Abstract
Objective Despite revascularization has been proven to be the optimal choice of moyamoya disease (MMD), many complications may occur in this context.. This study aimed to analyse the risk factors for new or expanded symptomatic infarctions of patients with MMD after revascularization.
Methods Patients with MMD who received revascularization in our department were retrospectively enrolled, new or expanded cerebral infarction within a month after revascularization was the main concern. Basic and clinical information, and intraoperative conditions were evaluated. Univariate and multivariate analyses were performed to evaluate the risk factors. Receiver operating characteristic curve analysis was conducted to evaluate the efficiency of the risk factors.
Results
Eventually, 108 consecutive patients received 174 surgeries were enrolled, experienced new or expanded infarction occured in 13 (7.47%) surgeries, which showed higher Suzuki stage on the non-operative side, more posterior cerebral artery (PCA) involvement, and more intraoperative hypotension compared to those without infarction(p < .05). The Suzuki stage on the non-operative side had the highest area under the curve (AUC) of 0.737, with a sensitivity of 0.692 and specificity of 0.783. Combination of the three factors showed better efficiency, with an AUC of 0.762, a sensitivity of 0.692, and a specificity of 0.907.
Conclusions
Revascularization was a safe option for patients with MMD, higher Suzuki stage on the non-operative side, PCA involvement, and intraoperative hypotension might be the risk factors for new or expanded infarction after revascularization in patients with MMD.
Introduction
Moyamoya disease (MMD), also known as spontaneous occlusion of the circle of Willis, is a rare cerebrovascular disease characterized by progressive occlusion of the terminal portion of internal carotid artery, proximal portion of the anterior cerebral artery, and middle cerebral artery (MCA), combining with the formation of abnormal vascular net[Citation1,Citation2]. The incidence of MMD is higher in East Asia compared to other regions, Japan has the highest incidence in East Asia, which led to earlier research here. MMD brings excessive harms and burdens to the patients and communities [Citation1], ischaemic stroke and hemorrhage are the two main manifestations of MMD. Pediatric patients, who usually manifest as cerebral ischemia, rarely present as hemorrhage. While in adults, hemorrhagic symptoms induced by rupture of compensatory vessels are the most common [Citation1].
Revascularization, which includes direct, indirect, and combined bypass, has been recognized as the optimal choice for MMD by improving symptoms and reducing the incidence of infarction or hemorrhage to the most [Citation3,Citation4]. However, many complications, such as infarction and hemorrhage, might occur postoperatively [Citation5–10], which would result in operation failure, unsatisfactory quality of life and even death [Citation11,Citation12]. Postoperative infarction can be divided into symptomatic and asymptomatic infarctions, which can be demonstrated by computed tomography (CT) perfusion or magnetic resonance imaging (MRI) examination [Citation3]. Increasing studies have focused on the research of this field [Citation5,Citation7–10], but the inclusion and exclusion criteria, results, and conclusions were different, and a consensus has yet to be reached. Here, we retrospectively explored the risk factors for postoperative new or expanded infarction in patients with MMD who received revascularization at our hospital, and the efficiencies of the factors were deeply analysed.
Methods
Patients
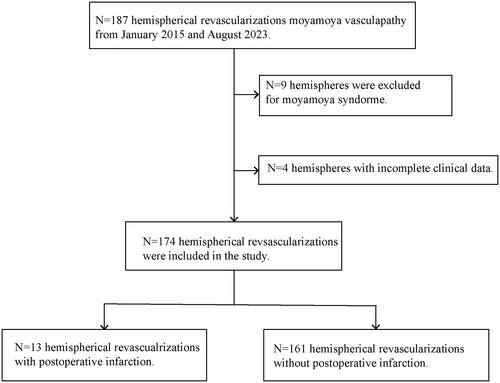
We enrolled patients with MMD who underwent direct revascularization (superficial temporal artery-MCA bypass, superficial temporal artery [STA]-MCA bypass), indirect revascularization (encephaloduro-arterio-myosynangiosis [EDAMS]), or combined revascularization (STA-MCA bypass combined with EDAMS) at our department between January 2015 and August 2023. This study was approved by the ethics committee of our hospital (ID:[2020]02-181-01), and the requirement for informed consent was waived due to the retrospective design of this study. MMD was diagnosed based on digital subtraction angiography and the diagnostic criteria of the Research Committee on Spontaneous Occlusion of the Circle of Willis [Citation13]. Patients who met the following criteria were enrolled in this study: 1) MMD was definitively diagnosed and received revascularization in our department; 2) All the necessary medical data were available. The following patients were excluded: 1) Patients with other diseases, such as vasculitis, moyamoya syndrome and so on; 2) Patients without complete imaging test and other clinical data ().
Data collections
Basic demographic characteristics (age, gender, smoking, alcohol use, etc.), clinical information (onset feature, hypertension, diabetes, bilateral Suzuki stages, posterior cerebral artery (PCA) involvement or not, lipid metabolism, antiplatelet drugs use), and operation conditions (duration of surgery, type of revascularization, duration of anaesthesia, and intraoperative hypotension) were all obtained in detail. For patients with symptoms of cerebral ischemia, antiplatelet drugs were administrated before (usually 100 mg aspirin and/or 75 mg clopidogrel per day) or after (usually 100 mg aspirin per day) bypass. The diagnosis of hypertension [Citation14] and diabetes [Citation15] were based on previous guideline. Of note, intraoperative hypotension in this study was defined as mean arterial pressure < 60 mmHg, systolic blood pressure < 90 mmHg, or the use of intermittent bolus or intravenous infusion of vasoactive drugs (such as ephedrine, dopamine, and norepinephrine) for more than 5 min [Citation16].
Surgical procedures and postoperative infarction
All the patients received digital subtraction angiography, CT angiography and perfusion imaging tests before revascularization, which included direct, indirect, and combined revascularization, as mentioned above. The STA was selected as the donor artery, and a small branch of the ipsilateral MCA, for example, the angular artery, was chosen as the recipient artery. Most of the patients received combined bypass, indirect bypass procedure was performed in patients who were deemed too young or who had excessively thin anastomotic vessels. Of note, pure direct bypass was performed in patient with temporalis hypertrophy. For patients who require bilateral bypasses, the more severe hemisphere was done firstly, and contralateral bypass was usually performed three months later. The first day after surgery, all patients received CT or MRI test, repeat imaging test was performed in patient with neurological deterioration or new neurological symptoms. Postoperative infarction, the primary observation indicator in current study, included new and expanded infarction based on CT perfusion and/or MRI [Citation3] (). However, some patients may receive just CT plain test during emergencies. For patients with post-surgical infarction, neurotrophic treatment, circulation improvement, and other treatments were did based on the patients’ symptoms and extent of cerebral infarction, and dynamic imaging tests were carried out accordingly.
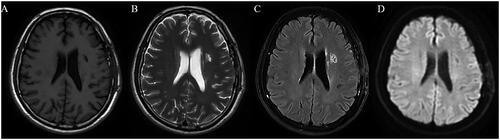
Figure 2. A 62-year-old male with MMD who underwent left revascularization, MRI test showed infarction in the left corona radiata and basal ganglia, accompanied by local liquefaction (A: T1 sequence, B: T2 sequence, C: flair sequence, D: diffusion weighted imaging sequence). MMD: moyamoya disease; MRI: magnetic resonance imaging.
Statistical analysis
SPSS (version 26.0) was used for statistical analyses, data were considered statistically significant when p < .05. Mean ± standard deviation or median (P25, P75) was used to express the measurement data. Chi-square test, t-test (normal distribution), or Mann-Whitney test (abnormal distribution) was used to analyse the differences between patients with postoperative infarction or not. The risk factors for cerebral infarction were evaluated by univariate logistic regression analysis, factors with statistical significance were then enrolled in the multivariate logistic analysis to evaluate the independent risk factors and construct the regression equation. A receiver operating characteristic (ROC) curve was used to evaluate the efficiency of risk factors.
Results
Baseline characteristics of the patients
A total of 108 patients with MMD underwent 174 hemispherical revascularizations were enrolled in this study. The characteristics of 108 patients (mean age, 47.81 ± 12.88; range, 4–74 years) were summarized (), which included 56 male patients (51.85%) and 52 female patients (48.15%). The majority of patients initially presented with cerebral ischemia, which accounted for 76.85% (83/108) of the cases, including 61 cases (56.48%) of cerebral infarction and 22 cases (20.37%) of chronic transient ischaemic attack. Furthermore, intracranial hemorrhage occurred in 23 patients (21.30%), and seizure in 2 patients (1.85%). Of note, 38 patients (35.16%) had hypertension, and 19 (17.59%) had diabetes. Other clinical information of the patients, such as comorbidities, smoking, alcohol use were all detailly listed in .
Table 1. Demographic and clinical characteristics of the 108 patients.
Risk factors for postoperative infarction
In summary, 108 patients underwent 174 sides of surgery, including 17 (9.77%) hemispherical direct bypass, 24 (13.79%) indirect bypass, and 133 (76.44%) combined bypass. Postoperatively, new or expanded infarctions were observed in 13 surgeries (7.47%), including 8 (4.60%) new infarctions and 5 (2.87%) expanded infarctions. Among the 13 cases, five (2.87%) surgeries occurred ipsilaterally, four (2.30%) occurred contralaterally, and four (2.30%) occurred bilaterally. No significant infarction was found in those patients, thus resulting in no significant deterioration of patients’ cognitive and neurological function. Only a few patients had residual mild neurological impairments after symptomatic treatment during follow up. Univariate regression analysis suggested that patients with higher Suzuki stage on the non-operative side, PCA involvement, and intraoperative hypotension had a higher probability of postoperative infarction (p < .05) (). Further multivariate logistic analysis revealed that the aforementioned three factors were all independent risk factors for postoperative new or expanded infarction (p < .05) ().
Table 2. Univariate analysis of patients with or without new or expanded infarction.
Table 3. Multivariate analyses for independent risk factors of new or expanded cerebral infarction.
Predictive value of the risk factors in postoperative infarction
Based on the results in , the binary regression equation was established: Logit (P) = −4.450 + 1.735 X1 + 1.350X2 + 1.687 X3, where X1, X2, and X3 were the Suzuki stage on the non-operative side, PCA involvement, and intraoperative hypotension, respectively. ROC was calculated for the three risk factors (), showing that Suzuki stage on the non-operative side had the highest diagnostic efficiency, with an area under the ROC curve (AUC) of 0.737, the sensitivity and specificity were 0.692 and 0.783, respectively. Intraoperative hypotension had the lowest AUC for predicting the occurrence of postoperative infarction, with an AUC of 0.701. Taking the above factors into consideration, the AUC reached 0.762, with a sensitivity of 0.692 and a specificity of 0.907 ().
Figure 3. ROC curves depicted the AUC of predictive equation. (A) ROC curves for Suzuki stage on the non-operative side, PCA involvement, and intraoperative hypotension. (B) The ROC curves for the predictive equation with combination of all the three factors. ROC: receiver operating characteristic; AUC: area under the ROC curve; PCA: posterior cerebral artery.
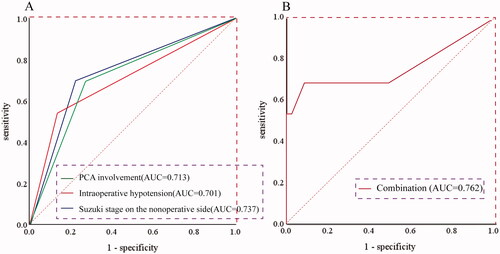
Table 4. Evaluation efficiency for the risk factors by ROC.
Posterior cerebral artery involvement and postoperative infarction
The PCA itself was usually enrolled to discuss in the postsurgical infarction of patients with MMD, and different viewpoints have been put forward [Citation17,Citation18]. We found 52 hemispheres (29.89%) of PCA involvement, and it was positively correlated with postoperative infarction (). In current study, patients with PCA involvement were older than those without PCA involvement (51.00 ± 10.22 vs 45.89 ± 13.47, p = .016), which might be ascribed to the PCA involvement was correlated with the duration of the disease. In addition, we found that more patients with PCA involvement had drinking (p = .013), and smoking history (p < .001), which might be due to that drinking and smoking habits could prompt the stenosis progression of susceptible arteries [Citation19]. The two subgroups were similar regarding other characteristics ().
Table 5. Comparison of characteristics between patients with or without PCA involvement.
Discussions
Revascularization has been widely used in patients with MMD [Citation20,Citation21], and moyamoya syndrome [Citation21,Citation22], many studies have provided compelling evidences regarding the efficacy of this surgical intervention [Citation4,Citation5,Citation23]. However, the early postoperative period following revascularization is characterized by heightened risk of various complications [Citation5,Citation24], infarction is one of the most common, which exerts a profound negative influence on body function, life quality and life expectancy [Citation11,Citation12]. According to the symptoms, infarction can be divided into symptomatic and asymptomatic types, and based on the locations of infarction lesions, it could be subtyped into peri-anastomosis infarction and remote infarctions [Citation6,Citation25]. The overall incidence of symptomatic infarction could reach 17.9% to 23.7% [Citation6,Citation25], it is estimated to be approximately 0.04% in the operated hemisphere of adult patients [Citation17]. Despite extensive attention has been paid to investigating the potential risk factors for post-surgical infarction, a consensus is yet to be reached. Moreover, many studies ignored the intraoperative and anaesthetic factors, for example intraoperative hypotension [Citation5,Citation7]. A postoperative infarction incidence of 7.47% was reported in current study, and the discrepancies in this rate can be attributed to the differences in the definition of postoperative infarction, the timeline of assessment, the sample selection and inclusion criteria. We found higher Suzuki stage on the non-operative side, PCA involvement, and intraoperative hypotension were independent risk factors for postoperative infarction. All three factors had good predictive value, the higher Suzuki stage on the non-operative side had the highest AUC, when the three factors were taken together, the AUC could reach 0.762.
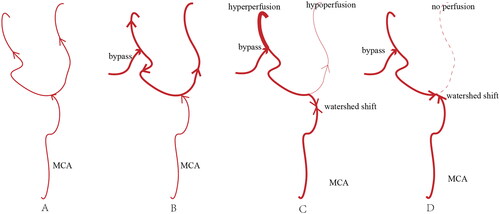
Patients with higher Suzuki stage or PCA involvement have worse vascular conditions and are usually associated with more postoperative infarction [Citation17,Citation18], as the leptomeningeal collateral of PCA is a crucial collateral circulation in high staged patients [Citation18], these patients were less likely to have compensate collateralization [Citation26]. The anterior branch of the MCA, which usually supplies the sphenoid and parietal bones, is easily damaged during craniotomy, and new infarction might occur in such condition [Citation27]. Moreover, intraoperative hypotension leads to hemodynamic fluctuation, which results in higher risk of postoperative infarction. Hoyeon Cho et al. found that low flow velocity in the MCA was a positive factor of infarction after bypass in adult patient [Citation28], blood flow competition could be a factor of postoperative infarction, as more than half of the infarctions occur remotely located from the anastomosis site, and these infarctions in the remote sites were usually more severe than peri-anastomosis infarction [Citation28]. Furthermore, the conflict between the flood from the bypass vessel and the MCA results in relative hypoperfusion and even no blood flow at the remote site [Citation29] (). For this, various factors should be carefully considered.
Figure 4. The figure illustrated the mechanism of infarction remotely located from the anastomosis site in patients with MMD after revascularization. (A) The branch of MCA and their distal perfusion in patients with moyamoya disease. (B) The ideal hemodynamic condition after revascularization, which was, however, challenging to achieve. (C) and (D) The watershed shift after bypass, where (C) depicted the watershed shift proximally at the bifurcation, with partial perfusion still present in the remote site, while (D) showed the watershed shift at the bifurcation, with minimal and even no perfusion in the remote site. MMD: moyamoya disease; MCA: middle cerebral artery.
The revascularization is inherently challenging and time-consuming, but it offers immediate restoration of blood flow. Nevertheless, brain protrusion may occur in some indirect bypass cases, and temporal muscle edema may contribute to increased intracranial pressure, thereby exerting a detrimental effect on blood flow [Citation30]. Studies found that combined or indirect revascularization is an independent risk factor for infarction [Citation6,Citation17]. Another study held a different viewpoint, adult patient who received direct or combined bypass suffered more infarction [Citation30], as collateral vessel development is less obvious in adults than pediatric patients [Citation30]. Moreover, a large multicentres study found that the symptomatic stroke outcomes between different bypass choices were comparable [Citation31]. We also found similar result.
Moreover, many studies demonstrated that patients with infarction history are more likely to suffer from postoperative infarction [Citation3,Citation17,Citation32,Citation33], as these patients were more likely to have hemodynamic conditions for insufficient brain reserve [Citation17,Citation32], but our study found that there is no direct correlation between the characteristics of disease onset and postoperative infarction. Although preoperative mRS score might be a protective factor, patients with worse mRS usually experienced neurological deficits and established flood compensations [Citation33], selection bias could also account for this condition, as patients with a history of infarction were more unwilling to undergo bypass than those without infarction history, let alone worst-off patients. While double craniotomies in a single surgery could be attempted in patients with more unstable hemodynamics and provide a broad space for revascularization, it requires a longer time and usually results in more blood loss, which is usually followed by more infarction [Citation34]. Furthermore, younger patients are more likely to suffer from cerebral ischemia and are usually associated with a faster deterioration condition, they tend to suffer from postoperative infarction [Citation25,Citation35]. As the elderly usually have underlying diseases, such as hypertension and diabetes, this group might have a higher possibility of postoperative infarction [Citation17], but in current study, we found no correlation between age and postoperative infarction. Of note, diabetes is also usually considered as the risk factor that might be relevant to the occurrence of infarction, the elevated blood glucose levels can elicit a myriad of detrimental effect on endothelial cell function, platelet membrane glycation, and subsequent aggregation, concomitant with increased synthesis of superoxide anions and thromboxane compounds. Simultaneously, the production of vasodilatory factors such as nitric oxide or prostacyclin is diminished, this intricate cascade of events culminate in oxidative stress and thrombus formation [Citation36], but our study demonstrated that diabetes didn’t correlate with the occurrence of postoperative infarction.
The MRI was used in the outcome evaluation after bypass [Citation37], as changes in neuroimaging appeared long before cerebrovascular events [Citation38], and improvement or change in the cerebrovascular reactivity was consistent with collateral formation and better angiographic responses [Citation39,Citation40], cerebral blood volume and time to peak on MRI can depict hemodynamic status after revascularization [Citation41]. Nevertheless, the clinical significance of MRI in the prediction of infarction after revascularization of MMD is still arguable. As for the geographic distribution difference of MMD, the race was usually discussed, Jorge Rios-Zermeno et al. found that Asian and White patients with MMD were more likely to receive operations compared to Black and Hispanic patients [Citation42,Citation43], which might be that MMD was historically affected Asian [Citation44]. What’s more, the Hispanic patients were also associated with increased hospital stay and cost [Citation43], but the race seemed did not affect the overall outcome after bypass [Citation40,Citation43,Citation45]. In addition to the factors mentioned above, perioperative management could also influence the occurrence of infarction, such as hypotension, hypercapnia, hypocapnia, and low haemoglobin [Citation46,Citation47].
Here, we enrolled patients with MMD from a relatively long interval to analyse the risk factors for postoperative infarction and identify the risk factors for post-surgical infarction. Therefore, it is imperative to thoroughly consider various factors to formulate a personalized surgical plan. Additionally, meticulous attention should be given to surgical and anaesthesia plan, as well as postoperative management. However, the study was subject to selection bias for all patients came from a single center, we couldn’t systematically analyse the influence factors of postoperative infarction. As most of the patients did not undergo a diffusion-weighted imaging test, and we couldn’t detect all infarction lesions, especially asymptomatic one, which could influence the true incidence of infarction. More studies are needed to better elucidate infarction after revascularization. Nevertheless, current study contributed to the exploration of the risk factors for early postoperative infarction in patients with MMD who received bypass.
Conclusions
We found that higher Suzuki stage on the non-operative side, PCA involvement, and intraoperative hypotension might be independent risk factors for postoperative infarction in MMD, and Suzuki stage on the non-operative side had the best predictive value among the three factors. Efforts should be made to implement effective interventions aimed to mitigate the incidence of postsurgical infarction in patients with MMD.
Author contributions
Hui Wang and Chuan Chen developed the study concept and design. Tao Sun, Lixin Huang, Baoyu Zhang and Cong Ling completed the acquisition of data. Tao Sun and Qiuhua Zeng made contributions to data analysis. Jun Sun and Zhimin Wu made contributions to data interpretation. Tao Sun, Qiuhua Zeng and Lixin Huang drafted the manuscript. All authors critically revised, read and approved the final version of the manuscript, and agreed to be accountable for the study.
Ethical approval
This study was approved by the ethics committee of the Third Affiliated Hospital of Sun Yat-sen University (ID:[2020]02-181-01) and conducted ethically in accordance with the World Medical Association Declaration of Helsinki.
Consent form
The requirement for informed consent was waived due to the retrospective design of the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data are available without restriction by contacting Tao Sun or Hui Wang.
Additional information
Funding
References
- Ihara M, Yamamoto Y, Hattori Y, et al. Moyamoya disease: diagnosis and interventions. Lancet Neurol. 2022;21(8):1–11. doi: 10.1016/S1474-4422(22)00165-X.
- Suzuki J, Takaku A. Cerebrovascular “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol. 1969;20(3):288–299. doi: 10.1001/archneur.1969.00480090076012.
- Ha EJ, Kim KH, Wang KC, et al. Long-term outcomes of indirect bypass for 629 children with moyamoya disease: longitudinal and cross-sectional analysis. Stroke. 2019;50(11):3177–3183. doi: 10.1161/STROKEAHA.119.025609.
- Jeon JP, Kim JE, Cho WS, et al. Meta-analysis of the surgical outcomes of symptomatic moyamoya disease in adults. J Neurosurg. 2018;128(3):793–799. doi: 10.3171/2016.11.JNS161688.
- Guo Q, Pei S, Wang QN, et al. Risk factors for preoperative cerebral infarction in infants with moyamoya disease. Transl Stroke Res. 2023. doi: 10.1007/s12975-023-01167-z.
- Takasu S, Kanamori F, Hatano N, et al. Effects of superficial temporal artery to middle cerebral artery bypass on postoperative infarction rates among young children ( < / = 5 years old) with moyamoya disease. Neurosurg Rev. 2023;46(1):87. doi: 10.1007/s10143-023-01999-1.
- Chen Y, Ma L, Lu J, et al. Postoperative hemorrhage during the acute phase after direct or combined revascularization for moyamoya disease: risk factors, prognosis, and literature review. J Neurosurg. 2019;133(5):1450–1459. doi: 10.3171/2019.7.JNS19885.
- Nomura S, Kawashima A, Ishiguro T, et al. Five-day bed rest reduces postoperative intracerebral hemorrhage after direct bypass for moyamoya disease. World Neurosurg. 2022;159:e267–e272. doi: 10.1016/j.wneu.2021.12.043.
- Pang CH, Lee SU, Lee Y, et al. Prediction of hemorrhagic cerebral hyperperfusion syndrome after direct bypass surgery in adult nonhemorrhagic moyamoya disease: combining quantitative parameters on rapid perfusion CT with clinically related factors. J Neurosurg. 2023;138(3):683–692. doi: 10.3171/2022.5.JNS212838.
- Kim KM, Kim JE, Cho WS, et al. Natural history and risk factor of recurrent hemorrhage in hemorrhagic adult moyamoya disease. Neurosurgery. 2017;81(2):289–296. doi: 10.1093/neuros/nyw179.
- Zhang Q, Yin Z, Zhu C, et al. Effectiveness of indirect revascularization for adult hemorrhagic moyamoya disease: a 10-year follow-up study. J Neurosurg. 2023;140(3):764–773. doi: 10.3171/2023.6.JNS23727.
- Guzman R, Lee M, Achrol A, et al. Clinical outcome after 450 revascularization procedures for moyamoya disease [Clinical article]. J Neurosurg. 2009;111(5):927–935. doi: 10.3171/2009.4.JNS081649.
- Health Labour Sciences Research Grant for Research on Measures for Infractable Diseases. Guidelines for diagnosis and treatment of moyamoya disease (spontaneous occlusion of the circle of Willis. Neurol Med Chir. 2012;52(5):245–266. doi: 10.2176/nmc.52.245.
- Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–3104. doi: 10.1093/eurheartj/ehy339.
- Elsayed NA, Aleppo G, Aroda VR, et al. On, 2. Classification and diagnosis of diabetes: standards of care in diabetes-2023. Diabetes Care. 2023;46(9):1715–1S40. doi: 10.2337/dc23-S002.
- Devereaux PJ, Yang H, Yusuf S, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (poise trial): a randomised controlled trial. Lancet. 2008;371(9627):1839–1847. doi: 10.1016/S0140-6736(08)60601-7.
- Wei W, Chen X, Yu J, et al. Risk factors for postoperative stroke in adults patients with moyamoya disease: a systematic review with meta-analysis. BMC Neurol. 2019;19(1):98. doi: 10.1186/s12883-019-1327-1.
- Park W, Ahn JS, Lee HS, et al. Risk factors for newly developed cerebral infarction after surgical revascularization for adults with moyamoya disease. World Neurosurg. 2016;92:65–73. doi: 10.1016/j.wneu.2016.03.053.
- Mineharu Y, Takagi Y, Koizumi A, et al. Genetic and nongenetic factors for contralateral progression of unilateral moyamoya disease: the first report from the Supra Japan Study Group. J Neurosurg. 2022;136(4):1005–1014. doi: 10.3171/2021.3.JNS203913.
- Scala M, Fiaschi P, Capra V, et al. When and why is surgical revascularization indicated for the treatment of moyamoya syndrome in patients with rasopathies? A systematic review of the literature and a single institute experience. Childs Nerv Syst. 2018;34(7):1311–1323. doi: 10.1007/s00381-018-3833-7.
- Fiaschi P, Scala M, Piatelli G, et al. Limits and pitfalls of indirect revascularization in moyamoya disease and syndrome. Neurosurg Rev. 2021;44(4):1877–1887. doi: 10.1007/s10143-020-01393-1.
- Scala M, Fiaschi P, Cama A, et al. Radiation-induced moyamoya syndrome in children with brain tumors: case series and literature review. World Neurosurg. 2020;135:118–129. doi: 10.1016/j.wneu.2019.11.155.
- Sun J, Li ZY, Chen C, et al. Postoperative neovascularization, cerebral hemodynamics, and clinical prognosis between combined and indirect bypass revascularization procedures in hemorrhagic moyamoya disease. Clin Neurol Neurosurg. 2021;208:106869. doi: 10.1016/j.clineuro.2021.106869.
- Hara S, Nariai T, Inaji M, et al. Imaging pattern and the mechanisms of postoperative infarction after indirect revascularization in patients with moyamoya disease. World Neurosurg. 2021;155:e510–e521. doi: 10.1016/j.wneu.2021.08.098.
- Araki Y, Uda K, Yokoyama K, et al. Risk factors for cerebral infarction early after revascularization in children younger than 5 years with moyamoya disease. World Neurosurg. 2022;160:e220–e226. doi: 10.1016/j.wneu.2021.12.115.
- Zhao M, Deng X, Zhang D, et al. Risk factors for and outcomes of postoperative complications in adult patients with moyamoya disease. J Neurosurg. 2018;130(2):531–542. doi: 10.3171/2017.10.JNS171749.
- Dauser RC, Tuite GF, Mccluggage CW. Dural inversion procedure for moyamoya disease. Technical note. J Neurosurg. 1997;86(4):719–723. doi: 10.3171/jns.1997.86.4.0719.
- Cho H, Jo KI, Yu J, et al. Low flow velocity in the middle cerebral artery predicting infarction after bypass surgery in adult moyamoya disease. J Neurosurg. 2017;126(5):1573–1577. doi: 10.3171/2016.3.JNS152256.
- Hayashi T, Shirane R, Fujimura M, et al. Postoperative neurological deterioration in pediatric moyamoya disease: watershed shift and hyperperfusion. J Neurosurg Pediatr. 2010;6(1):73–81. doi: 10.3171/2010.4.PEDS09478.
- Kazumata K, Ito M, Tokairin K, et al. The frequency of postoperative stroke in moyamoya disease following combined revascularization: a single-university series and systematic review. J Neurosurg. 2014;121(2):432–440. doi: 10.3171/2014.1.JNS13946.
- El NK, Chen CJ, Jabre R, et al. Direct versus indirect revascularization for moyamoya: a large multicenter study. J Neurol Neurosurg Psychiatry. 2023:jnnp-2022-329176. doi: 10.1136/jnnp-2022-329176.
- Hyun SJ, Kim JS, Hong SC. Prognostic factors associated with perioperative ischemic complications in adult-onset moyamoya disease. Acta Neurochir. 2010;152(7):1181–1188. doi: 10.1007/s00701-010-0638-1.
- Li J, Zhao Y, Zhao M, et al. High variance of intraoperative blood pressure predicts early cerebral infarction after revascularization surgery in patients with moyamoya disease. Neurosurg Rev. 2020;43(2):759–769. doi: 10.1007/s10143-019-01118-z.
- Kim SK, Wang KC, Kim IO, et al. Combined encephaloduroarteriosynangiosis and bifrontal encephalogaleo (periosteal) synangiosis in pediatric moyamoya disease. Neurosurgery. 2002;50(1):88–96. doi: 10.1097/00006123-200201000-00016.
- Miyatake S, Miyake N, Touho H, et al. Homozygous c.14576g > a variant of rnf213 predicts early-onset and severe form of moyamoya disease. Neurology. 2012;78(11):803–810. doi: 10.1212/WNL.0b013e318249f71f.
- Zhang XG, Zhang YQ, Zhao DK, et al. Relationship between blood glucose fluctuation and macrovascular endothelial dysfunction in type 2 diabetic patients with coronary heart disease. Eur Rev Med Pharmacol Sci. 2014;18(23):3593–3600.
- Yu Z, Bai X, Zhang Y, et al. Baseline hemodynamic impairment and revascularization outcome in newly diagnosed adult moyamoya disease determined by pseudocontinuous arterial spin labeling. World Neurosurg. 2022;165:e494–e504. doi: 10.1016/j.wneu.2022.06.084.
- Filimonova E, Ovsiannikov K, Rzaev J. Neuroimaging in moyamoya angiopathy: updated review. Clin Neurol Neurosur. 2022;222:107471. doi: 10.1016/j.clineuro.2022.107471.
- Watchmaker JM, Frederick BD, Fusco MR, et al. Clinical use of cerebrovascular compliance imaging to evaluate revascularization in patients with moyamoya. Neurosurgery. 2019;84(1):261–271. doi: 10.1093/neuros/nyx635.
- L WS, Garza M, Davis LT, et al. Presurgical magnetic resonance imaging indicators of revascularization response in adults with moyamoya vasculopathy. J Magn Reson Imaging. 2022;56(4):983–994. doi: 10.1002/jmri.28156.
- Yun TJ, Cheon JE, Na DG, et al. Childhood moyamoya disease: quantitative evaluation of perfusion MR imaging–correlation with clinical outcome after revascularization surgery. Radiology. 2009;251(1):216–223. doi: 10.1148/radiol.2511080654.
- Rios-Zermeno J, Ghaith AK, El HV, et al. Extracranial-intracranial bypass for moyamoya disease: the influence of racial and socioeconomic disparities on outcomes - a national inpatient sample analysis. World Neurosurg. 2023;182:e624–e634. doi: 10.1016/j.wneu.2023.12.005.
- Raygor KP, Phelps R, Rutledge C, et al. Socioeconomic factors associated with pediatric moyamoya disease hospitalizations: a nationwide cross-sectional study. J Neurosurg Pediatr. 2022;29(6):602–611. doi: 10.3171/2022.1.PEDS21339.
- Ghaffari-Rafi A, Ghaffari-Rafi S, Leon-Rojas J. Socioeconomic and demographic disparities of moyamoya disease in the United States. Clin Neurol Neurosurg. 2020;192:105719. doi: 10.1016/j.clineuro.2020.105719.
- Winkler EA, Yue JK, Deng H, et al. National trends in cerebral bypass surgery in the United States, 2002-2014. Neurosurg Focus. 2019;46(2):E4. doi: 10.3171/2018.11.FOCUS18530.
- Choi JW, Chong S, Phi JH, et al. Postoperative symptomatic cerebral infarction in pediatric moyamoya disease: risk factors and clinical outcome. World Neurosurg. 2020;136:e158–e164. doi: 10.1016/j.wneu.2019.12.072.
- Kim SH, Choi JU, Yang KH, et al. Risk factors for postoperative ischemic complications in patients with moyamoya disease. J Neurosurg. 2005;103(5 Suppl):433–438. doi: 10.3171/ped.2005.103.5.0433.