Abstract
Background
Literature on the safety of remdesivir in hospitalized COVID-19 patients with severe renal impairment is limited. We aimed to investigate the safety and effectiveness of remdesivir in this population.
Methods
We conducted a retrospective cohort study of adult hospitalized COVID-19 patients who received remdesivir between April 2022 and October 2022. Outcomes were compared between estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2 and ≥30 mL/min/1.73 m2 groups. The primary safety outcomes were acute kidney injury (AKI) and bradycardia, while the primary effectiveness outcomes included mortality in COVID-19-dedicated wards and hospital mortality. Secondary outcomes included laboratory changes, disease progression, and recovery time.
Results
A total of 1,343 patients were recruited, with 307 (22.9%) in the eGFR <30 group and 1,036 (77.1%) in the eGFR ≥30 group. Patients with an eGFR <30 had higher risks of AKI (adjusted hazard ratio [aHR] 2.92, 95% CI 1.93–4.44) and hospital mortality (aHR 1.47, 95% CI 1.06–2.05) but had comparable risks of bradycardia (aHR 1.15, 95% CI 0.85–1.56) and mortality in dedicated wards (aHR 1.43, 95% CI 0.90–2.28) than patients with an eGFR ≥30. Risk of disease progression was higher in the eGFR <30 group (adjusted odds ratio 1.62, 95% CI 1.16–2.26). No difference between the two groups in laboratory changes and recovery time.
Conclusions
Hospitalized COVID-19 patients receiving remdesivir with severe renal impairment had an increased risk of AKI, hospital mortality, and COVID-19 disease progression compared to patients without severe renal impairment.
Introduction
The Coronavirus Disease (COVID-19) has led to 770 million infections and 7 million deaths worldwide [Citation1]. Chronic kidney disease (CKD) imposes a substantial global burden, with an estimated prevalence of 9.1%, reflecting a 29.3% increase over the past three decades [Citation2]. Individuals with CKD have an increased susceptibility to severe COVID-19 and an increased risk of mortality [Citation3–5].
Remdesivir – an adenosine triphosphate analog and RNA-dependent RNA polymerase inhibitor – stands as the first antiviral drug to receive emergency use authorization from both the United States and European Union [Citation6,Citation7]. Notably, patients with an estimated glomerular filtration rate (eGFR) below 30 mL/min/1.73 m2 were excluded from the registrational trials of remdesivir [Citation8], rendering its usage not suitable for this group [Citation9]. Animal studies on repeat-dose toxicity have revealed the transient presence of the metabolite GS-704277, potentially contributing to adverse renal events following remdesivir administration [Citation10]. Additionally, caution in patients with renal impairment rose from concerns about the accumulation of the excipient sulfobutyl ether beta-cyclodextrin (SBECD) [Citation2, Citation11,Citation12]. However, despite these considerations, remdesivir is frequently employed in clinical settings involving patients with compromised renal function.
The majority of currently published studies examining remdesivir use in patients with an eGFR <30 encompass observational investigations, case reports, and case series studies [Citation13]. Virtually all studies indicate that remdesivir is relatively safe and well-tolerated among patients with severe renal impairment [Citation14–21]. It must be noted, however, that these studies are subject to various research limitations, including small sample sizes, absence of comparison groups, and failure to adequately control for potential confounding variables, which collectively contribute to the limited reliability of their findings. In contrast, studies utilizing the US FDA Adverse Events Reporting System (FAERS) and international pharmacovigilance post-marketing databases (VigiBase) have reported a higher incidence of kidney injury associated with remdesivir [Citation22–24].
There is an ongoing debate concerning the safety of remdesivir in patients with severe renal impairment. Hence, this study aimed to investigate the safety and effectiveness among hospitalized COVID-19 patients receiving remdesivir, stratified by their eGFR: those with an eGFR <30 mL/min/1.73 m2 and those with an eGFR ≥30 mL/min/1.73 m2.
Methods
Ethics statement
This study received approval from the Institutional Review Board at Taipei Veterans General Hospital (TPEVGH IRB; No. 2023-04-009AC) and was carried out in accordance with the principles outlined in the Declaration of Helsinki. As the investigation presented little risk to the participants and did not involve any procedures, the TPEVGH IRB waived the need for written informed consent from the patients.
Study design and data source
This single-center, retrospective cohort study was conducted at Taipei Veterans General Hospital, one of the largest medical centers in Taiwan. The hospital allows more than 2.5 million outpatient visits for 1.1 million patients each year. Data were collected by reviewing electronic medical records from the study hospital’s information system.
Study population
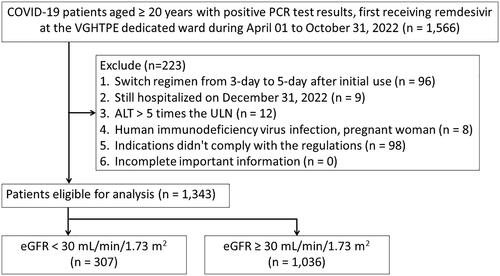
We identified all eligible patients aged 20 years and older who were confirmed COVID-19-positive by real-time polymerase chain reaction and received remdesivir for the first time for the treatment of COVID-19 at the COVID-19-dedicated wards between April 01, 2022, and October 31, 2022. Noteworthily, the research period coincided with the epidemic period of the Omicron variant strains. Remdesivir as a lyophilized powder (Veklury®) was provided free of charge by the Taiwan Centers for Disease Control (CDC) after review and approval by epidemic prevention physicians. Patients with any of the risk factors for severe illness (e.g. age ≥65 years, diabetes, CKD, cardiovascular disease, chronic pulmonary disease), not on oxygen, and within 7 days of onset of illness, are treated for 3 days. For patients with oxygen saturation less than 94% without oxygen therapy or, in severe cases, requiring oxygen therapy, the treatment period is 5 days. Patients were administered 200 mg of remdesivir on the first day, followed by 100 mg/day for 3 to 5 days.
Patients were excluded if one of the following events existed: had a regimen change from a 3-day to a 5-day regimen; still hospitalized on December 31, 2022; alanine aminotransferase (ALT) levels greater than 5 times the upper limit of the normal (ULN) range prior to the index date; had human immunodeficiency virus infection or were pregnant; whose indications did not comply with the regulations of Taiwan Centers for Disease Control; who had incomplete important information (e.g. age, sex). The index date is defined as the date of receiving the first dose of remdesivir. Patients whose remdesivir regimen was changed from 3 to 5 days were excluded from this study because it was not available to know whether the additional days were subsequently due to poor remdesivir efficacy or disease worsening.
Baseline covariates
The baseline characteristics collected included age, sex, body mass index, comorbidities, Charlson comorbidity index (CCI) [Citation25], smoking status, COVID-19 disease severity, cycle threshold value of SARS-CoV-2, concomitant nephrotoxic drugs, adjuvant COVID-19 therapies, antibiotics, number of days before starting remdesivir after diagnosing of COVID-19, duration of remdesivir treatment (3-day or 5-day regimen), oxygen requirement, and laboratory data (ALT, total bilirubin, eGFR, serum creatinine), heart rate, and COVID-19 vaccine doses. COVID-19 disease severity was classified as mild to moderate, severe, and critical according to the guidelines of the Taiwan Center for Disease Control [Citation26]. eGFR was calculated using the Modification of Diet in Renal Disease (MDRD) study equation [Citation27].
Ascertainment of exposure and outcomes
All qualified participants were categorized into two groups: eGFR less than 30 mL/min/1.73 m2 and eGFR greater than or equal to 30 mL/min/1.73 m2 group. Patients on dialysis were assigned to the eGFR < 30 group.
Primary safety outcomes were acute kidney injury (AKI) and bradycardia. AKI is defined as an increase in serum creatinine (SCr) of 0.3 mg/dL during the administration of remdesivir 48 h after the end of treatment or an increase in SCr exceeding 1.5 times the baseline value during the administration of remdesivir 7 days after the end of treatment according to Kidney Disease: Improving Global Outcomes (KDIGO) staging [Citation28]. Patients who were already undergoing dialysis were excluded from this analysis due to the potential instability of their SCr levels, which could result in false AKI outcomes. Bradycardia is defined as a heart rate of fewer than 60 beats per minute (bpm), except for patients whose heart rate was originally under 60 bpm [Citation29]. Heart rate was measured by an electronic blood pressure monitor. Secondary safety outcomes were changes in hepatic and renal laboratory data, including ALT, total bilirubin, eGFR, and SCr. Laboratory data were monitored from the index date to 7 days after the end of remdesivir treatment, and the worst value was recorded. Early discontinuation of remdesivir due to its side effects was also recorded.
Primary effectiveness outcomes were mortality in COVID-19-dedicated wards and mortality in the hospital. Mortality in dedicated wards was assessed from the index date to the occurrence of the event or the discharging from the dedicated wards, and mortality in the hospital from the index date to the occurrence of the event or discharge. The rules for transferring in and out of the dedicated wards were in accordance with Taiwan CDC regulations [Citation30,Citation31]. Patients diagnosed with COVID-19 may be admitted to a dedicated ward, except those who are asymptomatic or mildly ill, under 65 years of age, and able to function independently. If the patient’s fever has been suppressed for at least one day, symptoms have resolved, respiratory tests are PCR negative or the cycle threshold value of SARS-CoV-2 ≥ 30, and the attending physician evaluates the patient as suitable for transfer, the patient may be transferred out of the dedicated ward.
Secondary effectiveness outcomes were the progression of COVID-19 disease and the recovery time from COVID-19. The progression of COVID-19 disease is defined as the requirement for oxygen in patients receiving the 3-day remdesivir regimen or the need for advanced equipment to deliver supplemental oxygen in patients receiving the 5-day remdesivir regimen. Monitoring was conducted from the date of remdesivir discontinuation until the patients were transferred out of the dedicated wards. The order of equipment advancement is a nasal cannula, simple mask, venturi mask, nonrebreather mask, high-flow nasal cannula, bi-level positive airway pressure, and mechanical ventilator. Patients who received a 5-day regimen and were on an invasive ventilator at the index date were not included in the analysis of this outcome because the outcome did not occur in them. The recovery time from COVID-19 included the length of stay in dedicated wards and the length of oxygen requirement. The former measured the number of days from the index date to the date of discharge from the dedicated wards, while the latter measured the number of days from the index date to the removal of supplemental oxygen for patients following a 5-day regimen.
Statistical analyses
For baseline characteristics, categorical variables were presented as frequencies and percentages and compared using the chi-square test or Fisher’s exact test with P values. Continuous variables were summarized as mean with standard deviations (SD) for normally distributed data or median with interquartile range (IQR) for non-normally distributed data. The statistical significance of differences between the two groups was determined using the student’s t-test or Mann-Whitney U test with p values, respectively.
For primary outcomes, the incidence rate was calculated as events per 1,000 patient-days. The Kaplan-Meier curve was used to estimate the event-free survival, and the log-rank test was used to compare the curves between groups. Cox proportional hazard models were used to compare primary outcomes between groups, in which hazard ratios (HR) with 95% CIs were reported. For secondary outcomes, multivariate logistic regressions or multivariate linear regressions were used, in which odds ratios (OR) or effect sizes (coefficient β) with 95% CIs were reported. All outcomes were adjusted for relevant confounders, as shown in . Statistical significance was determined at a 2-sided α level of 0.05.
Table 1. Baseline characteristics of study population.
All data extracted from electronic medical records were logged into a formatted Microsoft Excel (Microsoft Corporation) template. These data were categorized and tabulated for further analysis. Statistical analyses were performed using the SPSS software (version 24; IBM Corp., Armonk, NY, USA) and the STATA software (version 17; StataCorp., College Station, Brazos, USA).
Sensitivity analyses
We performed sensitivity analyses to test the robustness of our main results. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula and the Cockcroft-Gault (CG) formula are widely utilized in clinical practice for calculating eGFR [Citation32–34]. Therefore, we first employed these two formulas to calculate eGFR to validate the obtained results. Second, considering the variations in AKI definitions across different guidelines, we employed the Acute Dialysis Quality Initiative (ADQI) staging and the Acute Kidney Injury Network (AKIN) staging to define the outcome of AKI [Citation35,Citation36].
Results
A total of 1,343 patients were enrolled, of whom 307 (22.9%) were in the eGFR < 30 mL/min/1.73 m2 group and 1,036 (77.1%) in the eGFR ≥ 30 mL/min/1.73 m2 group. The flow chart for the enrollment of the study population is depicted in .
Baseline patient characteristics
The baseline characteristics of the study population are presented in . For the eGFR < 30 and eGFR ≥ 30 groups, the median (IQR) age was 79.3 (69.0–89.9) and 81.2 (67.3–90.7) years, respectively. There were more males in the eGFR ≥ 30 group (eGFR < 30: 59.0% vs eGFR ≥ 30: 65.6%, p = 0.038). Compared with the eGFR ≥ 30 group, the eGFR < 30 group had higher CCI scores and a prevalence of hypertension, diabetes mellitus, and chronic heart disease but a lower prevalence of chronic liver disease. The two groups had differences in COVID-19 disease severity, concomitant nephrotoxic drug, tocilizumab use, antibiotics use, oxygen requirement, and baseline laboratory data.
Safety outcomes
Regarding the safety outcome, 39 (39/185 = 21.1%) of the eGFR < 30 group and 76 (76/1,036 = 7.3%) of the eGFR ≥ 30 group experienced acute kidney injury (incidence rate 28.6 versus 9.1 events per 1000 patient-days). The eGFR <30 group were more associated with a higher risk of acute kidney injury (aHR 2.92, 95% CI 1.93–4.44) than the eGFR ≥ 30 group. There was no difference in the bradycardia events and the changes in liver and kidney laboratory data between the two groups. Early discontinuation of remdesivir was observed in 7 (2.3%) and 9 patients (0.9%) in the eGFR < 30 group and the eGFR ≥ 30 group, respectively. The causes were bradycardia (5 in the eGFR < 30 group and 7 in the eGFR ≥ 30 group) and elevated liver enzymes (2 in the eGFR < 30 group and 2 in the eGFR ≥ 30 group).
Effectiveness outcomes
The incidence rates and adjusted hazard ratios (aHR) of outcomes are expressed in , the ORs/β are shown in , and the Kaplan-Meier survival curves of outcomes are depicted in . The incidence rate of mortality in dedicated wards was 12.6 and 6.9 per 1000 patient-days for eGFR < 30 and eGFR ≥ 30 groups, with a considerably higher risk of mortality in dedicated wards between the groups (log-rank p = 0.0062). However, eGFR < 30 was not associated with a higher risk of mortality in dedicated wards (aHR 1.43, 95% CI 0.90–2.28) after adjustment for potential confounders in multivariate Cox regression analysis. The in-hospital mortality rate for all patients was 14.1% (190/1343). The incidence rate of mortality in the hospital was 11.6 and 7.5 per 1000 patient-days for eGFR < 30 and eGFR ≥ 30 groups, with a considerably higher risk of mortality in dedicated wards between the groups (log-rank p = 0.0058). After adjustment, eGFR < 30 was associated with a higher risk of mortality in the hospital [aHR 1.47, 95% CI 1.06–2.05).
Figure 2. Kaplan-Meier survival curves of the COVID-19 cohort receiving remdesivir (A) acute kidney injury (B) bradycardia (C) mortality in COVID-19 dedicated ward (D) Mortality in hospital.
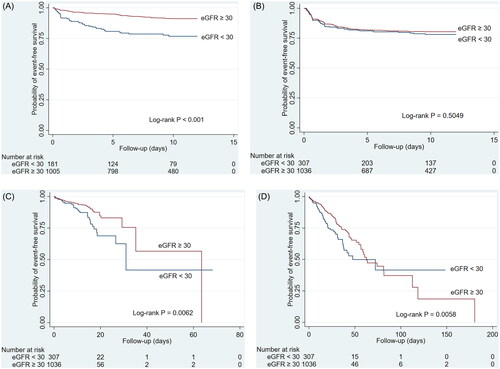
Table 2. Incidence rates and hazard ratios for primary outcomes.
Table 3. Secondary outcomes.
The risk of COVID-19 disease progression was higher in the eGFR < 30 group than the eGFR ≥ 30 group (aOR 1.62, 95% CI 1.16–2.26). After stratifying by remdesivir treatment, only in the 5-day regimen that the eGFR <30 group exhibited a higher risk of COVID-19 disease progression (31.4% vs. 20.6%, aOR 1.73, 95% CI 1.17–2.55]. There was no difference in the length of stay in dedicated wards and the length of oxygen requirement between the two groups.
Sensitivity analyses
Results were generally consistent across sensitivity analyses. When the CKD-EPI or CG formula was used to calculate eGFR, the risk estimate of all outcomes did not change apparently from those in the main analysis using the MDRD formula (Supplementary Table S1). The incidence of AKI varied depending on the definition used for AKI outcomes. In the eGFR < 30 group, the incidence of AKI was 21.5% according to the KDIGO definition, 8.8% according to the ADQI definition, and 12.7% according to the AKIN definition (Supplementary Table S2). However, the aHRs for AKI remained consistent with the primary analysis, showing an increased risk of AKI in the eGFR < 30 group regardless of the AKI definition used.
Discussion
In this retrospective cohort study, we discovered that among hospitalized COVID-19 patients receiving remdesivir, those with eGFR < 30 were linked to a significant increase in the risk of AKI, hospital mortality, and COVID-19 disease progression compared to patients with eGFR ≥ 30. There was no difference between the two groups in terms of bradycardia, mortality in COVID-19 dedicated wards, changes in laboratory data, and the time of recovery from COVID-19. To the best of our knowledge, this is the largest study to investigate the safety and effectiveness of remdesivir in patients with an eGFR of < 30 mL/min.
The present study provides insight into the potential safety issues of remdesivir in patients with severe renal impairment, suggesting remdesivir may be used cautiously in this population after weighing risks and benefits. Evidence regarding remdesivir’s safety and effectiveness profiles in other vulnerable groups, such as the elderly, those with multiple comorbidities, children, pregnant/lactating women, and immunocompromised transplant patients, remains limited [Citation37–39]. Future studies should prioritize evaluating remdesivir in these underrepresented populations to ensure proper treatment guidance is available.
The incidence of AKI in patients with eGFR < 30 in our study (21.5%) was notably higher than that reported by Ackley et al. (5%) and Sunny et al. (11%) [Citation14,Citation15], but significantly lower than the 35.2% reported by Wang et al. [Citation17] This discrepancy may arise from variations in the renal function formulas employed and the definitions used for AKI outcomes. Had we applied the same AKI definition, our incidence of AKI would likely have been quite comparable. When utilizing the ADQI criteria, we observed rates of 8.8%, as opposed to Ackley’s 5%, while the use of the AKIN criteria resulted in rates of 12.7%, compared to Sunny’s 11%. In addition, even with the same AKI definition according to KDIGO criteria, there was a significant discrepancy between our study and that of Wang et al. suggesting the characteristics of the patient cohort and the study period may have a certain impact. Recognizing that these operational definitions can significantly impact study outcomes, we conducted sensitivity testing to provide a more profound understanding of the limitations and uncertainties inherent in our findings. This approach allows for a more comprehensive and nuanced interpretation of results, thereby enhancing the reliability and robustness of our conclusions.
We observed a heightened risk of AKI in the eGFR <30 group compared to the ≥30 group, which contradicts the findings of previous literature that reported no significantly increased risk [Citation14,Citation15,Citation20]. This disparity can likely be attributed to the limited sample sizes in those earlier studies, where the number of individuals with AKI in the eGFR <30 group fell below 5, resulting in insufficient statistical power to detect differences.
Furthermore, our study utilized the incidence rate to assess AKI risks, while previous studies relied solely on incidence. The use of both the incidence rate and hazard ratio in our analysis minimizes potential biases when evaluating the risk of drug-related adverse events [Citation40].
Previous studies have identified several risk factors for AKI in COVID-19 patients, including age, male gender, obesity, the use of diuretics and vasopressors, as well as underlying comorbidities, such as CKD, hypertension, and diabetes [Citation41–43]. In our present study, we considered these possible confounders and adjusted them in the statistical models to increase the robustness of the results. We finally observed a higher risk of AKI in the severe renal impairment group. These findings suggest that the use of remdesivir in individuals with severe renal impairment may carry potential renal side effects, necessitating a careful evaluation of the drug’s benefits and risks before its administration. If remdesivir is deemed necessary in patients with severe renal impairment, close monitoring of renal function is imperative. Additionally, early consideration of adjunctive therapies, including dialysis and infusion management, is advisable in the event of declining renal function, while addressing the underlying cause of AKI remains essential.
Our study further revealed that patients with eGFR < 30 had comparable dedicated ward mortality but a higher risk of hospital mortality than patients with eGFR ≥30. A unique aspect of this study was the consideration of death in the dedicated ward as an outcome, which could potentially offer a more accurate measure of remdesivir’s effectiveness. Our hospital mortality rate of 14.1% closely mirrors the WHO Solidarity 2022s rate of 14.5% among all patients receiving remdesivir [Citation44]. Prior investigations into 30-day mortality among remdesivir-treated patients have reported no differences or even higher mortality rates in the eGFR < 30 group compared to the eGFR ≥ 30 group [Citation14,Citation15,Citation20]. However, these studies varied in terms of patient populations, healthcare settings, practices, and study designs, making direct comparisons challenging. Numerous studies have consistently demonstrated that severe renal impairment ranks among the most significant comorbid medical conditions associated with COVID-19-related mortality [Citation4,Citation5].
Moreover, we hypothesize that the increased risk of hospital mortality in patients with an eGFR of <30 is predominantly attributed to their compromised renal function. Our findings highlight the need for vigilant monitoring of clinical parameters, including vital signs and oxygen requirements, in individuals with impaired renal function treated with remdesivir and the need to adjust treatment and medical support accordingly. These results underscore the importance of including this vulnerable population in clinical trials evaluating COVID-19 antiviral therapy.
In our report, the incidence of bradycardia among remdesivir recipients was 19.0%, which falls at the lower end of the 17–74% range reported in other studies [Citation45–49]. The incidence in the cohort with an eGFR <30 was 20.5%, compared to 39% in a small study involving only 31 patients [Citation50]. Our results showed that the risk of bradycardia in the eGFR <30 cohort was comparable to that in the eGFR ≥30 cohort. Current reports have almost exclusively compared remdesivir users with non-users, and limited studies compared the same groups as our condition [Citation51,Citation52]. Furthermore, previous studies have been inconsistent regarding whether renal function may be a risk factor for bradycardia in remdesivir recipients; while Ai et al. suggested that CKD was associated with bradycardia, Hajimoradi et al. found no association between CKD and bradycardia [Citation51,Citation53]. In clinical practice, patients receiving remdesivir should be aware of the potential risk of bradycardia, and appropriate monitoring strategies should be implemented. If bradycardia occurs, prompt intervention should be undertaken to prevent further progression to severe arrhythmias and to ensure the safety of remdesivir recipients.
Strengths and limitations
The current study has several notable strengths. First, it represents the largest cohort study conducted to date, focusing on the safety and effectiveness of RDV in patients with severe renal impairment. Second, our sensitivity analyses explored various scenarios, bolstering the robustness of our findings. Lastly, we employed rigorous statistical methods to control for potential confounding variables, thereby enhancing the internal validity of our study.
Nonetheless, this research is not without its limitations. First, due to its retrospective observational study design, we cannot entirely rule out the possibility of residual or unmeasured confounding factors. Second, the reliance on data from a single center may restrict the generalizability of our findings to other settings or populations. These limitations necessitate caution when extrapolating and applying the results to broader clinical contexts. More extensive prospective studies are necessary to confirm these issues further.
Conclusion
Among hospitalized COVID-19 patients treated with RDV, those with severe renal impairment exhibited a heightened risk of acute kidney injury, hospital mortality, and COVID-19 disease progression compared to patients without severe renal impairment.
Author contributions
Chang HY, Hsu CC, Hu LF, Lu CC, and Chang LJ formulated the study concept and design. Chang HY and Hsu CC performed the statistical analysis and wrote the manuscript. All authors contributed to the discussion; reviewed, edited, and approved the final manuscript; and agreed to submission.
Supplemental Material
Download MS Word (34.1 KB)Acknowledgements
We thank Yaa-Hui Dong from the College of Pharmaceutical Sciences, National Yang Ming Chiao Tung University, for her valuable and constructive suggestions during the planning and development of this research work. We also thank Shang-Liang Wu from the Biostatistics Task Force, Taipei Veterans General Hospital, for statistical assistance.
Disclosure statement
The authors report no conflict of interest.
Data availability statement
The datasets created and examined in the present study cannot be accessed by the public due to the regulations of the Institutional Review Board at Taipei Veterans General Hospital. The dataset can be obtained from the corresponding author or IRB of TVGH upon request (email: [email protected]). Data can only be utilized for research purposes.
Additional information
Funding
References
- World Health Organization. WHO Coronavirus (COVID-19). Dashboard. https://covid19.who.int/. (accessed October, 7 2023).
- Collaboration GBDCKD. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2020;395(10225):1–12. doi:10.1016/S0140-6736(20)30045-3.
- Cheng Y, Luo R, Wang K, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838.
- Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi:10.1038/s41586-020-2521-4.
- Council E-E, Group EW, ERACODA Working Group. Chronic kidney disease is a key risk factor for severe COVID-19: a call to action by the ERA-EDTA. Nephrol Dial Transplant. 2021;36(1):87–94. doi:10.1093/ndt/gfaa314.
- Rubin D, Chan-Tack K, Farley J, et al. FDA approval of Remdesivir - A step in the right direction. N Engl J Med. 2020;383(27):2598–2600. doi:10.1056/NEJMp2032369.
- Kmietowicz Z. Covid-19: selected NHS patients will be treated with remdesivir. BMJ. 2020;369:m2097. doi:10.1136/bmj.m2097.
- Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of covid-19 - final report. N Engl J Med. 2020;383(19):1813–1826. doi:10.1056/NEJMoa2007764.
- Gilead Sciences, Inc. Remdesivir (Veklury) [package insert]. https://www.gilead.com/-/media/files/pdfs/medicines/covid-19/veklury/veklury_pi.pdf. (accessed 14 Augest 2023).
- European Medicines Agency: Summary on compassionate use. Remdesivir Gilead. 2020. Available at: https://www.ema.europa.eu/en/documents/other/summary-compassionate-use-remdesivir-gilead. _en. pdf. (accessed 14 August 2023).
- Stella VJ, Rajewski RA. Sulfobutylether-beta-cyclodextrin. Int J Pharm. 2020;583:119396. doi:10.1016/j.ijpharm.2020.119396.
- Luke DR, Tomaszewski K, Damle B, et al. Review of the basic and clinical pharmacology of sulfobutylether-beta-cyclodextrin (SBECD). J Pharm Sci. 2010;99(8):3291–3301. doi:10.1002/jps.22109.
- Davoudi-Monfared E, Ahmadi A, Karimpour-Razkenari E, et al. Remdesivir administration in COVID-19 patients with renal impairment: a systematic review. Am J Ther. 2022;29(5):e520–e33. doi:10.1097/MJT.0000000000001543.
- Ackley TW, McManus D, Topal JE, et al. A valid warning or clinical lore: an evaluation of safety outcomes of remdesivir in patients with impaired renal function from a multicenter matched cohort. Antimicrob Agents Chemother. 2021;65(2):e02290-20. doi:10.1128/AAC.02290-20.
- Sunny S, Samaroo-Campbell J, Abdallah M, et al. Is remdesivir safe in patients with renal impairment? Experience at a large tertiary urban medical center. Infection. 2023;51(1):247–252. doi:10.1007/s15010-022-01850-7.
- Seethapathy R, Wang Q, Zhao S, et al. Effect of remdesivir on adverse kidney outcomes in hospitalized patients with COVID-19 and impaired kidney function. PLoS One. 2023;18(2):e0279765. doi:10.1371/journal.pone.0279765.
- Wang S, Huynh C, Islam S, et al. Assessment of safety of remdesivir in covid - 19 patients with estimated glomerular filtration rate (eGFR) < 30 ml/min per 1.73 m;2. J Intensive Care Med. 2022;37(6):764–768. doi:10.1177/08850666211070521.
- Babar ZU, Dodani SK, Nasim A, et al. Outcome of COVID-19 and tolerance of remdesivir in patients with renal failure: a single center experience from Pakistan. J Infect Dev Ctries. 2023;17(6):812–818. doi:10.3855/jidc.17136.
- Stancampiano F, Jhawar N, Alsafi W, et al. Use of remdesivir for COVID-19 pneumonia in patients with advanced kidney disease: a retrospective multicenter study. Clin Infect Pract. 2022;16:100207.
- Umemura T, Mutoh Y, Mizuno T, et al. Safety evaluation of remdesivir for COVID-19 patients with eGFR < 30 mL/min without renal replacement therapy in a japanese single-center study. Healthcare (Basel). 2022;10(11):2299.
- Cheng M, Fowler R, Murthy S, et al. Remdesivir in patients with severe kidney dysfunction: a secondary analysis of the CATCO randomized trial. JAMA Netw Open. 2022;5(8):e2229236. doi:10.1001/jamanetworkopen.2022.29236.
- Silva NAO, Zara A, Figueras A, et al. Potential kidney damage associated with the use of remdesivir for COVID-19: analysis of a pharmacovigilance database. Cad Saude Publica. 2021;37(10):e00077721. doi:10.1590/0102-311X00077721.
- Wu B, Luo M, Wu F, et al. Acute kidney injury associated with remdesivir: a comprehensive pharmacovigilance analysis of COVID-19 reports in FAERS. Front Pharmacol. 2022;13:692828. doi:10.3389/fphar.2022.692828.
- Gerard AO, Laurain A, Fresse A, et al. Remdesivir and acute renal failure: a potential safety signal from disproportionality analysis of the WHO safety database. Clin Pharmacol Ther. 2021;109(4):1021–1024.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi:10.1016/0021-9681(87)90171-8.
- Taiwan Centers for Disease Control. Guidelines for Clinical Management of Novel Coronavirus (SARS-CoV-2) Infection. May. 2022;26 https://www.cdc.gov.tw/File/Get/ZA_eJYJTL7Zy0kVG7f24Qg (accessed May 6 2024).
- Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Intern Med. 1999;130(6):461–470. doi:10.7326/0003-4819-130-6-199903160-00002.
- Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–84. doi:10.1159/000339789.
- Kusumoto FM, Schoenfeld MH, Barrett C, et al. 2018 ACC/AHA/HRS guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society. Circulation. 2019;140(8):e382–e482. doi:10.1161/CIR.0000000000000721.
- Taiwan Centers for Disease Control. Management of confirmed cases of severe specific infectious pneumonia and conditions for de-isolation treatment. April 3, 2022. https://www.cdc.gov.tw/Uploads/4a75642a-55f7-4e9f-91a7-91104aac26d0.pdf. (accessed May 6 2024).
- Taiwan Centers for Disease Control. Hospital Medical Contingency Plan for COVID-19. April 6, 2022. https://www.chshb.gov.tw/sites/default/files/2022-04/%E9%86%AB%E9%99%A2%E5%9B%A0%E6%87%89COVID-19%E9%86%AB%E7%99%82%E6%87%89%E8%AE%8A%E6%8E%AA%E6%96%BD1110406.pdf. (accessed May 6 2024).
- Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi:10.1159/000180580.
- Rostoker G, Andrivet P, Pham I, et al. A modified Cockcroft-Gault formula taking into account the body surface area gives a more accurate estimation of the glomerular filtration rate. J Nephrol. 2007;20(5):576–585.
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi:10.7326/0003-4819-150-9-200905050-00006.
- Bellomo R, Ronco C, Kellum JA, et al. Acute dialysis quality initiative w. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the second international consensus conference of the acute dialysis quality initiative (ADQI) group. Crit Care. 2004;8(4):R204–12. doi:10.1186/cc2872.
- Mehta RL, Kellum JA, Shah SV, et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi:10.1186/cc5713.
- Molaei E, Molaei A, Hayes AW, et al. Remdesivir: treatment of COVID-19 in special populations. Naunyn Schmiedebergs Arch Pharmacol. 2024;397(6):3829–3855. doi:10.1007/s00210-023-02927-2.
- Elec F, Magnusson J, Elec A, et al. COVID-19 and kidney transplantation: the impact of remdesivir on renal function and outcome - a retrospective cohort study. Int J Infect Dis. 2022;118:247–253.
- Solera JT, Árbol BG, Mittal A, et al. Longitudinal outcomes of COVID-19 in solid organ transplant recipients from 2020 to 2023. Am J Transplant. 2024. Published online Mar 17, 2024. doi:10.1016/j.ajt.2024.03.011.
- Ho CC, Wu SL, Tsai HY, et al. A retrospective cohort study on the cardiotoxicity incidence rates of immune checkpoint inhibitors for oncology patients. J Chin Med Assoc. 2023;86(5):499–505. doi:10.1097/JCMA.0000000000000910.
- Chan L, Chaudhary K, Saha A, et al. AKI in hospitalized patients with COVID-19. J Am Soc Nephrol. 2021;32(1):151–160.
- Contreras-Villamizar K, Barbosa O, Munoz AC, et al. Risk factors associated with acute kidney injury in a cohort of hospitalized patients with COVID-19. BMC Nephrol. 2023;24(1):140. doi:10.1186/s12882-023-03172-8.
- Zhang J, Pang Q, Zhou T, et al. Risk factors for acute kidney injury in COVID-19 patients: an updated systematic review and meta-analysis. Ren Fail. 2023;45(1):2170809.
- Consortium W, WHO Solidarity Trial Consortium. Remdesivir and three other drugs for hospitalised patients with COVID-19: final results of the WHO solidarity randomised trial and updated meta-analyses. Lancet. 2022;399(10339):1941–1953. doi:10.1016/S0140-6736(22)00519-0.
- Bistrovic P, Manola S, Lucijanic M. Bradycardia during remdesivir treatment might be associated with improved survival in patients with COVID-19: a retrospective cohort study on 473 patients from a tertiary Centre. Postgrad Med J. 2022;98(1161):501–502. doi:10.1136/postgradmedj-2021-141079.
- Alsowaida YS, Shehadeh F, Kalligeros M, et al. Incidence and potential risk factors for remdesivir-associated bradycardia in hospitalized patients with COVID-19: a retrospective cohort study. Front Pharmacol. 2023;14:1106044. doi:10.3389/fphar.2023.1106044.
- Umeh C, Giberson C, Kumar S, et al. A multicenter retrospective analysis on the etiology of bradycardia in COVID-19 patients. Cureus. 2022;14(1):e21294. doi:10.7759/cureus.21294.
- Schreiber A, Bauzon JS, Batra K, et al. Clinical characteristics and implications of bradycardia in COVID-19 patients treated with remdesivir: a single-center retrospective cohort study. Clin Drug Investig. 2022;42(9):763–774. doi:10.1007/s40261-022-01187-x.
- Pantazopoulos I, Mavrovounis G, Dimeas G, et al. Remdesivir-induced bradycardia is not associated with worse outcome in patients with COVID-19: a retrospective analysis. Am J Cardiovasc Drugs. 2022;22(6):705–710. doi:10.1007/s40256-022-00547-4.
- Seethapathy R, Zhao S, Long JD, et al. A propensity Score-Matched observational study of remdesivir in patients with COVID-19 and severe kidney disease. Kidney360. 2022;3(2):269–278. doi:10.34067/KID.0006152021.
- Ai MY, Chang WL, Yang CJ. Remdesivir-Induced bradycardia and mortality in SARS-CoV-2 infection, potential risk factors assessment: a systematic review and Meta-Analysis. J Clin Med. 2023;12(24):7518. doi:10.3390/jcm12247518.
- Ishisaka Y, Aikawa T, Malik A, et al. Association of remdesivir use with bradycardia: a systematic review and meta-analysis. J Med Virol. 2023;95(8):e29018. doi:10.1002/jmv.29018.
- Hajimoradi M, Sharif Kashani B, Dastan F, et al. Remdesivir associated sinus bradycardia in patients with COVID-19: a prospective longitudinal study. Front Pharmacol. 2022;13:1107198. doi:10.3389/fphar.2022.1107198.