarian ultrasound image (HO);
With hyperandrogenism and polycystic ovaries observed through ultrasound, but without ovulation disorders (HP);
With ovulation disorders and polycystic ovaries, but without evidence of hyperandrogenism (OP) [Citation6,Citation13,Citation34].
Apart from the Rotterdam criteria, there are two other definitions of PCOS. Androgen Excess Society (2006) considers hyperandrogenism as the fundamental PCOS disorder and a prerequisite for diagnosis, in combination with one of the remaining Rotterdam criteria. The National Institutes of Health (NIH) criteria from 2009 involve the identification of clinical or biochemical hyperandrogenism and chronic ovulation disorders. It is crucial to exclude other conditions such as Cushing’s syndrome, congenital adrenal hyperplasia and androgen-secreting tumours before diagnosing PCOS [Citation34].
Despite the apparent clarity of these criteria, the etiology of PCOS remains unknown, and standardized treatment protocols have yet to be established. As a result, PCOS continues to be an active area of research and scientific inquiry. This paper explores the postulated causes and potential consequences of the clinical and biochemical syndrome, along with currently accepted and emerging therapeutic approaches.
Symptoms, signs and complications
The symptoms of PCOS are individual. Not all the symptoms may be present in every case. Most common symptoms and complications of PCOS are:
Reduced fertility and menstrual disorders manifested by irregular/painful/heavy periods: Women with PCOS often experience menstrual irregularities, such as amenorrhea (absence of periods) or oligomenorrhea (infrequent periods). These abnormalities can contribute to reduced fertility/difficult to conceive which occurs in about 73–74% of cases [Citation6,Citation13,Citation35].
Hirsutism: Increased androgen levels can lead to excessive hair growth, especially on the face, chest, back or abdomen. Excessive hair growth, known as hirsutism, is a common symptom in PCOS and affects 85–90% of women with PCOS [Citation13,Citation36].
Acne: Women with PCOS may be prone to acne, particularly during adolescence.
Oily skin and hair: There may be a tendency for the skin and hair to be excessively oily.
Receding hairline or baldness.
Lowered voice timbre.
Clinical symptoms of PCOS also include:
Excessive weight gain: Although some women with PCOS may also have a normal body weight, excess body weight can worsen PCOS symptoms, increase the risk of metabolic disorders and have a negative impact on fertility. Forty to sixty percent of women with PCOS are classified as obese or overweight, further contributing to metabolic disturbances and increasing cardiovascular risk [Citation6,Citation13,Citation30,Citation35–36],
Change of body shape,
Hypertension,
Increased blood sugar levels.
Dark skin pigmentation: Some women with PCOS may develop dark patches of skin around the groin, anus and armpits.
Breast tenderness: Breast soreness or tenderness may be experienced by some women.
Cravings for food/sweets: Some women with PCOS may experience intense food cravings, especially for sweets.
Sleep problems: Sleep difficulties or sleep disorders may be present in some women with PCOS [Citation6,Citation13,Citation36].
Type 2 diabetes: The prevalence of metabolic disorders, is higher in women with PCOS compared to the general population (3–7 times higher), especially among Indians [Citation26].
Metabolic syndrome is present in about 40% of women with PCOS.
Lipid disorders: Abnormal lipid levels, such as high cholesterol or TGs.
Arterial hypertension: Approximately, 20% of women with PCOS experience high blood pressure (arterial hypertension) [Citation13,Citation35,Citation36] ().
Furthermore, chronic hyperandrogenaemia (elevated levels of androgens) in PCOS can contribute to the development of hormone-dependent tumours, such as endometrial, breast or ovarian neoplasms [Citation3].
During gynaecological and ultrasound examinations, enlarged and tender ovaries may be observed. The temperature chart may show flat and hypotonic patterns. Additionally, elevated TG levels and increased VLDL fraction can be seen in the blood [Citation37].
Routine oral glucose tolerance testing (OGTT) is recommended for obese women with PCOS to assess their risk of developing diabetes. However, it is not necessary for women with normal body weight. Women diagnosed with PCOS are considered at risk of developing diabetes according to the standards of the Polish Diabetes Association [Citation38]. It is important to emphasize that PCOS symptoms are highly individualized, and not all symptoms will be present in every case [Citation3].
Mental health
PCOS can have an impact on mental well-being and increase the risk of depression. In addition to these physical abnormalities, there is a close correlation between PCOS and mental disorders. Women with PCOS have a higher frequency of depression, drug-related and bipolar disorders, as well as eating disorders such as bulimia, anorexia or non-specific dietary disorders. These mental health issues can significantly impact the quality of life of individuals with PCOS [Citation8]. Each individual should feel comfortable in their own body, accepting and appreciating themselves while emphasizing their strengths and attributes. Hormonal changes that manifest in a patient’s appearance can disrupt their self-esteem and self-acceptance. This is particularly dangerous for young girls who are still shaping their system of values and learning self-acceptance during the transition from adolescence to adulthood. Visible symptoms can even lead to breakdowns, depression and even suicidal thoughts [Citation39]. The outwardly manifesting symptoms have a significant impact on the psychosexual development of young girls. It is essential to provide psychological care when a patient requires it. As indicated by Sonino et al. women suffering from hirsutism show higher levels of anxiety and tension, and they are at a higher risk of developing social phobias [Citation40]. These patients describe their bodies as a ‘prison that robs them of their identity’ and use highly deprecating terms to refer to themselves [Citation41]. Mental health is a crucial aspect of overall well-being, and metabolic and endocrine disorders that involve weight fluctuations can strongly influence a patient’s self-esteem, potentially leading to the development of mental illnesses. Patients may resort to alternative diets, often very restrictive and detrimental to their bodies. This can also result in the development of eating disorders.
Reduced fertility also has a negative impact on the psyche of patients with PCOS. It leads to frustration, depression and difficulties in self-acceptance. Women from an early age are prepared and socialized for the role of mothers. Problems with giving birth to offspring may affect mental health. The diagnosis of reduced fertility causes psychological distress (‘infertility stress’) [Citation42].
Medical treatment
Therapy should be individually fitted to meet the needs of the patient and should also aim to prevent long-term treatment complications. For overweight and obese patients, it is recommended to adopt healthy eating habits, a low-energy diet and physical activity adjusted to the body’s capacity, as even a small weight loss (2–5% of initial body weight) translates into tangible health benefits. Reducing IR has a positive impact on normalizing androgen levels, which can result in spontaneous regulation of menstrual cycles. These are the most cost-effective and straightforward non-pharmacological treatment methods [Citation4].
International guidelines for PCOS include recommendations regarding the treatment of symptoms such as irregular menstrual cycles, hirsutism and lack of ovulation. The first line of treatment is lifestyle modification – proper diet, physical activity, sleep and stress reduction to normalize hyperinsulinaemia. Hormonal therapies are only considered when lifestyle modifications do not achieve the desired effects. Among medical interventions, combined oral contraceptives (COCs) have been shown to be effective in treating irregular cycles, hirsutism and acne compared to preparations containing only progestogens. The choice of COC can be based on patient preferences and minimizing side effects, as there is no evidence of superiority of a specific oestrogen–progestogen combination. It is worth noting that the World Health Organization (WHO) recommends the use of a combination of 35 μg of oestrogens and cyproterone acetate (CPA) as second-line treatment only in cases of severe acne and hirsutism due to the increased risk of thromboembolic events associated with these preparations. It is worth emphasizing that contraceptives are not appropriate in women with metabolic syndrome or severe IR [Citation43].
To regulate menstrual cycles in patients, pharmaceuticals containing oestrogen–progestin contraceptive preparations are used. They act on the HPO axis, increasing the level of SHBG. Some of them contain substances that block the action of androgens by inhibiting the receptors of target organs, thereby reducing its free fraction. [Citation4].
Metformin therapy leads to only mild to moderate improvement in menstrual cycle regularity and is less effective than COCs. Direct evidence suggests minimal improvement in menstrual cycle regulation with metformin-lifestyle compared with lifestyle [Citation10,Citation43,Citation44]. However, updated guidelines suggest an innovative approach, encouraging the combination of metformin with COCs, especially in women with overweight or obesity coexisting with PCOS. Regardless of age, the combination of metformin and hormonal therapy yields satisfactory results. Data on antiandrogenic medications are limited due to a lack of high-quality studies, and existing evidence does not show significant benefits of combining antiandrogenic drugs with COCs. A placebo-controlled randomized trial also confirmed this, as it demonstrated only minimal additional benefits from combining bicalutamide, an androgen receptor antagonist, with COCs over 12 months [Citation43].
Metformin lowers the levels of insulin and androgens while normalizing the levels of gonadotropins. It also weakens the secretion of AMH by the ovaries, leading to the regulation of androgen metabolism. In patients with PCOS, metformin influences the reduction of androgen production in the adrenal glands and ovaries, as well as the increase in the production of SHBG. Metformin has a mild to moderate impact on IR and a minimal to moderate impact on improving the lipid profile. Future research should focus on evaluating the effectiveness of combined therapies, such as metformin and GLP-1 receptor agonists, as well as predicting the availability of these anti-obesity drugs in low-income countries and communities [Citation43].
Pharmaceutical treatment
Various pharmaceutical treatment methods have been proposed for PCOS. However, they have certain drawbacks such as adverse effects, low efficacy, poor adherence to long-term pharmacological treatment, and contraindications in half of cases. Therefore, complementary treatment may be a suitable alternative [Citation36,Citation45–47].
Currently, oral contraceptives are the most commonly chosen option in the treatment of PCOS. They work by reducing the levels of free androgens in the blood and inhibiting the secretion of gonadotropins. Clomiphene citrate, a nonsteroidal selective oestrogen receptor modulator (SERM), and letrozole, an aromatase inhibitor, are widely used for ovulation induction. Clomiphene citrate is the first-line therapy, although emerging evidence suggests that letrozole may have higher efficacy and safety for both the mother and the foetus [Citation3,Citation44,Citation48–50].
The progestogenic component can consist of a derivative of 17-alpha-hydroxyprogesterone or 19-nortestosterone, including many 13-ethylgonans and 13-methylgonans [Citation51]. The structure of the progestogen molecule determines its pro- or anti-androgenic activity, its affinity for progesterone and receptors, and its interaction with the sex hormone binding globulin (SHBG). Based on these factors, different ‘generations’ of progestogens with similar properties can be identified.
Derivatives of 17-alpha-hydroxyprogesterone include, among others, CPA, which has anti-androgenic effects and acts as a direct antagonist of the androgen receptor [Citation15,Citation52]. Chlormadinone acetate (CMA), a halogenated derivative of 17-hydroxyprogesterone, serves as a competitive inhibitor of testosterone and dihydrotestosterone at the doses used in HŚA, exhibiting anti-androgenic properties through the inhibition of 5-alpha-reductase activity. However, higher doses of CMA may lead to androgenic effects [Citation53].
Derivatives of 19-nortestosterone, such as levonorgestrel (LNG), desogestrel, gestodene and norgestimate, are 13-ethylgonans with androgenic activity [Citation54]. However, dienogest, also a derivative of 19-nortestosterone, exhibits anti-androgenic activity (DNG) [Citation54,Citation55]. Nomegestrol acetate (NOMAC), a derivative of 17-alpha-hydroxy-19-norprogesterone, possesses high affinity and selectivity for the progesterone receptor but lacks agonist activity towards other steroid receptors [Citation52].
If a PCOS patient is considering pregnancy, testing the anti-Müllerian hormone AMH level may be helpful. It allows you to determine the state of the so-called ovarian reserve, and thus indirectly assess how many egg cells a woman has in her ‘free reserves’. The study therefore allows us to approximately estimate how much time a woman has left to become a mother.
Pharmaceutical treatment – new options
Semaglutide therapy deserves special attention. According to a study conducted by Carmina and Longo, three months of semaglutide therapy significantly reduced the values of BMI, body weight, fasting glucose, insulin and HOMA-IR (p < .01). Nearly, 80% of obese PCOS patients who did not respond to lifestyle changes achieved at least 5% weight loss, which resulted in significant improvements in basal glucose and IR (calculated by HOMA-IR). The average weight loss after semaglutide therapy was greater than after metformin or liraglutide, with minimal side effects [Citation56].
Another promising new drug is tirzepatid. It is a newly FDA-approved drug that significantly reduces body weight and improves insulin sensitivity in patients both with and without diabetes. Although this drug may cause some gastrointestinal side effects, the severity of these side effects is lower than with GLP-1RA drugs alone due to its dual mechanism of action. Tirzepatid may constitute a new therapeutic strategy in the treatment of PCOS, especially in the context of weight control and insulin sensitivity. However, robust clinical trials with tirzepatid in PCOS patients are needed to confirm this [Citation57].
Pharmaceutical treatment – metformin
Metformin is used in the treatment of IR in PCOS patients. Overall, treating IR along with excessive androgenization improves hormonal, metabolic and reproductive functions. The mechanism of action of metformin is a decrease in glucose production in the liver, reduced intestinal absorption and an increase in insulin sensitivity. Undesirable side effects include diarrhoea, nausea and vomiting, flatulence, weakness, indigestion, abdominal discomfort or headache. Contraindications to metformin treatment may include severe renal failure, known hypersensitivity to metformin hydrochloride, acute or chronic metabolic acidosis, including diabetic ketoacidosis with or without coma [Citation3,Citation44,Citation48,Citation58–60].
Pharmaceutical treatment – antiandrogens
Contraceptives that are not recommended for a woman with metabolic syndrome or IR. A much safer solution is the use of anti-androgens. Antiandrogens have been recognized as an effective therapeutic strategy to reduce excess androgens and thereby alleviate the clinical symptoms associated with elevated androgen levels. Taking an antiandrogen results in reduced IR [Citation59–62].
Two types of antiandrogens have been proposed for the treatment of PCOS: androgen receptor antagonists such as spironolactone and flutamide and 5-alpha reductase inhibitors such as finasteride. However, data on the clinical effects of antiandrogen and combination therapy in adolescents with PCOS are limited [Citation59,Citation62]. AAs are more effective than metformin in reducing the symptoms of PCOS and protecting the endometrium. Their combination with metformin may also be a solution in the treatment of obesity (reducing BMI). Additionally, this therapy improves glucose tolerance [Citation60,Citation61].
Spironolactone (Aldactone®) is an antiandrogen used in the treatment of hirsutism and acne. It has a competitive antagonistic effect on aldosterone receptors, which causes potassium retention and sodium and water excretion. A side effect of this drug is gynecomastia, contraindications include hyperkalaemia, Addison’s disease and the use of eplerenone. Spironolactone is a drug for PCOS because it affects all factors of the pathogenesis of the disease, such as blockade of the androgen receptor, direct action on the ovaries and adrenal glands at 17HSD, which reduces androgen synthesis, as well as peripheral transformation of androstenedione to testosterone. This drug has strong anti-inflammatory and anti-aldosterone effects [Citation60,Citation63].
Another antiandrogen is finasteride (Propecia®). It is also often used in the treatment of hirsutism and acne. It has a slightly different effect; it is a type II inhibitor of 5-alpha-reductase, an enzyme that converts androgen testosterone in DHT. Side effects of this drug include decreased libido, erectile dysfunction and ejaculation disorders, contraindications include pregnancy or hypersensitivity to any of the ingredients in the preparation of this product. When using this medicine, the risk of fulminant hepatitis should be taken into account [Citation54,Citation60].
Diet
An important dietary recommendation for patients with PCOS is to follow a low glycaemic index (GI) diet and control carbohydrate intake. This is especially important because women with PCOS often suffer from obesity, and reducing energy consumption can lead to weight reduction, which in turn can impact menstrual cycle regulation, restore ovulation, reduce chronic inflammation, and have a beneficial effect on the cardiovascular system and reduce the risk of cancer [Citation9].
In addition to dietary therapy, regular physical activity is also significant in the weight reduction process. Studies have shown that individuals following a low GI diet experienced greater weight loss and fat tissue reduction compared to a control group without such recommendations [Citation64]. Even a slight weight loss of just 5% of initial body weight can lead to improvements in lipid parameters, carbohydrate metabolism and insulin profile [Citation65].
The basic principles of dietetics for patients with PCOS include reducing the consumption of simple carbohydrates, controlling lipid profile, reducing saturated fatty acid intake, and increasing fibre intake. It is important to have balanced meals in terms of protein, fat and carbohydrates, and to maintain adequate fluid balance [Citation64,Citation66].
A study conducted on the diet of patients with PCOS showed excessive consumption of saturated fatty acids, cholesterol and simple sugars, especially sucrose, which has a negative impact on the cardiovascular system and may contribute to the development of diabetes. In the same study, a majority of patients had insufficient dietary fibre levels and did not consume adequate amounts of cobalamin, calcium, zinc and magnesium [Citation66,Citation67].
It is worth mentioning that the ketogenic diet, which is a high-fat diet, is not specifically recommended for PCOS weight loss. The popularity of high-fat diets as a solution for combating obesity is not supported by evidence. Currently, there is no evidence to suggest that the ketogenic diet is more effective than other low-calorie diets for weight loss. The ketogenic diet is a very restrictive diet that requires careful monitoring and supplementation. It may not be suitable for everyone and can have a negative impact on mental well-being. However, some studies have indicated the potential of using short-term ketogenic diets along with calorie restriction in the treatment of PCOS, but further research is needed to explore this topic [Citation68,Citation69].
In conclusion, a low GI diet with controlled carbohydrate intake, along with regular physical activity, is recommended for patients with PCOS. It is important to reduce the consumption of simple carbohydrates, control lipid profile and increase fibre intake. The ketogenic diet is not specifically recommended for PCOS weight loss and should be approached with caution and scepticism.
Gut microbiota
PCOS is a complex endocrine and metabolic disorder. It has been found that several genes associated with PCOS are linked to carbohydrate metabolism and steroid synthesis pathways, suggesting a significant correlation between metabolic factors and the pathological mechanism of PCOS. Gut microbiota (GM) participates in various metabolic functions and are therefore closely related to the pathogenesis and clinical symptoms of PCOS. Torres et al. [Citation70] conducted a study on 73 patients with PCOS and found that compared to normal women, patients with PCOS had fewer types of GM, and this trend was associated with increased androgen levels. To investigate the impact of GM on the host phenotype of PCOS, faecal samples from healthy women and PCOS patients were transplanted into two groups of mice via oral flushing. Compared to control group of mice’s, mices transplanted with faecal samples from PCOS patients developed IR, also had higher levels of testosterone and LH, had an increased number of ovarian cyst-like follicles, reduced corpora lutea. Fertility tests were also conducted, counting the number of offspring in the first litters after mating. Mice transplanted with faecal samples from women with PCOS had fewer offspring compared to healthy control mice [Citation13]. In other words, the diversity of GM was lower in patients with PCOS. Another study Lindheim et al. [Citation17] found that in a mouse model of PCOS induced by letrozole, the abundance of firmicutes (which are associated with obesity and metabolic syndrome) increased, while overall GM diversity decreased. Based on these experiments, it can be inferred that GM may play a role in the occurrence and development of PCOS. This may lead to a new therapeutic strategy in the clinical diagnosis and treatment of PCOS. Of course, the precise mechanism of action of GM in PCOS requires further research and scientific evidence [Citation6].
Changes in gut microbiota in women with PCOS
The human GM is composed of a vast number of microorganisms, including bacteria, viruses, protozoa, archaea and fungi. The GM, consisting of various species and strains, plays a crucial role in physiology, metabolism, nutrition and immune functions. Studies have investigated the association between PCOS and changes in GM and have identified significant differences in the composition of GM between women with PCOS and healthy individuals. The diversity of the GM, indicated by alpha and beta diversity, is altered in patients with PCOS [Citation71]. A balanced GM is essential for preventing the development of diseases. Additionally, an imbalance in specific bacterial species, such as Bacteroidetes and Firmicutes, has been observed in PCOS. These changes may affect the production of short-chain fatty acids, impacting metabolism, gut barrier integrity and immune response. For example, studies have shown an increase in Escherichia, Shigella or Bacteroides vulgatus in women with PCOS, while others have reported variations in Prevotella abundance. Beneficial bacteria like Lactobacilli and Bifidobacteria, which support immunity and nutrient absorption, are often reduced in PCOS [Citation23]. However, the modifications in GM in PCOS are diverse, sometimes contradictory and not fully understood. Studies have explored the relationship between GM and PCOS, particularly focusing on the association with IR and level of sex hormones [Citation72].
Gut microbiota and insulin resistance
Insulin resistance is intricately linked to GM, as demonstrated by studies conducted on animals and humans. Transplanting a healthy gut flora into germ-free mice has been found to promote adipose tissue growth and induce IR. On the other hand, it is hypothesized that transplanting a healthy microbiota into ill individuals could improve clinical parameters [Citation72]. For instance, a study by Vrieze et al. showed that after six weeks of transplanting GM from healthy individuals to patients with metabolic syndrome, there was an increase in insulin sensitivity and a decrease in BMI in the recipients [Citation73]. These findings suggest that GM may contribute to the development of IR. The mechanisms linking IR, GM and PCOS are diverse and have been summarized in a review by He and Li. An imbalance in gut flora, leading to increased gut permeability, could contribute to chronic low-grade inflammation through immune system activation. Proinflammatory cytokines can disrupt insulin receptor function, resulting in IR [Citation74].
Probiotics, prebiotics and symbiotic
According to the WHO, probiotics are living microorganisms that, when consumed in appropriate quantities, provide health benefits to the host [Citation75]. These beneficial microorganisms naturally occur in fermented foods and exhibit various properties such as antioxidant, antimicrobial and anti-inflammatory effects. They also improve metabolic parameters, regulate gut flora and modulate the immune system. Using probiotics as a form of therapy involves the proper supplementation of these microorganisms to treat diseases [Citation72].
Although the precise mechanism remains unclear, probiotic therapy has shown promising results in positively influencing the metabolic profile of women with PCOS [Citation76–80]. For instance, Ahmadi et al. conducted a study in which PCOS patients were supplemented with probiotics (L. acidophilus, L. casei and B. bifidum) for a duration of 12 weeks. The results revealed a significant decrease in body weight and BMI compared to the placebo group. Additionally, improvements were observed in glycaemic control, TG levels and very low-density lipoprotein (VLDL) cholesterol [Citation76]. Similar outcomes were reported in studies supplementing women with PCOS with L. casei, L. acidophilus, L. rhamnosus, L. bulgaricus, B. breve, B. longum and Streptococcus thermophiles for 8 weeks, which resulted in notable reductions in serum glucose and insulin levels [Citation77]. Furthermore, Rashad et al. demonstrated that probiotic supplementation with L. delbrueckii and L. fermentum for 12 weeks significantly lowered IR (HOMA-IR) levels and improved lipid profiles [Citation79]. Another study found that probiotic therapy using L. acidophilus, L. plantarum, L. fermentum and L. gasseri for 12 weeks may play a role in modulating inflammatory processes in women with PCOS [Citation72].
A meta-analysis conducted by Heshmati et al., encompassing seven randomized controlled trials (RCTs), did not observe significant effects of probiotic supplementation on anthropometric indicators such as body weight, BMI, waist circumference, HOMA-IR and LDL in PCOS patients when compared to a placebo. However, it did reveal a major influence on glycaemic control, insulin levels and lipid metabolism, including a decrease in serum TG levels and an increase in HDL cholesterol. These findings suggest that probiotic supplementation could serve as an adjunct therapy for PCOS. The impact of probiotic therapy on the hormonal profile of women with PCOS remains less extensively studied [Citation78]. In a recent meta-analysis by Shamasbi et al., significant improvements were observed in hormonal and inflammatory markers, including a reduction in free androgens (FAI) and malondialdehyde (MDA) levels, as well as an increase in SHBG and nitric oxide (NO) binding.
Furthermore, another study indicated that probiotic supplementation for 12 weeks in women with PCOS had a beneficial effect on total testosterone levels, SHBG and MDA but did not significantly affect other metabolic profiles [Citation78]. It is important to note that there is considerable variation in the probiotic strains and dosages used across these studies, underscoring the need for future research to establish standardized guidelines in this regard. Among prebiotics, fructooligosaccharides (FOS), inulin, galactooligosaccharides (GOS) and lactulose are the most well-known [Citation75]. These substances modulate the composition of the gut microbiome, exerting positive effects on the overall health of the host.
Several studies have demonstrated that prebiotics can have a positive impact on metabolic markers and possess immunomodulatory properties by promoting the growth of beneficial bacteria, such as Bifidobacterium and Lactobacillus. This leads to significant reductions in fasting glucose, serum TG levels, total cholesterol and LDL cholesterol, as well as substantial increases in HDL cholesterol. Recent research by Fernandes et al. revealed that certain prebiotics directly contribute to controlling hyperglycaemia and IR (HOMA-IR). These findings suggest that the combination of prebiotics with probiotics may enhance their effectiveness in reducing LDL cholesterol levels. Moreover, probiotics (or symbiotic) have the potential to lower TG levels, as indicated by a mean difference of −17.51 mg/dL (95% CI −29.65 to −5.36) [Citation81]. Additionally, sustained consumption of dextrin by women with PCOS has shown improvement in androgen levels, hirsutism and menstrual cycle irregularities. However, further research is needed to elucidate and compare the effectiveness of different probiotic strains and dosages, determine the optimal treatment duration, and establish the health benefits of probiotics, prebiotics and symbiotic in relation to clinical outcomes in PCOS [Citation72].
Supplementation
Supplements, the most important vitamins (folic acid, vitamin D, etc.) taken for too long a period or in too high a dose may pose a potential threat that may cause the opposite toxic effect [Citation82–85].
Vitamin D
The molecular mechanism between vitamin D and PCOS is unclear and there is strong need to make more research at the future.
Yildizhan et al. claim that obese women with PCOS had significantly lower levels of 25-hydroxyvitamin D [Citation85].
Jafari-Sfidvajani et al. observed improved biochemical parameters in women with PCOS after vitamin D3 supplementation, suggesting that vitamin D3 may prevent the progression of inflammation in PCOS pathogenesis. On the other hand, Jafari-Sfidvajani et al. did not find significant differences in androgen profiles after vitamin D supplementation, except for an improvement in menstrual frequency [Citation86].
Study conducted by Lerchbaum et al. found in healthy women without PCOS that baseline oestradiol levels were significantly lower in the vitamin D group compared to the placebo group. No significant differences were found between vitamin D and placebo groups in PCOS in terms of other baseline characteristics, as well as in healthy women [Citation87].
A meta-analysis conducted by Akbari et al. in seven RCTs found that vitamin D supplementation in women with PCOS resulted in improved concentrations of high-sensitivity C-reactive protein (hs-CRP), MDA and total antioxidant capacity (TAC), but had no effect on NO and total glutathione (GSH) levels [Citation88].
A study conducted by Krul-Poel et al. confirmed the role of vitamin D in adverse metabolic events in PCOS. Lower vitamin D levels were associated with IR due to the complex pathophysiology of PCOS [Citation82].
Another study conducted by Razavi et al. with vitamin D–calcium supplementation showed a tendency towards a decrease in LH, while there was no significant effect of vitamin D–calcium supplementation on prolactin, FSH, 17-OH progesterone, markers of inflammation, and GSH levels [Citation38].
Vitamin D improves oxidative stress levels in PCOS [Citation89–91]. A study conducted by Kyei et al. found that the combination of vitamin D3 and MitoQ10 significantly reduced markers of oxidation, superoxide dismutase (SOD) and MDA, as well as hormonal markers including oestradiol, progesterone, FSH, LH and LH/FSH. The study also observed improvements in histomorphology characteristics of the ovaries with multiple healthy follicles and atretic follicles after simultaneous administration [Citation83,Citation84].
Vitamin E
Vitamin E functions as a scavenger of free radicals and balances the ratio of oxidants to antioxidants. Studies conducted by Cicek et al. suggest that vitamin E may have a beneficial effect on the endometrial lining in infertile women due to its antithrombotic and antioxidant properties [Citation92].
According to the findings of Bahmani et al., supplementation with folate at a dose of 5 mg per day leads to a decrease in the levels of Hcy, HOMA-B, hs-CRP and MDA in the serum, as well as an increase in the levels of TAC and GSH compared to the placebo group [Citation93]. Simultaneous supplementation of omega-3 fatty acids and vitamin E, as demonstrated, reduces the expression of lipoprotein (a), mRNA and oxidized mRNA-LDL genes. Furthermore, it significantly decreases the levels of TGA, VLDL, LDL cholesterol and the ratio of total cholesterol to HDL in the serum of patients with PCOS. There is also an increase in TAC values in the serum and a decrease in MDA levels compared to the placebo group [Citation29].
In a 12-week study conducted by Mirmasoumi et al., investigating supplementation with flaxseed oil rich in omega-3 fatty acids, a significant reduction in insulin levels, HOMA-IR and mFG indices, and an increase in the quantitative insulin sensitivity check index were observed. Additionally, compared to the placebo group, a decrease in serum levels of TGA, VLDL cholesterol and hs-CRP was observed. Supplementation with omega-3 flaxseed oil did not show a significant impact on hormonal and lipid profiles or NO levels in the serum [Citation13]. Consumption of omega-3 fatty acids leads to a significant reduction in serum insulin levels, HOMA-IR, testosterone levels and hirsutism, while increasing the Quantitative Insulin Sensitivity Index (QUICKI). Furthermore, compared to the placebo, omega-3 supplementation decreases the levels of hs-CRP and MDA, while increasing the total level of GSH in the serum. Other metabolic parameters remain unchanged [Citation94].
Inositol
Myo-inositol (MI) is commonly present in nature, both in mammals and other animals. It is naturally consumed with food, from fruits, beans, grains and nuts.
There is a complex relationship between glucose metabolism and inositol. Inositol inhibits glucose absorption in the duodenum and reduces blood glucose levels by influencing the same transport system [Citation89,Citation95]. On the other hand, glucose can limit cellular uptake of inositol and deplete MI through the activation of the glucose–sorbitol pathway. Studies have shown that inhibiting aldose reductase restores inositol levels in cultured cells, counteracting the depleting effects of sorbitol [Citation96]. Sodium–glucose transporter inhibitors prevent the uptake of both glucose and inositol, suggesting that both molecules utilize the same transporter system [Citation95]. Additionally, both hyperglycaemia and IR affect the proportions of different inositol isomers present in tissues.
Myo-inositol stimulates FSH signalling as a second messenger, while d-chiro-inositol (DCI) participates in androgen synthesis and acts as an aromatase inhibitor. In a properly functioning ovary, the balance between these two isomers supports the proper secretion of hormones and ovarian function. Under physiological conditions, the MI/DCI ratio is approximately 100:1 in follicular fluid [Citation97–99]. In patients with PCOS and IR, hyperinsulinaemia leads to a higher DCI to MI ratio [Citation37]. MI may influence aromatase activity in an opposite manner to DCI [Citation6,Citation100]. Higher MI/DCI ratios promote aromatase activity in the granulosa layer, increasing oestrogen levels, while lower MI/DCI ratios stimulate androgen production in the cells [Citation6,Citation101]. This may explain why DCI supplementation increases testosterone levels and simultaneously decreases oestrogen levels [Citation93]. The result is hyperandrogenism and suppression of FSH signalling. This mechanism engages in the so-called ‘ovarian paradox’ which was defined by Carlomagno et al. According to this hypothesis, increased epimerase activity in the ovaries of patients with PCOS leads to a local deficiency of MI, which in turn affects the inadequate quality of oocytes. In these conditions, glucose uptake and metabolism in oocytes and follicular cells are impaired [Citation101].
The various beneficial effects of inositol on follicular development, hormonal regulation and glucose homeostasis indicate its potential application as a therapy for patients with PCOS. There are studies confirming the positive impact of inositol on metabolic, hormonal and reproductive disorders in PCOS, both as a standalone substance and in combination with other agents that enhance its therapeutic effects [Citation98,Citation100].
Flavonoids and isoflavones
Both flavonoids and isoflavones consist of polyphenols with antioxidant, antidiabetic and anti-inflammatory properties. Flavonol consumption is effective in treating metabolic syndrome, improve lipid profile and total cholesterol levels and a decrease in LDL levels. There are no changes observed in anthropometric characteristics, hormonal environment, menstrual cycles and overall glycaemic metabolism [Citation102].
Alternative medicine
Herbal medicine
According to studies, a wide range of herbs can be used to reduce the symptoms of PCOS and improve various aspects of life for PCOS patients. Plants, as natural remedies, may bring beneficial effects to PCOS patients, but further research is necessary to understand their mechanisms of action and safety [Citation103].
Cinnamomum verum deserves special attention. Cinnamon, in combination with Glycyrrhiza spp., Paeonia lactiflora Pall. and Hypericum perforatum L., reduced BMI and insulin levels without significant impact on fasting blood sugar (FBS). Administration of Cinnamomum zeylanicum Blume and Nigella sativa L. led to a significant decrease in insulin levels, IR, as well as cholesterol, TGs and low-density lipoprotein (LDL), and lowered LH levels. However, it did not show significant effects on FSH levels or testosterone levels. Additionally, the use of Lagerstroemia speciosa L. and Cinnamomum burmannii over six months significantly reduced the BMI of patients [Citation103–106].
It seems that cinnamon in all its forms (extract, powder and supplement) affected hormonal status, carbohydrate metabolism and menstrual cycles. It should be noted that the improvement in the studied parameters occurred after consuming different forms of Cinnamomum cassia, including powdered supplements and extracts. We can argue that the form of administration is not significant in the therapeutic process [Citation103–106].
Terminalia chebula, commonly known as ‘Haritaki/Myrobalan’, has long been used in herbal medicine and traditional medicine. It has antioxidant, anti-proliferative, antimicrobial, pro-apoptotic, anti-diabetic, anti-aging, hepatoprotective, epileptic and anti-epileptic properties. Importantly, in the case of PCOS, Terminalia chebula has been found to have beneficial effects on glucose and lipid metabolism and prevents atherogenesis and endothelial dysfunction. The fruits, seeds and bark contain bioactive compounds such as chebulic acid, chebulinic acid and chebulaginic acid, which have shown valuable pharmacological properties through extensive scientific research. These compounds exhibit antioxidant, anti-inflammatory, anti-diabetic, cardioprotective and anti-aging effects [Citation107].
A histopathological study of pancreatic tissue in mice, conducted by Agrawal and Kulkarni showed that the extract had a protective effect against damage caused by hyperglycaemia. Immunohistochemistry of pancreatic tissue demonstrated increased SIRT1 expression in diabetic animals treated with the extract. The results of these studies indicate that the extract of T. chebula has a significant effect in the treatment of type 2 diabetes and, therefore, has a beneficial impact on IR [Citation108].
Other herbs with confirmed therapeutic value include:
Trigonella foenum-graecum L.;
Danzhi xiaoyao (Xiao Yao San is one of the most used herbal formulations in Traditional Chinese Medicine. The formula contains herbs such as Chinese angelica, white peony and bupleurum);
Flaxseed powder (Linum usitatissimum L.);
Grifola frondosa (a species of fungus in the Polyocracies family);
Unkei-to (a Japanese herb);
Marjoram (marjoram infusion);
Vitex agnus-castus (similar benefits to oral contraceptives in regulating menstrual cycles, with significantly fewer side effects).
They may contribute to regulating menstrual cycles and improving fertility. Herbal remedies not only improve reproductive disorders but also play a significant role in balancing hormonal status and menstrual cycles [Citation103].
Other herbs, when combined with medications, may impact fertility. Cimicifuga racemosa, in combination with clomiphene, may increase fertility potential in PCOS patients [Citation42]. Another study showed that long-term use of Cimicifuga racemosa as a phytoestrogen may be an alternative to clomiphene [Citation109]. On the other hand, the combination of berberine (from Coptidis rhizome) with letrozole did not show significant positive effects on fertility [Citation110], while berberine administration in combination with metformin yielded promising results [Citation111].
Limited research has been conducted on the anti-androgenic effects of herbs and their impact on hirsutism. It has been found that tea made from Anethum graveolens L., Asparagus racemosus Willd. and Matricaria chamomilla L. may reduce testosterone levels and the severity of hirsutism. More research is needed to clarify the potential effects of herbs on hirsutism [Citation103].
Experimental studies in animals have shown that Nigella sativa (black cumin) may have potential activity against PCOS [Citation66,Citation112–115]. Studies on rats with PCOS by Kohzadi et al. showed that oral administration of a water-alcoholic extract of N. sativa for 63 days resulted in a significant improvement in the total level of antioxidants (TAC) and a reduction in the concentration of MDA in ovarian tissue. Additionally, administration of thymoquinone (active ingredient of black cumin) improved ovarian morphology, function and induced ovulation in rats with PCOS, similarly to metformin [Citation66].
A study by Nafiu et al. in female rats with letrozole-induced PCOS showed that administration of N. sativa oil for 7 weeks led to an increase in the number of rats undergoing regular menstrual cycles, the appearance of the corpus luteum, and a decrease in the number of follicular cysts. Additionally, there was a decrease in the level of hormones such as LH, testosterone, FSH and biochemical parameters such as glucose, TGs, LDL cholesterol and MDA, as well as an increase in the level of HDL-c, SOD and GPX [Citation113].
Other studies [Citation112,Citation114,Citation115] have shown that administering N. sativa extract or black cumin seed extract along with honey to rats with PCOS resulted in improvements in the levels of sex hormones such as LH, FSH, oestrogens, testosterone and progesterone. Additionally, a reduction in IR, glucose concentration and an improvement in the body’s antioxidant function were observed. These conclusions suggest that black cumin may have therapeutic potential in the treatment of PCOS by improving ovarian morphology and function, regulating sex hormone levels, and having anti-inflammatory and antioxidant effects [Citation112,Citation114,Citation115].
Glycyrrhiza glabra L., commonly known as licorice, is a member of the Fabaceae family and has been utilized in traditional medicine for its healing properties on wounds, pain relief, alleviating cough and treating gastritis. This plant is valued for the presence of essential medicinal compounds in its roots, including flavonoids, sterols, gums, starches and essential oils. The sterols or phytoestrogens found in licorice have been associated with the reduction of TGs and cholesterol. Glycerin, the primary active component in licorice root, possesses a sweetness level approximately 50 times greater than sucrose. Studies have revealed that glycerin exhibits a mineralocorticoid-like impact by obstructing the 11beta-hydroxysteroid dehydrogenase type 2 (11betaHSD2) enzyme. This inhibition raises the levels of glucocorticoids in the bloodstream. Moreover, glucocorticoids stimulate the secretion of insulin, thereby contributing to a reduction in blood sugar levels [Citation116–118].
Acupuncture
In addition to pharmacological treatment and lifestyle changes such as diet, supplementation and physical activity, PCOS treatment process can include vagus nerve stimulation through acupuncture. Apart from its effectiveness in treating epilepsy, refractory epilepsy and autoimmune disorders, vagus nerve stimulation plays a significant role in transmitting information about peripheral pro-inflammatory cytokines to the brain (NTS) because it is sensitive to the presence of interleukins and prostaglandins. In turn, the NTS transmits this information to various levels, including the hypothalamus, limbic system and pituitary gland, leading to the activation of the hypothalamic-pituitary-adrenal (HPA) axis, which results in the release of cortisol from the adrenal cortex, a key component in PCOS treatment [Citation119,Citation120].
The anti-inflammatory role of the vagus nerve occurs through the vagal reflex, inhibiting the release of cytokines such as TNFα from macrophages, commonly referred to as the cholinergic anti-inflammatory pathway. These anti-inflammatory capabilities of the vagus nerve make it a target for modulating VNS to influence intestinal inflammatory conditions such as inflammatory bowel disease (IBD), rheumatoid arthritis (RA), sepsis, cardiovascular diseases, Alzheimer’s disease and most importantly, diabetes associated with PCOS [Citation119,Citation120].
Depression is a problem affecting women with PCOS. The use of vagus nerve stimulation in the treatment of treatment-resistant depression was approved in 2005. This approval followed several controlled and uncontrolled studies that observed improvements in standardized mood assessments after VNS treatment in patients with treatment-resistant depression [Citation42].
Transcutaneous auricular vagus nerve stimulation (taVNS) is a non-invasive method of delivering transcutaneous stimulation directly to the auricular branch of the vagus nerve. It involves acupuncture at specific ear points. taVNS has been shown to be an effective, safe and cost-effective method. It has broad potential in the treatment of patients with disorders of consciousness (DOC) and has a positive impact on human emotions and cognitive functions, including cortical arousal and alertness. However, it remains unclear how taVNS specifically affects cortical arousal and alertness [Citation42,Citation107,Citation119].
Results and conclusions
In summary, the treatment of PCOS focuses on achieving specific goals tailored to the individual needs of each patient. PCOS has a varied course; therefore, there are effective therapeutic approaches. Each treatment should be appropriately customized to the patient’s body requirements. Although an ideal solution would be causal treatment, the full understanding of PCOS pathogenesis is still not completely known and requires further research. Nevertheless, knowledge in this field is continuously evolving, and new findings about various therapeutic options are emerging.
A comprehensive approach to treating PCOS includes regular check-up visits, lifestyle changes (such as a healthy diet, physical activity and stress reduction), conventional therapy (such as hormonal treatment, metformin, dietary supplementation) and alternative treatments (herbal medicine, acupuncture). It is important to effectively combine these different methods to maximize therapeutic benefits.
Moreover, prevention of long-term complications of the disease is also significant. Patients with PCOS are at a higher risk of developing other complications, such as metabolic syndrome, diabetes or cardiovascular diseases. Therefore, it is essential to take actions aimed at minimizing these long-term effects of the disease, such as adopting a healthy diet, maintaining an active lifestyle, and reducing stress. Lifestyle changes will not only help in the current situation but also prevent future complications.
Author contributions
Stańczak Natalia Anna – substantial contributions to the conception or design of the work; the acquisition, analysis or interpretation of data for the work, drafting the work, reviewing it critically for important intellectual content. Grywalska Ewelina – final approval of the version to be published. Dudzińska Ewa – substantial contributions to the conception or design of the work; analysis, or interpretation of data for the work. Final approval of the version to be published. All authors are agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors agree to be accountable for all aspects of the work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from FAIRsharing upon reasonable request. Restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. However, data are available from the authors upon reasonable request and with permission of corresponding author, Natalia Anna Stańczak (Department of Dietary and Nutritional Education, Medical University of Lublin, Chodźki 7, 20-093 Lublin, Poland).
Additional information
Funding
References
- Kelly C, Lyall H, Petrie J, et al. Low grade chronic inflammation in women with polycystic ovarian syndrome. J Clin Endocrinol Metab. 2001;86(6):2453–2455. doi: 10.1210/jcem.86.6.7580.
- Kuligowska-Jakubowska M, Dardzińska J, Rachoń D. Zaburzenia gospodarki węglowodanowej u kobiet z zespołem wielotorbielowatych jajników (PCOS). Diabetol Klin. 2012;5:185–195.
- Laber S. Diagnoza lekarska w zespole policystycznych jajników. Food Forum. 2018;2:7–10.
- Lerchbaum E, Theiler-Schwetz V, Kollmann M, et al. Effects of vitamin D supplementation on surrogate markers of fertility in PCOS women: a randomized controlled trial. Nutrients. 2021;13(2):547. doi: 10.3390/nu13020547.
- Szydlarska D, Grzesiuk W, Bar-Andziak E. Kontrowersje wokół patogenezy zespołu policystycznych jajników, Endokrynologia. Otyłość i Zaburzenia Przemiany Materii. 2010;3:141–146.
- Wołczyński S, Zgliczyński W. Abnormalities of the menstrual cycle. In: Zgliczyński W, editor. Large interna – endocrinology. 2nd ed. Warsaw, Poland: Medical Tribune; 2012. p. 561–567.
- Echiburú B, Pérez-Bravo F, Galgani JE, et al. Enlarged adipocytes in subcutaneous adipose tissue associated to hyperandrogenism and visceral adipose tissue volume in women with polycystic ovary syndrome. Steroids. 2018;130:15–21. doi: 10.1016/j.steroids.2017.12.009.
- Nowotnik A. Wielowymiarowość doświadczenia zespołu policystycznych jajników u kobiet w wieku rozrodczym: przegląd badań. Now Lek. 2012;81(3):268–272.
- Kłósek P, Grosicki S, Całyniuk B. Dietoterapia w zespole policystycznych jajników – zalecenia praktyczne. For Zab Metabl. 2017;4:148–154.
- Sadłocha M, Skrzypulec-Pilnta V. Insulinozależny model patofizjologii zespołu policystycznych jajników. Rola metforminy w procesie leczniczym. Forum Położnictwa i Ginekologii luty/marzec. 2016;32:20–27.
- Kharitonenkov A, Adams AC. Inventing new medicines: the FGF21 story. Mol Metab. 2014;3(3):221–229. doi: 10.1016/j.molmet.2013.12.003.
- Schiffer L, Arlt W, O’Reilly MW. Understanding the role of androgen action in female adipose tissue. Front Horm Res. 2019;53:33–49. doi: 10.1159/000494901.
- Mirmasoumi G, Fazilati M, Foroozanfard F, et al. The effects of flaxseed oil omega-3 fatty acids supplementation on metabolic status of patients with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Exp Clin Endocrinol Diabetes. 2018;126(4):222–228. doi: 10.1055/a-1736-9017.
- Rudnicka E, Suchta K, Grymowicz M, et al. Chronic low grade inflammation in pathogenesis of PCOS. Int J Mol Sci. 2021;22(7):3789. doi: 10.3390/ijms22073789.
- Schindler AE, Campagnoli C, Druckmann R, et al. Classification and pharmacology of progestins. Maturitas. 2003;46 Suppl. 1(1–2):S7–S16. doi: 10.1016/j.maturitas.2003.09.014.
- Ye W, Xie T, Song Y, et al. The role of androgen and its related signals in PCOS. J Cell Mol Med. 2021;25:1825–1837.
- Lindheim L, Bashir M, Münzker J, et al. Alterations in gut microbiome composition and barrier function are associated with reproductive and metabolic defects in women with polycystic ovary syndrome (PCOS): a pilot study. PLOS One. 2017;12(1):e0168390. doi: 10.1371/journal.pone.0168390.
- Jang SH, Cho MJ. Transcutaneous auricular vagus nerve stimulation in disorders of consciousness: a mini-narrative review. Medicine. 2022;101(50):e31808. doi: 10.1097/MD.0000000000031808.
- Xu Y, Qiao J. Association of insulin resistance and elevated androgen levels with polycystic ovarian syndrome (PCOS): a review of literature. J Healthc Eng. 2022;2022:9240569.
- Andhalkar S, Chaware V, Redasani V. A review on medicinal plants of natural origin for treatment of polycystic ovarian syndrome (PCOS). Asian J Pharm Res Dev. 2021;9(3):76–81. doi: 10.22270/ajprd.v9i3.949.
- Moran LJ, Misso ML, Wild RA, et al. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2010;16(4):347–363. doi: 10.1093/humupd/dmq001.
- Moller DE, Flier JS. Detection of an alteration in the insulin-receptor gene in a patient with insulin resistance, acanthosis nigricans, and the polycystic ovary syndrome (type A insulin resistance). N Engl J Med. 1988;319(23):1526–1529. doi: 10.1056/NEJM198812083192306.
- El-Saber Batiha G, Magdy Beshbishy A, El-Mleeh A, et al. Traditional uses, bioactive chemical constituents, and pharmacological and toxicological activities of Glycyrrhiza glabra L. Biomolecules. 2020;10(3):35. doi: 10.3390/biom10030352.
- Ferranti SD, Gauvreau K, Ludwig DS, et al. Prevalence of the metabolic syndrome in American adolescents: findings from the third national health and nutrition examination survey. Circulation. 2004;110(16):2494–2497. doi: 10.1161/01.CIR.0000145117.40114.C7.
- Luque-Ramírez M, Nattero-Chávez L, Ortiz Flores AE, et al. Combined oral contraceptives and/or antiandrogens versus insulin sensitizers for polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2018;24(2):225–241. doi: 10.1093/humupd/dmx039.
- Reckelhoff Jane F, Shawky Noha M, Romero Damian G, et al. Polycystic ovary syndrome: insights from preclinical research. Kidney360. 2022;3(8):1449–1457.
- Pache TD, Fauser BC. Polycystic ovaries in female-to-male transsexuals. Clin Endocrinol. 1993;39(6):702–703.
- Sobstyl M, Tkaczuk-Włach J, Sobstyl J, et al. Zespół metaboliczny a zespół policystycznych jajników. Prz Menopauzalny. 2011;1:74–78.
- Sunita JR, Ghongane BB, Ramanand JB, et al. Clinical characteristics of polycystic ovary syndrome in Indian women. Indian J Endocrinol Metab. 2013;17(1):138–145.
- Naseran SN, Mokhtari M, Abedinzade M, et al. Evaluation of the effect of Nigella sativa hydro-alcoholic extract and honey on gonadotropins and sex hormones level in the polycystic ovarian syndrome model of Wistar rat. Sci J Kurd Univ Med Sci. 2020;25(1):117–129.
- Morotti E, Battaglia B, Fabbri R, et al. Cigarette smoking and cardiovascular risk in young women with polycystic ovary syndrome. Int J Fertil Steril. 2014;7(4):301–312.
- Norman R, Masters L, Milner C, et al. Relative risk of conversation from normoglycaemia to impaired glucose tolerance or non-insulin dependent diabetes mellitus in polycystic ovarian syndrome. Hum Reprod. 2001;16(9):1995–1998. doi: 10.1093/humrep/16.9.1995.
- Rahmani E, Samimi M, Ebrahimi FA, et al. The effects of omega-3 fatty acids and vitamin E co-supplementation on gene expression of lipoprotein (a) and oxidized low-density lipoprotein, lipid profiles and biomarkers of oxidative stress in patients with polycystic ovary syndrome. Mol Cell Endocrinol. 2017;439:247–255. doi: 10.1016/j.mce.2016.09.008.
- Anderson RA, McLaughlin M, Wallace WH, et al. The immature human ovary shows loss of abnormal follicles and increasing follicle developmental competence through childhood and adolescence. Hum Reprod. 2014;29(1):97–106. doi: 10.1093/humrep/det388.
- Barbosa-Desongles A, Hernandez C, Simo R, et al. Testosterone induces cell proliferation and cell cycle gene overexpression in human visceral preadipocytes. Am J Physiol Cell Physiol. 2013;305(3):C355–C359.
- Dicker A, Rydén M, Näslund E, et al. Effect of testosterone on lipolysis in human pre-adipocytes from different fat depots. Diabetologia. 2004;47(3):420–428. doi: 10.1007/s00125-003-1324-0.
- O’Reilly MW, Kempegowda P, Walsh M, et al. AKR1C3-mediated adipose androgen generation drives lipotoxicity in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102(9):3327–3339. doi: 10.1210/jc.2017-00947.
- The Rotterdam ESHRE/ASRM-sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41–47. doi: 10.1093/humrep/deh098.
- Fica S, Albu A, Constantin M, et al. Insulin resistance and fertility in polycystic ovary syndrome. J Med Life. 2008;1(4):415–422.
- Sidhwani S, Scoccia B, Sunghay A, et al. PCOS is associated with atherogenic changes in lipoprotein particle number and size independent of body weight. Clin Endocrinol. 2011;75(1):76–82. doi: 10.1111/j.1365-2265.2011.04015.x.
- Romer T. Zespół policystycznych jajników (ZPJ) – czynnościowy hiperandrogenizm jajników (CHJ) w Endokrynologia Kliniczna dla ginekologa internisty i pediatry Red. Wyd PWN Warszawa. 1998;1:167–181.
- Razavi M, Jamilian M, Karamali M, et al. The effects of vitamin D-K-calcium co-supplementation on endocrine, inflammation, and oxidative stress biomarkers in vitamin D-deficient women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Horm Metab Res. 2016;48(7):446–451. doi: 10.1055/s-0042-104060.
- Kitzinger C, Willmott J. The thief of womanhood: women’s experience of polycystic ovarian syndrome. Soc Sci Med. 2002;54(3):349–361. doi: 10.1016/s0277-9536(01)00034-x.
- Sonino N, Fava GA, Mani E, et al. I wsp.: quality of life of hirsute women. Postgrad Med J. 1993;69(809):186–189. doi: 10.1136/pgmj.69.809.186.
- Ekback M, Wijma K, Benzein E. “It is always on my mind”: women’s experiences of their bodies when living with hirsutism. Health Care Women Int. 2009;30(5):358–372. doi: 10.1080/07399330902785133.
- O’Reardon JP, Cristancho P, Peshek AD. Vagus nerve stimulation (VNS) and treatment of depression: to the brainstem and beyond. Psychiatry. 2006;3(5):54–63.
- Kathleen M, Dokras A, Piltonen T. Update on PCOS: consequences, challenges, and guiding treatment. J Clin Endocrinol Metabol. 2021;106(3):1071–1083.
- Milewicz A. Reimbursement of metformin for polycystic ovary syndrome. Endokrynol Pol. 2013;64(5):409–414. doi: 10.5603/EP.2013.0025.
- Taghvaee Javanshir S, Yaghmaei P, Hajebrahimi Z. Thymoquinone ameliorates some endocrine parameters and histological alteration in a rat model of polycystic ovary syndrome. Int J Reprod Biomed. 2018;16(4):275–284.
- Chen Y, Lu X, Hu L. Transcutaneous auricular vagus nerve stimulation facilitates cortical arousal and alertness. Int J Environ Res Public Health. 2023;20(2):1402. doi: 10.3390/ijerph20021402.
- Helvaci N, Yildiz BO. Oral contraceptives in polycystic ovary syndrome. Minerva Endocrinol. 2014;39(3):175–187.
- Messinis IE. Ovulation induction: a mini review. Hum Reprod. 2005;20(10):2688–2697. doi: 10.1093/humrep/dei128.
- Tannus S, Burke YZ, Kol S. Treatment strategies for the infertile polycystic ovary syndrome patient. Womens Health. 2015;11(6):901–912. doi: 10.2217/whe.15.40.
- Milewicz T. Metformina w ginekologii. Forum Położnictwa i Ginekologii. 2016;25:36–45.
- Bednarska S, Siejka A. The pathogenesis and treatment of polycystic ovary syndrome: what’s new? Adv Clin Exp Med. 2017;26(2):359–367. doi: 10.17219/acem/59380.
- Shahin AY, Mohammed SA. Adding the phytoestrogen Cimicifugae racemosae to clomiphene induction cycles with timed intercourse in polycystic ovary syndrome improves cycle outcomes and pregnancy rates – a randomized trial. Gynecol Endocrinol. 2014;30(7):505–510. doi: 10.3109/09513590.2014.895983.
- Mazurek A, Kuć P, Laudański T. Progestins in hormonal replacement therapy and contraception. Prz Menopauz. 2003;4:40–45.
- Giatti S, Melcangi RC, Pesaresi M. The other side of progestins: effects in the brain. J Mol Endocrinol. 2016;57(2):R109–R126.
- Druckmann R. Profile of the progesterone derivative chlormadinone acetate – pharmocodynamic properties and therapeutic applications. Contraception. 2009;79(4):272–281. doi: 10.1016/j.contraception.2008.10.017.
- Propecia® drug information; 2021 [cited 2021 Sep 4]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020788s020s021s0 23lbl.pdf
- Ruan X, Seeger H, Mueck AO. The pharmacology of dienogest. Maturitas. 2012;71(4):337–344. doi: 10.1016/j.maturitas.2012.01.018.
- Carmina E, Longo RA. Semaglutide treatment of excessive body weight in obese PCOS patients unresponsive to lifestyle programs. J Clin Med. 2023;12(18):5921. doi: 10.3390/jcm12185921.
- Anala AD, Saifudeen ISH, Ibrahim M, et al. The potential utility of tirzepatide for the management of polycystic ovary syndrome. J Clin Med. 2023;12(14):4575.
- Al Khalifah RA, Florez ID, Zoratti MJ, et al. Efficacy of treatments for polycystic ovarian syndrome management in adolescents. J Endocr Soc. 2021;5(1):bvaa155. doi: 10.1210/jendso/bvaa155.
- Sadeghi HM, Adeli I, Calina D, et al. Polycystic ovary syndrome: a comprehensive review of pathogenesis, management, and drug repurposing. Int J Mol Sci. 2022;23(2):583. doi: 10.3390/ijms23020583.
- Sobkiewicz S. Indukcja owulacji w zespole policystycznych jajników. Forum Położnictwa i Ginekologii. 2016;30:24–30.
- Łagowska K, Bajerska J, Jamka M. The role of vitamin D oral supplementation in insulin resistance in women with polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2018;10(11):1637. doi: 10.3390/nu10111637.
- Reiser E, Lanbach J, Böttcher B, et al. Non-Hormonal treatment options for regulation of menstrual cycle in adolescents with PCOS. J Clin Med. 2023;12:67.
- Highlights of prescribing information-Aldactone® (Spironolactone) tablets, for oral use; 2021 [cited 2021 Apr 10]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/012151s075lbl.pdf
- Adamek B, Korzonek-Szlacheta I, Nowak J, et al. Dietoprofilaktyka zaburzeń miesiączkowania, Dietoprofilaktyka chorób żywieniowo zależnych red. Katowice: Wyd. SUM; 2017.
- Qi X, Yun C, Sun L, et al. Gut microbiota–bile acid–interleukin-22 axis orchestrates polycystic ovary syndrome. Nat Med. 2019;25(9):1459–1233. doi: 10.1038/s41591-019-0509-0.
- Peckenpaugh NJ. Insulinooporność i zespół metaboliczny [w:] Podstawy żywienia i dietoterapii. Wrocław: Edra Urban & Partner; 2015. p. 194–203.
- Kohzadi R, Nejati V, Razi M, et al. Effects hydro-alcoholic extract of (Nigella sativa L.) on the level of malondialdehyde (MDA) and total antioxidant capacity (TAC) of the ovary tissue in a rat model of PCOS. J Anim Environ. 2017;9(3):85–92.
- Szczurko M, Skowronek M, Zapalowska-Chwyc M, et al. A quantitative assessment of nutrition in patients with polycystic ovary syndrome (PCOS). Roczniki Państwowego Zakładu Higieny. 2016;4:419–426.
- Ahmed AF, Sharkawi SS, AbdelHameed SS, et al. Ketogenic diet restores hormonal, apoptotic/proliferative balance and enhances the effect of metformin on a letrozole-induced polycystic ovary model in rats. Life Sci. 2023;313:121285. doi: 10.1016/j.lfs.2022.121285.
- Paoli A, Mancin L, Giacona MC, et al. Effects of a ketogenic diet in overweight women with polycystic ovary syndrome. J Transl Med. 2020;18(1):104. doi: 10.1186/s12967-020-02277-0.
- Torres PJ, Siakowska M, Banaszewska B, et al. Gut microbial diversity in women with polycystic ovary syndrome correlates with hyperandrogenism. J Clin Endocrinol Metab. 2018;103(4):1502–1511. doi: 10.1210/jc.2017-02153.
- Zhao X, Jiang Y, Xi H, et al. Exploration of the relationship between gut microbiota and polycystic ovary syndrome (PCOS): a review. Geburtshilfe Frauenheilkd. 2020;80(2):161–171. doi: 10.1055/a-1081-2036.
- Giampaolino P, Foreste V, Di Filippo C, et al. Microbiome and PCOS: state-of-art and future aspects. Int J Mol Sci. 2021;22(4):2048. doi: 10.3390/ijms22042048.
- Vrieze A, Van Nood E, Holleman F, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143(4):913–916.e7. doi: 10.1053/j.gastro.2012.06.031.
- He F-F, Li Y-M. Role of gut microbiota in the development of insulin resistance and the mechanism underlying polycystic ovary syndrome: a review. J Ovarian Res. 2020;13:1–13.
- Guarner F, Khan AG, Garisch J, et al. World gastroenterology organisation global guidelines. J Clin Gastroenterol. 2012;46(6):468–481. doi: 10.1097/MCG.0b013e3182549092.
- Ahmadi S, Jamilian M, Karamali M, et al. Probiotic supplementation and the effects on weight loss, glycaemia and lipid profiles in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Hum Fertil. 2017;20(4):254–261. doi: 10.1080/14647273.2017.1283446.
- Askari G, Shoaei T, Tehrani HG, et al. Effects of probiotic supplementation on pancreatic β-cell function and C-reactive protein in women with polycystic ovary syndrome: a randomized double-blind placebo-controlled clinical trial. Int J Prev Med. 2015;6(1):27. doi: 10.4103/2008-7802.153866.
- Heshmati J, Farsi F, Yosaee S, et al. The effects of probiotics or synbiotics supplementation in women with polycystic ovarian syndrome: a systematic review and meta-analysis of randomized clinical trials. Probiot Antimicrob Proteins. 2019;11(4):1236–1247. doi: 10.1007/s12602-018-9493-9.
- Rashad NM, El-Shal AS, Amin AI, et al. Effects of probiotics supplementation on macrophage migration inhibitory factor and clinical laboratory feature of polycystic ovary syndrome. J Funct Foods. 2017;36:317–324. doi: 10.1016/j.jff.2017.06.029.
- Shamasbi SG, Ghanbari-Homayi S, Mirghafourvand M. The effect of probiotics, prebiotics, and synbiotics on hormonal and inflammatory indices in women with polycystic ovary syndrome: a systematic review and meta-analysis. Eur J Nutr. 2019;59(2):433–450. doi: 10.1007/s00394-019-02033-1.
- Fernandes R, Rosario VAD, Mocellin MC, et al. Effects of inulin-type fructans, galacto-oligosaccharides and related synbiotics on inflammatory markers in adult patients with overweight or obesity: a systematic review. Clin Nutr. 2017;36(5):1197–1206. doi: 10.1016/j.clnu.2016.10.003.
- Krul-Poel YH, Snackey C, Louwers Y, et al. The role of vitamin D in metabolic disturbances in polycystic ovary syndrome: a systematic review. Eur J Endocrinol. 2013;169(6):853–865. doi: 10.1530/EJE-13-0617.
- Kyei G, Sobhani A, Nekonam S, et al. Assessing the effect of MitoQ10 and vitamin D3 on ovarian oxidative stress, steroidogenesis and histomorphology in DHEA induced PCOS mouse model. Heliyon. 2020;6(7):e04279. doi: 10.1016/j.heliyon.2020.e04279.
- Xue Y, Xu P, Xue K, et al. Effect of vitamin D on biochemical parameters in polycystic ovary syndrome women: a meta-analysis. Arch Gynecol Obstet. 2017;295(2):487–496. doi: 10.1007/s00404-016-4247-y.
- Yildizhan R, Kurdoglu M, Adali E, et al. Serum 25-hydroxyvitamin D concentrations in obese and non-obese women with polycystic ovary syndrome. Arch Gynecol Obstet. 2009;280(4):559–563. doi: 10.1007/s00404-009-0958-7.
- Jafari-Sfidvajani S, Ahangari R, Hozoori M, et al. The effect of vitamin D supplementation in combination with low-calorie diet on anthropometric indices and androgen hormones in women with polycystic ovary syndrome: a double-blind, randomized, placebo-controlled trial. J Endocrinol Invest. 2018;41(5):597–607. doi: 10.1007/s40618-017-0785-9.
- Lim SS, Kakoly NS, Tan JWJ, et al. Metabolic syndrome in polycystic ovary syndrome: a systematic review, meta-analysis and meta-regression. Obes Rev. 2019;20(2):339–352. doi: 10.1111/obr.12762.
- Akbari M, Ostadmohammadi V, Lankarani KB, et al. The effects of vitamin D supplementation on biomarkers of inflammation and oxidative stress among women with polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled trials. Horm Metab Res. 2018;50(4):271–279. doi: 10.1055/s-0044-101355.
- Chukwuma CI, Ibrahim MA, Islam S. Myo-inositol inhibits intestinal glucose absorption and promotes muscle glucose uptake: a dual approach study. J Physiol Biochem. 2016;72(4):791–801. doi: 10.1007/s13105-016-0517-1.
- Dubey P, Reddy S, Boyd S, et al. Effect of nutritional supplementation on oxidative stress and hormonal and lipid profiles in PCOS-affected females. Nutrients. 2021;13(9):2938. doi: 10.3390/nu13092938.
- Lenarcik A, Milewicz A. Zespół wielotorbielowatych jajników w Endokrynologia w codziennej praktyce lekarskiej Red. Wyd. Pomorskiej Akademii Medycznej w Szczecinie, Szczecin. 2009;1:413–420.
- Cicek N, Eryilmaz OG, Sarikaya E, et al. Vitamin E effect on controlled ovarian stimulation of unexplained infertile women. J Assist Reprod Genet. 2012;29(4):325–328. doi: 10.1007/s10815-012-9714-1.
- Bahmani F, Karamali M, Shakeri H, et al. The effects of folate supplementation on inflammatory factors and biomarkers of oxidative stress in overweight and obese women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled clinical trial. Clin Endocrinol. 2014;81(4):582–587. doi: 10.1111/cen.12451.
- Amini M, Bahmani F, Foroozanfard F, et al. The effects of fish oil omega-3 fatty acid supplementation on mental health parameters and metabolic status of patients with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. J Psychosom Obstet Gynaecol. 2018;12:2000.
- Bevilacqua A, Bizzarri M. Inositols in insulin signaling and glucose metabolism. Int J Endocrinol. 2018;2018:1968450. doi: 10.1155/2018/1968450.
- Cammarata PR, Chen HQ, Yang J, et al. Modulation of myo-[3H]inositol uptake by glucose and sorbitol in cultured bovine lens epithelial cells. II. Characterization of high- and low-affinity myo-inositol transport sites. Investig Ophthalmol Vis Sci. 1992;33:3572–3580.
- Facchinetti F, Dante G, Neri I. The ratio of MI to DCI and its impact in the treatment of polycystic ovary syndrome: experimental and literature evidences. Front Gynecol Endocrinol. 2016;3:103–109.
- Kalra B, Kalra S, Sharma JB. The inositols and polycystic ovary syndrome. Indian J Endocrinol Metab. 2016;20(5):720–724. doi: 10.4103/2230-8210.189231.
- Unfer V, Carlomagno G, Papaleo E, et al. Hyperinsulinemia alters myoinositol to d-chiroinositol ratio in the follicular fluid of patients with PCOS. Reprod Sci. 2014;21(7):854–858. doi: 10.1177/1933719113518985.
- Unfer V, DiNicola S, Laganà AS, et al. Altered ovarian inositol ratios may account for pathological steroidogenesis in PCOS. Int J Mol Sci. 2020;21(19):7157. doi: 10.3390/ijms21197157.
- Carlomagno G, Unfer V, Roseff S. The d-chiro-inositol paradox in the ovary. Fertil Steril. 2011;95(8):2515–2516. doi: 10.1016/j.fertnstert.2011.05.027.
- Romualdi D, Costantini B, Campagna G, et al. Is there a role for soy isoflavones in the therapeutic approach to polycystic ovary syndrome? Results from a pilot study. Fertil Steril. 2008;90:1826–1833.
- Moini Jazani A, Nasimi Doost Azgomi H, Nasimi Doost Azgomi A, et al. A comprehensive review of clinical studies with herbal medicine on polycystic ovary syndrome (PCOS). Daru. 2019;27(2):863–877. doi: 10.1007/s40199-019-00312-0.
- Borzoei A, Rafraf M, Niromanesh S, et al. Effects of cinnamon supplementation on antioxidant status and serum lipids in women with polycystic ovary syndrome. J Tradit Complement Med. 2018;8(1):128–133. doi: 10.1016/j.jtcme.2017.04.008.
- Hajimonfarednejad M, Nimrouzi M, Heydari M, et al. Insulin resistance improvement by cinnamon powder in polycystic ovary syndrome: a randomized double-blind placebo controlled clinical trial. Phytother Res. 2018;32(2):276–283. doi: 10.1002/ptr.5970.
- Kort DH, Lobo RA. Preliminary evidence that cinnamon improves menstrual cyclicity in women with polycystic ovary syndrome: a randomized controlled trial. Am J Obstet Gynecol. 2014;211(5):487.e1–487.e6. doi: 10.1016/j.ajog.2014.05.009.
- Jokar A, Masoomi F, Sadeghpour O, et al. Potential therapeutic applications for Terminalia chebula in Iranian traditional medicine. J Tradit Chin Med. 2016;36(2):250–254. doi: 10.1016/s0254-6272(16)30035-8.
- Agrawal OD, Kulkarni YA. Treatment with Terminalia chebula extract reduces insulin resistance, hyperglycemia and improves SIRT1 expression in type 2 diabetic rats. Life. 2023;13(5):1168. doi: 10.3390/life13051168.
- Kamel HH. Role of phyto-oestrogens in ovulation induction in women with polycystic ovarian syndrome. Eur J Obstet Gynecol Reprod Biol. 2013;291(1):212. doi: 10.1016/j.ejogrb.2023.09.027.
- Wu X-K, Wang Y-Y, Liu J-P, et al. Randomized controlled trial of letrozole, berberine, or a combination for infertility in the polycystic ovary syndrome. Fertil Steril. 2016;106(3):757–765.e1.
- An Y, Sun Z, Zhang Y, et al. The use of berberine for women with polycystic ovary syndrome undergoing IVF treatment. Clin Endocrinol. 2014;80(3):425–431.
- Eini F, Joharchi K, Kutenaei MA, et al. Improvement in the epigenetic modification and development competence in PCOS mice oocytes by hydro-alcoholic extract of Nigella sativa during in-vitro maturation: an experimental study. Int J Reprod Biomed. 2020;18(9):733–746.
- Nafiu A, Alimi S, Babalola A, et al. Anti-androgenic and insulin-sensitizing actions of Nigella sativa oil improve polycystic ovary and associated dyslipidemia and redox disturbances. J Complement Med Res. 2019;10(4):186–199. doi: 10.5455/jcmr.20190613045154.