Abstract
Background
Little is known how individual time-in-therapeutic-range (TTR) impacts the effectiveness and safety of warfarin therapy compared to direct oral anticoagulants (DOACs) in patients with atrial fibrillation (AF).
Objective
To compare the effectiveness and safety of standard dose DOACs to warfarin in patients with AF, while categorizing warfarin treated patients into quartiles based on their individual TTR.
Materials and methods
We conducted a nationwide study including all patients with new-onset AF between 2011 and 2018 in Finland. Hazard ratios (HR) were calculated using Cox regression analysis with the inverse probability of treatment weighted method to assess the risks of ischaemic stroke (IS), intracranial haemorrhage (ICH) and mortality for users of apixaban (n = 12,426), dabigatran (n = 4545), rivaroxaban (n = 12,950) and warfarin (n = 43,548).
Results
The median TTR for warfarin users was 72%. Compared to the second best TTR quartile (reference), the risk of IS was higher in the two poorest TTR quartiles, and lower in the best TTR quartile and on rivaroxaban [2.35 (95% confidence interval, 1.85–2.85), 1.44 (1.18–1.75), 0.60 (0.47–0.77) and 0.72 (0.56–0.92)]. These differences were non-significant for apixaban and dabigatran. HR of ICH was 6.38 (4.88–8.35) and 1.87 (1.41–2.49) in the two poorest TTR groups, 1.44 (1.02–1.93) on rivaroxaban, and 0.58 (0.40–0.85) in the best TTR group compared to the reference group. Mortality was higher in the two poorest TTR groups and lowest in the best TTR group.
Conclusions
The outcome was unsatisfactory in the two lowest TTR quartiles – in half of the patients treated with warfarin. The differences between the high TTR groups and standard dose DOACs were absent or modest.
Introduction
The prevalence of atrial fibrillation (AF) continues to increase, and AF stands as the leading cause of ischaemic stroke (IS) [Citation1,Citation2]. However, the risk of stroke can be considerably mitigated through appropriate oral anticoagulation (OAC) therapy, using either warfarin or direct oral anticoagulants (DOACs) [Citation3,Citation4]. Effective anticoagulation with warfarin necessitates diligent monitoring of the international normalized ratio (INR) [Citation5,Citation6]. Consequently, DOACs are now preferred over warfarin for the treatment of non-valvular AF due in part to their simpler dosing regimen [Citation7–9]. Nevertheless, there remains a subset of AF patients who continue to receive warfarin treatment for various reasons, such as its lower cost.
While the differences in the thromboembolic risk between patients on DOACs and warfarin have been relatively minor, DOAC therapy has shown a significantly reduced risk of intracranial bleeding. These observations are supported by robust evidence from both randomized controlled trials (RCTs) and real-life observational studies [Citation4,Citation10].
Anticoagulation control for patients with AF on warfarin has traditionally been gauged using the time-in-therapeutic-range (TTR) value, with TTR levels below 60% considered inadequate [Citation11]. Inadequate warfarin control has been associated with an elevated risk of both thromboembolic and bleeding events [Citation12–15]. RCTs have demonstrated that DOACs exhibit superior efficacy and safety, particularly in settings where warfarin control is suboptimal compared to centres with well-managed warfarin control [Citation16–19]. However, there is limited real-life patient-level data available for comparing the quality of anticoagulation control between warfarin and DOAC treatments [Citation20–22].
In this study, we assessed the effectiveness and safety of warfarin treatment stratified by different individual TTR levels and compared it with DOACs. We utilized a large nationwide cohort of OAC-naïve patients with new-onset AF, representing all levels of healthcare.
Materials and methods
Study population
The Finnish AntiCoagulation in Atrial Fibrillation (FinACAF) Study, identified by the ClinicalTrials Identifier NCT04645537 and ENCePP Identifier EUPAS29845, is a comprehensive nationwide retrospective register-based cohort study. The study encompassed all patients diagnosed with AF between 2004 and 2018 in Finland. The patients were identified, and patient data were collected from three national health care registers covering the complete Finnish population: the Care Register of Health Care (HILMO) for hospitalizations and outpatient specialist visits, the Care Register of Health Care (AvoHILMO) for primary health care visits, and the National Reimbursement Register maintained by the Social Insurance Institution (KELA) for drug prescriptions. The study design and methods have been previously reported [Citation23]. To assess individual TTR values, laboratory data, including INR, was obtained from the six largest national central laboratories. For accurate patient data integration, the Finnish population and health care registers were linked using the national identification code assigned to all Finnish residents.
The study included patients aged 20 years or older with new-onset, non-valvular AF and who initiated OAC therapy between 1 January 2011 and 31 December 2018. The patient collection process is described in the Supplementary material online, Figure S1, and the used health care registers are summarized in Supplementary Table S1.
Treatment and outcome measures
For patients on warfarin, drug exposure began on the date of the first purchase if there was an INR measurement within 30 days prior. Intervals between INR measurements were required to be <60 days, and the drug exposure period concluded 60 days after the last INR measurement [Citation13]. The exposure period was also terminated if 180 days passed without a new warfarin purchase or the purchase of any DOAC. The detailed protocol for assessing drug exposure is depicted in Supplementary material online, Figure S2.
The individual TTR value was calculated during the exposure period of warfarin treatment. This period started seven days after the initiation of exposure and ended at the exposure’s conclusion, using Rosendaal’s method [Citation24]. Following interpolation of the method, the percentage of time during which the observed and calculated INR values fell between 2.0 (≥) and 3.0 (≤) was determined [Citation24]. TTR was calculated for patients who had at least three INR measurements taken with intervals shorter than 60 days and patients were divided into TTR quartiles from the lowest to the highest TTR value [Citation13].
For patients receiving DOACs, the drug exposure period commenced on the first date of drug purchase, assuming standard dosing (dabigatran 150 mg bd., apixaban 5 mg bd. and rivaroxaban 20 mg od.). The drug exposure period continued until 30 days after the estimated depletion of the drugs if no subsequent purchases were made. If a patient switched to another OAC or another dose of the used DOAC, the exposure period with DOACs immediately terminated.
The outcomes of interest in this study were IS, intracranial haemorrhage (ICH), any bleeding and all-cause mortality, following the initiation of OAC exposure, were obtained from the register of Health Care (HILMO) for hospitalizations (codes used for the comorbidities and outcome measures, Supplementary material online, Table S2). Dates of death were retrieved from the National Cause of Death Register. For patients with a history of IS, ICH or bleeding before starting OAC exposure, a new or recurrent event was considered an endpoint event if it met the following criteria: (1) the event was the main diagnosis of hospitalization and (2) a minimum of 90 days had elapsed since the previous diagnosed event.
Participants were followed for the occurrence of IS, ICH or bleeding events until 31 December 2018, or until death, or until the OAC exposure reached a maximum duration of 730 days, whichever came first. The follow-up period was capped at 730 days due to the limited number of patients with DOAC exposure exceeding 730 days (Supplementary material online, Figure S2).
To predict the risk of poor INR control in patients receiving warfarin treatment, we utilized the modified SAMe-TT2R2 score, a clinical tool for predicting the quality of warfarin therapy by individual TTR based on patient characteristics [Citation11,Citation25]. As smoking status and ethnicity information (more than 90% of the Finnish population are ethnic Finns) were not available, the modified SAMe-TT2R2 score was calculated excluding these factors, considering sex (female: 1 point), age (<60 years: 1 point), medical history (history of more than two of the following: hypertension, diabetes, coronary artery disease, peripheral artery disease, heart failure, stroke; pulmonary, hepatic or kidney disease: 1 point) and treatment (amiodarone: 1 point).
Study ethics
The study protocol was approved by the Research Ethics Committee of the Faculty of Medicine of Helsinki University, Helsinki, Finland and granted research permission from the Helsinki University Hospital. The study was conducted without any direct patient involvement or contact in any phase of the study. Therefore, no patient consent is needed according to Finnish legislation and the consent was waived off due to the retrospective nature of this study. Patients’ identification numbers were pseudonymized, and the research group received individualized but unidentifiable data. The study conforms to the Declaration of Helsinki as revised in 2013 and to European General Data Protection Regulation (EGDPR), and followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement [Citation26].
Statistical methods
Crude and weighted event rates per 100 patient years (py) were reported for different OAC groups. Cox regression analysis was utilized to estimate event hazard ratios (HRs) with 95% confidence intervals (95% CIs) between the OAC groups. Upon visual inspection, the second-best TTR quartile was found to be the closest to the DOAC groups, thus selected as the reference group. To account for potential variations in baseline characteristics among the patient groups, an inverse probability of treatment weighting was employed. The treatment weights were derived using a generalized boosted model with 10,000 regression trees, aimed at achieving balanced treatment populations that represent the average treatment effect for the overall population. The covariates used in the weighting process included age, sex, previous stroke or transient ischaemic attack, vascular disease, hypertension, diabetes, cancer, use of statins or antithrombotic drugs, CHA2DS2-VASc score and HAS-BLED-score. To assess the balance between the populations, standardized mean differences between the groups were examined, with a threshold of 0.1 set to determine acceptable balance between the weighted groups. The statistical analyses were conducted using R version 4.2.1 (R Foundation for Statistical Computing, Vienna, Austria), and a significance threshold of .05 was considered for the p values.
Results
In total, 74,008 patients with a new diagnosis of AF between 2011 and 2018 were included in the study (Supplementary material online, Figure S1). Overall, 43,548 (58.8%) patients initiated OAC therapy with warfarin, 12,950 (17.5%) with rivaroxaban, 12,426 (16.8%) with apixaban, 4545 (6.1%) with dabigatran and 539 (0.7%) with edoxaban (). Among patients on warfarin, the mean and median TTR over the exposure period were 66% and 72%, respectively. The mean TTR values (range) in the warfarin quartiles were 32% (0–52%) in the first quartile, 65% (52–72%) in the second quartile, 77% (72–83%) in the third quartile and 90% (83–100%) in the highest quartile. Supplementary material online, Figure S3, depicts the trends in the initiation of OACs in patients with new-onset AF in Finland from 2011 to 2018.
Table 1. Baseline characteristics of the study cohort according to oral anticoagulant categories.
The study cohort consisted of 49.6% females, with a mean age of 73.0 years. Hypertension was the most prevalent comorbidity (78.8%). Patients on warfarin were, in turn, slightly older and had slightly higher CHA2DS2-VASc scores compared to patients on apixaban, edoxaban or rivaroxaban (). Patients initiating dabigatran treatment, in turn, were more often male and younger, with lower CHA2DS2-VASc scores compared to the other treatment groups. The mean follow-up times ranged from 273 days with edoxaban to 400 days with warfarin.
Edoxaban was excluded from the main analyses due to the small number of patients and limited exposure time. Supplementary material online, Figure S4, illustrates the cumulative incidence of the study endpoints, including also edoxaban.
Ischaemic stroke and intracranial haemorrhage
During the follow-up period, there were 1002 IS and 673 ICH events in the study population. Among patients on warfarin, the rates of IS in TTR quartiles were 3.45, 1.79, 1.18 and 0.74/100 py from the lowest to the highest quartile, respectively (, ). The rates of IS among patients on standard dose DOACs were 1.08/100 py for apixaban, 0.73/100 py for dabigatran and 0.83/100 py for rivaroxaban. Compared to the third (second best) TTR group (reference), the weighted risk of IS was significantly higher in the two poorest TTR groups (HR 2.35, 95% CI 1.85–2.85 and HR 1.44, 1.18–1.75) (). On the other hand, the best TTR quartile and rivaroxaban group had slightly lower risks of IS, and the risks were similar for apixaban, dabigatran and the second best TTR groups.
Figure 1. Crude cumulative incidence curves of (a) ischaemic stroke, (b) intracranial haemorrhage and (c) all-cause mortality in the studied oral anticoagulation groups. TTR: time-in-therapeutic-range.
Figure 2. Inverse-probability-of-treatment-weighted Cox hazard ratios of calculated incidence rates per 100 patient years for direct oral anticoagulants compared with warfarin in different time-in-therapeutic-range groups for ischaemic stroke, intracranial haemorrhage, any bleeding and death endpoints. Reference group (1) = warfarin patients with the second best (TTR group 3) time-in-therapeutic-range (TTR).
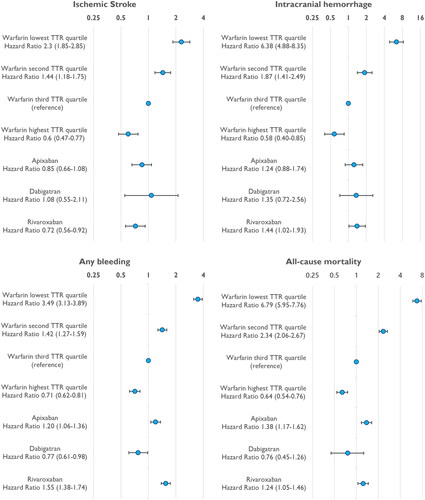
Table 2. Crude and weighted rates of outcome events according to the treatment categories.
The crude incidence rates of ICH were 3.60, 0.99, 0.51 and 0.32/100 py from the lowest to the highest TTR quartile, respectively (). The corresponding rates of ICH were 0.62, 0.47 and 0.67/100 py for apixaban, dabigatran and rivaroxaban, respectively. The weighted risk of ICH was significantly higher in the two poorest TTR groups compared to the second best TTR group (). The weighted risk of ICH was also slightly higher for rivaroxaban, while the risk was similar for apixaban, dabigatran and the second best TTR groups. Notably, the best TTR group had the lowest risk of ICH.
Bleeding events
The crude incidence rates of any bleeding events were highest in the poorest warfarin TTR group and lowest in the dabigatran group and the best warfarin TTR group (, ). The weighted risk of bleeding was significantly higher in the poorest TTR group compared to the second best TTR group (HR 3.49, 95% CI 3.13–3.89) and all the DOAC groups (). The crude and weighted risk of bleeding was lower with dabigatran compared to all the other DOACs.
All-cause mortality
The all-cause mortality was highest in the two lowest TTR groups (, ). Among patients on standard dose DOACs, the rate of death was 2.80/100 py for apixaban, 1.51/100 py for dabigatran and 2.08/100 py for rivaroxaban. The Cox weighted risk of death was significantly higher (HR 6.79, 5.95–7.76 and HR 2.34, 2.06–2.67) in the two poorest TTR groups and significantly lower in the best TTR group when compared to the second best TTR group (HR 0.64, 0.54–0.76; ).
Warfarin patients analysed as one group
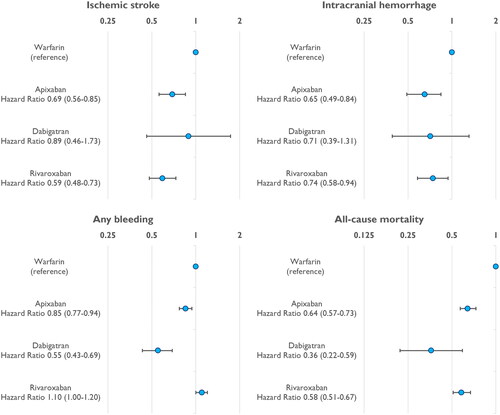
When warfarin patients were analysed as a single group, the weighted risk of IS was significantly lower (HR 0.69, 0.56–0.85 and HR 0.59, 0.48–0.73) in patients on apixaban and rivaroxaban compared to the warfarin group (). The weighted risk of ICH was also significantly lower in patients on apixaban and rivaroxaban compared to the warfarin group. Furthermore, the risk of death was lower in all DOAC groups compared to warfarin ().
Pre-estimation of the choice of optimal oral anticoagulation
Supplementary Tables S3 and S4 and Figure S5 provide information on the modified SAMe-TT2R2-score in the four TTR groups of patients on warfarin and patients on the DOACs. Patients in the highest TTR group had the lowest proportion of patients in modified SAMe-TT2R2-score classes 2 or higher, primarily due to the lower prevalence of heart failure in this group. However, the overall difference between the TTR groups was only modest. Patients on apixaban had the highest proportion of patients in the modified SAMe-TT2R2-score classes 2 or higher compared to the other DOAC groups.
Discussion
In our comprehensive nationwide study, encompassing all patients with new-onset AF in Finland across various health care settings, we identified a significant association between individual TTR and the effectiveness and safety of warfarin therapy. Specifically, we observed that half of the patients treated with warfarin, with suboptimal individual TTR (<72%), faced a considerably higher risk of IS, ICH and all-cause mortality when compared to patients with adequate TTR or those receiving DOACs. Notably, the lowest TTR quartile demonstrated a substantially elevated risk of adverse events compared to other patients on OAC.
Utilization of all OACs increased in patients with AF in Finland during the 2010s. The introduction of DOACs probably enhanced this process, although their widespread use was somewhat delayed due to the national reimbursement practices [Citation9]. It is important to note that we observed significant differences in baseline characteristics between the DOAC and warfarin groups. Dabigatran, as the first available DOAC for AF treatment, was predominantly initiated by younger patients with a lower prevalence of coronary heart disease and heart failure. Conversely, the differences in comorbidities were relatively modest between patients on warfarin, apixaban or rivaroxaban.
The overall risk of IS among AF patients receiving OAC was generally low; the crude IS rate in all patients in our study was 1.31/100 py, with a slightly higher rate of 1.54/100 py in warfarin-treated patients. Reported IS rates in real-life studies and RCTs [Citation4,Citation27,Citation28] have varied, ranging from 1 to 2/100 py, but higher rates of 2 to 4/100 py have been more commonly observed [Citation20–22,Citation29–31]. Our study conducted an on-treatment analysis of patients who were on an OAC, based on either continuous INR measurements (for warfarin-treated patients) or uninterrupted drug purchases in DOAC users, which likely contributed to the observed lower IS rate. However, the relative risks between DOACs and warfarin in our study align with previously published data [Citation4,Citation10,Citation31,Citation32].
While the overall effectiveness of warfarin was comparable to previous reports, the risk of stroke exhibited significant variation depending on the quality of anticoagulation. Notably, the risk of IS was 2–4 times higher in the two poorest TTR groups compared to patients with TTR >72% or those on DOAC treatment.
In general, both the crude and relative rates of ICH between warfarin and DOACs in our study were consistent with earlier reports [Citation10,Citation16,Citation17,Citation20–22,Citation27–31]. For warfarin-treated patients, the crude rate of ICH ranged from 0.30 to 3.6/100 py across the best to the poorest TTR groups. It is well documented that warfarin use carries a higher risk of ICH compared to DOACs and antiplatelet therapy [Citation3,Citation4,Citation31,Citation33,Citation34]. However, our results underscore that while the average risk of ICH is increased in patients on warfarin, the range of risk from the lowest to highest TTR is quite extensive, with a 10-fold difference observed in our patient population. In the best TTR group, the rate of ICH was remarkably low, 0.30/100 py, one of the lowest reported, including RCTs. This marks the first instance where any patient group on warfarin has demonstrated a lower risk of ICH compared to any other treatment. Furthermore, our individually assessed TTR setup provides a novel finding compared to RCTs, where centre-average-based TTR has been utilized. In the centre-based setup, warfarin patients, even in centres with good average TTR, had higher rate of ICH compared to patients on DOACs [Citation16–19].
We observed the highest bleeding rate in the poorest TTR group on warfarin, while the lowest bleeding rates were seen in the best TTR group and in patients on dabigatran. The bleeding rates for the other groups were very similar. With the exception of apixaban, our findings regarding bleeding events align well with previous literature [Citation10,Citation31,Citation35].
The overall mortality was higher for patients on warfarin compared to those on DOACs, but, similar to the results of IS, ICH and bleeding events, this finding was driven by the patients in the poor TTR quartiles.
Studies conducted before the era of DOACs indicated that suboptimal INR control during warfarin treatment is associated with a higher risk of thromboembolic and bleeding events [Citation12,Citation13]. Subsequent RCTs revealed that the efficacy and safety of DOACs compared to warfarin decline when the quality of warfarin therapy improves, as assessed by centre-based average TTR [Citation16–19]. However, only three small studies have explored how the quality of warfarin anticoagulation, assessed by TTR of individual patients, correlates with the treatment outcomes of DOACs in real-life situations [Citation20–22].
A study from China, involving 1428 patients on warfarin with a median TTR of 38.8%, revealed that a higher TTR was associated with better outcomes. However, even patients in the highest TTR group (TTR >56.2%) had a higher risk of stroke and ICH compared to patients on dabigatran [Citation20]. In another study from Taiwan, where the target INR for calculating TTR was 1.5–2.5 due to the Asian population, the achieved mean TTR was also low (44.4%) in 5197 patients and only 23.5% of patients had a TTR >70%, in contrast to the median TTR of 72% in the present study. The effectiveness and safety of warfarin and DOACs were comparable when TTR was >50%, but if TTR was below 50%, DOACs were significantly better [Citation21]. In the most recent real-world study from the US on 7163 patients on warfarin, apixaban, dabigatran or rivaroxaban, the median TTR was 55% and the risk of stroke and bleeding was markedly lower if TTR was ≥60% compared to patients with TTR <60% [Citation22]. However, the study was underpowered to find significant differences in outcomes between warfarin and individual DOACs in propensity score analyses when patients on warfarin were divided into <60% and ≥60% TTR groups [Citation22].
The SAMe-TT2R2-score is a validated tool which can help to find patients who are less likely to achieve a good TTR on warfarin [Citation11,Citation25]. The differences on the modified SAMe-TT2R2-score in our study between the warfarin TTR groups were only modest. Therefore, it appears unlikely that this or any other tool could effectively predict optimal anticoagulation control during warfarin treatment in non-valvular AF.
Our study has several strengths. First, the nationwide basis and large unselected cohort, covering all levels of healthcare, represent the most important strength. In Finland, all citizens have healthcare coverage, ensuring treatment accessibility for all, funded by taxation-based support.
Second, this study is the largest to compare warfarin with individual TTR data to DOACs in patients with AF. The slightly better quality of warfarin treatment in Finland compared to most previous reports ensures that warfarin is analysed fairly under optimal conditions [Citation12,Citation15]. Third, the results comparing warfarin as a whole to DOACs are consistent with previous reports. Nevertheless, our findings related to different TTR groups highlight a wide range of outcomes, from very poor to good, among patients on warfarin treatment. If warfarin is used, TTR must be as good as possible, and preferably more than 80% as seen in the present study.
We also acknowledge some limitations. The most crucial limitation is the retrospective register-based study design, subject to the general limitations of such approaches. Data accuracy depends on the quality of recording. However, the Finnish register for hospitalizations, from which all endpoints were gathered, has a long history of high quality and is well validated [Citation36,Citation37].
Since this is not a randomized study, caution must be exercised in interpreting our results. Although we used weighting methods to balance measured confounders, there may still be unmeasured or unknown confounders that could impact the results.
Finally, our register data did not include information about smoking and ethnicity. As a result, the SAMe-TT2R2-score had to be calculated without this information, which may have weakened the assessment of whether the SAMe-TT2R2-score could be a reliable tool for predicting treatment outcomes. Also, based on limitations of the administrative data, we were unable to detail the reasons for the poor TTR.
Conclusions
In this nationwide study encompassing 74,008 patients with AF in Finland, we have established a robust association between the effectiveness and safety of warfarin and the quality of treatment. While the overall quality of warfarin treatment was acceptable, half of the patients on warfarin – the two poorest TTR groups – did not receive adequate anticoagulation treatment. The rates of IS, ICH and mortality were 2–10 times higher among patients on warfarin in the two lowest TTR quartiles, whereas the differences between the highest TTR groups and standard dose DOACs were only modest.
Author contributions
MLe, AL, JHau, JP, MLi, PM, MN, ALA, JHar and KEJA planned the work. MLe, AL, OH, JHau, JP, MLi, JK, EK, PT, KT and SI-S had access to the data, and MLe, OH, JHau and OL contributed to the statistical analyses. MLe drafted the manuscript. All authors contributed to the design of the study, have critically revised the draft, and have approved the final version of the submitted manuscript.
Ethical approval
The study protocol was approved by the Ethics Committee of the Medical Faculty of the University of Helsinki, Helsinki, Finland (nr. 15/2017).
Supplemental Material
Download Zip (438.1 KB)Disclosure statement
M.Le.: consultant: BMS-Pfizer-alliance, Bayer, Boehringer-Ingelheim and MSD; speaker: BMS-Pfizer-alliance, Bayer, Boehringer Ingelheim, MSD, Terve Media and Orion Pharma. Research grants: Aarne Koskelo Foundation, Yrjö Jahnsson Foundation, The Finnish Foundation for Cardiovascular Research, Helsinki and Uusimaa Hospital District research fund; A.L.: research grants: Pulsus Foundation; O.H.: none; J.Hau.: research grants: The Finnish Foundation for Cardiovascular Research, and EU Horizon 2020, EU FP7. Advisory Board Member: BMS-Pfizer-alliance, Novo Nordisk, Amgen. Speaker: Cardiome, Bayer; J.P.: Consultant/speaker: Boehringer-Ingelheim, Bayer, BMS-Pfizer, Portola, Amgen, Herantis Pharma; other: Terve Media, Vital Signum, Abbott, Bayer; M.Li.: speaker: BMS-Pfizer-alliance; pm: consultant: Roche, BMS-Pfizer-alliance, Novartis Finland, Boehringer Ingelheim, MSD Finland, Bayer, Boehringer-Ingelheim; J.K.: none, O.L.: none; K.T.: research grants: The Finnish Foundation for Cardiovascular Research; P.T.: none; E.K.: none; S.I-S.: none; M.N.: consultant: Orion Pharma; A.L.A.: research grants: Finnish Foundation for Cardiovascular Research, Sigrid Juselius Foundation. Speaker: Abbott, Johnson & Johnson, Sanofi, Bayer, Boehringer-Ingelheim; J.Har.: research grants: The Finnish Foundation for Cardiovascular Research, Advisory Board Member: BMS-Pfizer-alliance, Novo Nordisk, Amgen. Speaker: Cardiome, Bayer; K.E.J.A.: research grants: The Finnish Foundation for Cardiovascular Research; speaker: Bayer, BMS-Pfizer-alliance and Boehringer-Ingelheim. Member in the advisory boards: Bayer, BMS-Pfizer-alliance and Astra-Zeneca. No other potential competing interests are reported by the authors.
Data availability statement
The data underlying this article were provided by the Finnish Institute for Health and Welfare (THL), the Population Register Center and the Social Insurance Institution (KELA) under license. Based on the contracts with the Finnish registries, restrictions apply to the availability of these data, and so they are not publicly available. Data might be, however, available from the authors upon reasonable request and with permissions needed from the Finnish Institute for Health and Welfare (THL), the Population Register Center and the Social Insurance Institution (KELA).
Additional information
Funding
References
- Lehto M, Haukka J, Aro A, et al. Comprehensive nationwide incidence and prevalence trends of atrial fibrillation in Finland. Open Heart. 2022;9(2):e002140. doi: 10.1136/openhrt-2022-002140.
- Björck S, Palaszewski B, Friberg L, et al. Atrial fibrillation, stroke risk, and warfarin therapy revisited: a population-based study. Stroke. 2013;44(11):3103–3108. doi: 10.1161/STROKEAHA.113.002329.
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146(12):857–867. doi: 10.7326/0003-4819-146-12-200706190-00007.
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955–962. doi: 10.1016/S0140-6736(13)62343-0.
- Hallinen T, Soini EJ, Asseburg C, et al. Warfarin treatment among Finnish patients with atrial fibrillation: retrospective registry study based on primary healthcare data. BMJ Open. 2014;4(2):e004071. doi: 10.1136/bmjopen-2013-004071.
- Hallinen T, Soini E, Asseburg C, et al. Cost-Effectiveness of apixaban versus other direct oral anticoagulants and warfarin in the prevention of thromboembolic complications among Finnish patients with non-valvular atrial fibrillation. Clin Outcomes Res. 2021;13:745–755.
- Wong ES, Done N, Zhao M, et al. Comparing total medical expenditure between patients receiving direct oral anticoagulants vs warfarin for the treatment of atrial fibrillation: evidence from VA-Medicare dual enrollees. J Manag Care Spec Pharm. 2021;27(8):1056–1066. doi: 10.18553/jmcp.2021.27.8.1056.
- Grymonprez M, Simoens C, Steurbaut S, et al. Worldwide trends in oral anticoagulant use in patients with atrial fibrillation from 2010 to 2018: a systematic review and meta-analysis. Europace. 2022;24(6):887–898. doi: 10.1093/europace/euab303.
- Teppo K, Airaksinen KEJ, Jaakkola J, et al. Trends in treatment and outcomes of atrial fibrillation during 2007–17 in Finland. Eur Heart J Qual Care Clin Outcomes. 2023;9(7):673–679. doi: 10.1093/ehjqcco/qcac086.
- Buckley BJR, Lane DA, Calvert P, et al. Effectiveness and safety of apixaban in over 3.9 million people with atrial fibrillation: a systematic review and meta-analysis. J Clin Med. 2022;11(13):3788. doi: 10.3390/jcm11133788.
- Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373–498. doi: 10.1093/eurheartj/ehaa612.
- Mearns ES, White CM, Kohn CG, et al. Quality of vitamin K antagonist control and outcomes in atrial fibrillation patients: a meta-analysis and meta-regression. Thromb J. 2014;12(1):14. doi: 10.1186/1477-9560-12-14.
- Lehto M, Niiranen J, Korhonen P, et al. Quality of warfarin therapy and risk of stroke, bleeding, and mortality among patients with atrial fibrillation: results from the nationwide FinWAF Registry: quality of warfarin therapy. Pharmacoepidemiol Drug Saf. 2017;26(6):657–665. doi: 10.1002/pds.4194.
- Vestergaard AS, Skjøth F, Larsen TB, et al. The importance of mean time in therapeutic range for complication rates in warfarin therapy of patients with atrial fibrillation: a systematic review and meta-regression analysis. PLOS One. 2017;12(11):e0188482. doi: 10.1371/journal.pone.0188482.
- Carmo J, Ferreira J, Costa F, et al. Non-vitamin K antagonist oral anticoagulants compared with warfarin at different levels of INR control in atrial fibrillation: a meta-analysis of randomized trials. Int J Cardiol. 2017;244:196–201. doi: 10.1016/j.ijcard.2017.06.004.
- Wallentin L, Yusuf S, Ezekowitz MD, et al. Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE-LY trial. Lancet. 2010;376(9745):975–983. doi: 10.1016/S0140-6736(10)61194-4.
- Wallentin L, Lopes RD, Hanna M, et al. Efficacy and safety of apixaban compared with warfarin at different levels of predicted international normalized ratio control for stroke prevention in atrial fibrillation. Circulation. 2013;127(22):2166–2176. doi: 10.1161/CIRCULATIONAHA.112.142158.
- Piccini JP, Hellkamp AS, Lokhnygina Y, et al. Relationship between time in therapeutic range and comparative treatment effect of rivaroxaban and warfarin: results from the ROCKET AF Trial. J Am Heart Assoc. 2014;3(2):e000521. doi: 10.1161/JAHA.113.000521.
- Lixiana; 2024. Available from: http://lixiana-epar-public-assessment-report_en.pdf
- Ho C-W, Ho M-H, Chan P-H, et al. Ischemic stroke and intracranial hemorrhage with aspirin, dabigatran, and warfarin: impact of quality of anticoagulation control. Stroke. 2015;46(1):23–30. doi: 10.1161/STROKEAHA.114.006476.
- Chan Y-H, Lee K-T, Kao Y-W, et al. The comparison of non-vitamin K antagonist oral anticoagulants versus well-managed warfarin with a lower INR target of 1.5 to 2.5 in Asians patients with non-valvular atrial fibrillation. PLOS One. 2019;14(3):e0213517. doi: 10.1371/journal.pone.0213517.
- Yao X, Abraham NS, Sangaralingham LR, et al. Effectiveness and safety of dabigatran, rivaroxaban, and apixaban versus warfarin in nonvalvular atrial fibrillation. J Am Heart Assoc. 2016;5(6):e003725. doi: 10.1161/JAHA.116.003725.
- Lehto M, Halminen O, Mustonen P, et al. The nationwide Finnish AntiCoagulation in Atrial Fibrillation (FinACAF): study rationale, design, and patient characteristics. Eur J Epidemiol. 2022;37(1):95–102. doi: 10.1007/s10654-021-00812-x.
- Rosendaal FR, Cannegieter SC, Meer FJM, et al. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69(3):236–239. doi: 10.1055/s-0038-1651587.
- Apostolakis S, Sullivan RM, Olshansky B, et al. Factors affecting quality of anticoagulation control among patients with atrial fibrillation on warfarin. Chest. 2013;144(5):1555–1563. doi: 10.1378/chest.13-0054.
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Epidemiology. 2007;18(6):800–804. doi: 10.1097/EDE.0b013e3181577654.
- Själander S, Sjögren V, Renlund H, et al. Dabigatran, rivaroxaban and apixaban vs. high TTR warfarin in atrial fibrillation. Thromb Res. 2018;167:113–118. doi: 10.1016/j.thromres.2018.05.022.
- Lip GYH, Keshishian A, Li X, et al. Effectiveness and safety of oral anticoagulants among nonvalvular atrial fibrillation patients: the ARISTOPHANES study. Stroke. 2018;49(12):2933–2944. doi: 10.1161/STROKEAHA.118.020232.
- Larsen TB, Skjøth F, Nielsen PB, et al. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. 2016;353:i3189. doi: 10.1136/bmj.i3189.
- Rutherford O-CW, Jonasson C, Ghanima W, et al. Comparison of dabigatran, rivaroxaban, and apixaban for effectiveness and safety in atrial fibrillation: a nationwide cohort study. Eur Heart J Cardiovasc Pharmacother. 2020;6(2):75–85. doi: 10.1093/ehjcvp/pvz086.
- Liu F, Yang Y, Cheng W, et al. Reappraisal of non-vitamin K antagonist oral anticoagulants in atrial fibrillation patients: a systematic review and meta-analysis. Front Cardiovasc Med. 2021;8:757188. doi: 10.3389/fcvm.2021.757188.
- Ntaios G, Papavasileiou V, Makaritsis K, et al. Real-world setting comparison of nonvitamin-K antagonist oral anticoagulants versus vitamin-K antagonists for stroke prevention in atrial fibrillation: a systematic review and meta-analysis. Stroke. 2017;48(9):2494–2503. doi: 10.1161/STROKEAHA.117.017549.
- Aguilar MI, Hart R. Oral anticoagulants for preventing stroke in patients with non-valvular atrial fibrillation and no previous history of stroke or transient ischemic attacks. Cochrane Database Syst Rev. 2005;2005(3):CD001927. doi: 10.1002/14651858.CD001927.pub2.
- Smith J, Forster A, House A, et al. Oral anticoagulants versus antiplatelet therapy for preventing stroke in patients with non-valvular atrial fibrillation and no history of stroke or transient ischemic attacks. Cochrane Database Syst Rev. 2007;2007(3):CD001919. doi: 10.1002/14651858.CD006186.pub2.
- Zhang J, Wang X, Liu X, et al. Comparative effectiveness and safety of direct acting oral anticoagulants in nonvalvular atrial fibrillation for stroke prevention: a systematic review and meta-analysis. Eur J Epidemiol. 2021;36(8):793–812. doi: 10.1007/s10654-021-00751-7.
- Rapola JM, Virtamo J, Korhonen P, et al. Validity of diagnoses of major coronary events in national registers of hospital diagnoses and deaths in Finland. Eur J Epidemiol. 1997;13(2):133–138. doi: 10.1023/a:1007380408729.
- Sund R. Quality of the Finnish Hospital Discharge Register: a systematic review. Scand J Public Health. 2012;40(6):505–515. doi: 10.1177/1403494812456637.