Abstract
Background
Polycystic ovary syndrome (PCOS) is one of the most common endocrine and metabolic disorders in women of reproductive age. It is frequently comorbid with obesity and negative emotions. Currently, there are few reports on the relationship between obesity and negative emotions in patients with PCOS. Here we performed both basic and clinical studies to study the relationship between obesity and negative emotions in PCOS.
Methods
We performed a cross-sectional study including 608 patients with PCOS and 184 healthy participants to assess the mental health status of people with different body mass indices (BMI). Self-rated anxiety, depression, and perceived stress scales were used for subjective mood evaluations. Rat PCOS models fed 45 and 60% high-fat diets were used to confirm the results of the clinical study. Elevated plus maze and open field tests were used to assess anxiety- and depression-like behaviors in rats.
Results
We observed overweight/obesity, increased depression, anxiety, and perceived stress in women with PCOS, and found that anxiety and depression were negatively correlated with BMI in patients with severe obesity and PCOS. Similar results were confirmed in the animal study; the elevated plus maze test and open field test demonstrated that only 60% of high fat diet-induced obesity partly reversed anxiety- and depression-like behaviors in PCOS rats. A high-fat diet also modulated rat hypothalamic and hippocampal luteinizing hormone and testosterone levels.
Conclusion
These results reveal a potential relationship between obesity and negative emotions in PCOS and prompt further investigation. The interactions between various symptoms of PCOS may be targeted to improve the overall well-being of patients.
KEY MESSAGES
Obesity was negatively correlated with negative emotions in patients with PCOS.
Obesity may affect the downregulation of LH and testosterone and participate in the regulation of emotions.
Increased BMI may be beneficial for patients with PCOS in terms of the psychological aspects.
Introduction
Polycystic ovary syndrome (PCOS) is the most common disorder in women of reproductive age, with a worldwide prevalence of 9–18% [Citation1]. It is characterized by hyperandrogenism, hyperinsulinemia, menstrual dysfunction, hirsutism, and infertility [Citation2,Citation3]. All these disruptions are important stress factors in the lives of women, increasing psychological stress and the risk of anxiety and depression in women with PCOS [Citation4]. Compared with healthy people, women with PCOS reported a higher prevalence of depression, anxiety, and perceived stress [Citation5]. Emotional disorders may further cause endocrine dysfunction, such as obesity, insulin resistance, menstrual irregularity, and even infertility [Citation6]. It is believed that the clinical and psychological symptoms may influence each other in PCOS [Citation7].
Approximately 50–80% of women with PCOS have comorbid obesity [Citation8]. Obesity is associated with a poor metabolic profile in women with PCOS [Citation9,Citation10]. Previous studies have shown that obesity is associated with depressive and anxiety symptoms in patients with PCOS [Citation11,Citation12]. Weight loss results in significant improvements in mental health domains, including depression and anxiety [Citation13]. Obesity treatment appears to be an effective therapeutic strategy for patients with PCOS [Citation14]. However, the relationship between obesity and negative emotions in patients with PCOS remains unclear. A thorough understanding of the relationship between obesity and negative emotions will help guide the clinical treatment of PCOS.
In the present study, we performed an epidemiological investigation to evaluate the psychological state of patients with PCOS. We then attempted to identify the crucial factors affecting the psychological state of patients with PCOS. Additionally, we performed animal experiments to observe the effects of a high-fat diet on negative emotions in a dehydroepiandrosterone (DHEA)-induced PCOS rat model. Finally, the potential molecular mechanisms underlying high-fat diet consumption were explored.
Materials and methods
Ethical approval
The clinical study was approved by the Regional Ethical Review Board of Peking University Third Hospital (PKU3-IRB-2016-212-02) and registered at ClinicalTrials.gov (Registration number NCT04264832). All participants were informed of the purpose, content, and risks of study participation. Written informed consent was obtained before the trial commencement. All experimental procedures were approved by the Animal Care and Use Committee of Peking University Third Hospital (PKU3-IRB-2019-029-02).
Participants
A large-scale epidemiological investigation was conducted in women of reproductive age (19–45 years); 608 women with PCOS and 184 age-matched controls were included at the Peking University Third Hospital from March 2016 to December 2021. Patients were eligible if they met at least two of the following 2003 Rotterdam diagnostic criteria: (1) infrequent ovulation or anovulation; (2) hyperandrogenism or clinical manifestations of high blood androgen; (3) ultrasound findings of polycystic ovaries with ≥12 follicles measuring 2–9 mm in diameter in at least one ovary, and/or ovarian volume >10 mL [Citation15]. Individuals were excluded from the study if they had other endocrine disorders, such as non-classic adrenal hyperplasia (17-hydroxyprogesterone <3 nmol/L), thyroid dysfunction, hyperprolactinemia (thyroid-stimulating hormone <0.55 or >4.78 mIU/mL), type I diabetes or poorly controlled type II diabetes, stage 2 hypertension (resting blood pressure ≥160/100 mmHg), psychiatric diagnoses, or use of psychiatric medications; none of the women had accepted any pharmacological or operative treatment (except allergy medications and occasional pain medications) within 12 weeks before entering the study.
The control women were healthy, had no history of endocrine disorders, lacked clinical or biochemical evidence of hyperandrogenism (total testosterone <60 ng/mL, free testosterone <2 ng/mL, DHEAS <271 μg/dL), and had normal ovarian morphology on ultrasonography [Citation15]. Patients were excluded if they had menstrual irregularities, signs of hyperandrogenism (Ferriman-Gallwey score >4), or evidence of polycystic ovary morphology on ultrasonography.
Clinical data collection
All study participants completed a questionnaire and underwent physical and transvaginal ultrasonographic examinations. Blood samples were collected from a subsample of women (PCOS, n = 608; Control, n = 184) for analysis of metabolic markers and hormones. Based on the Rotterdam PCOS criteria, hyperandrogenism, chronic anovulation, and polycystic ovaries were assessed.
Basic characteristics
Participants were carefully characterized regarding their general health history, medical history, and clinical, demographic (age, race/ethnicity), and anthropomorphic measurements (body mass index, waist circumference, and hip circumference) using questionnaires, interviews, and physical examinations [Citation16]. The questionnaire also requested details regarding age at menarche, menstrual cycle history, related family history, skin problems, metabolic diseases, and possible gynecologic diseases. The participants were assessed for blood pressure, breast, thyroid, premature alopecia, and any possible uterine and/or ovarian issues by physical or pelvic examination.
Transvaginal ultrasound examination
Transvaginal ultrasound scans were performed to determine the number of follicles and ovarian volume. Polycystic ovary is defined as ≥12 follicles in either ovary, measuring 2–9 mm in diameter, and/or an increased ovarian volume of each ovary >10 mL [Citation17].
Hormone profiles
Hormonal profiles of estrogen (E2, pmol/L), luteinizing hormone (LH, mIU/mL), serum follicle-stimulating hormone (FSH, mIU/mL), prolactin (PRL, ng/mL), total testosterone (T, nmol/L), and androstenedione (A4, nmol/L) in humans were measured using a Siemens Immulite 2000 immunoassay system (Siemens Healthcare Diagnostics, Siemens, Germany) [Citation18].
Psychological measures
Psychological state was assessed using four validated questionnaires. The Perceived Stress Scale [Citation19], Self-Rating Anxiety Scale (SAS) [Citation20], and Self-Rating Depressive Scale (SDS) [Citation21] were used to assess mental health status.
Animals and grouping
Female Sprague–Dawley rats (aged 22 days) were obtained from the Department of Laboratory Animal Science, Peking University Health Science Center. The animal experimentation was in accordance with the ARRIVE guidelines. All the rats were housed in standard plastic rodent cages and maintained in a regulated environment with ad libitum access to a normal chow diet and water. The rats were randomly divided into four groups: Control, DHEA, DHEA + 45% high-fat diet (HFD), and DHEA + 60% HFD. The DHEA group received subcutaneous DHEA injections daily (6 mg/100 g body weight dissolved in 0.1 ml of sesame oil) from day 27 to day 46 to establish a PCOS model [Citation22]. The DHEA + 45 and 60% HFD groups received the same DHEA injections and 45% (D12451) and 60% (D12492) fat-containing diets (45 and 60% of calories from fat; Research Diets, New Brunswick, NJ, USA), respectively. The control and DHEA (without high fat diet) groups received a normal rodent diet containing 10% fat and daily subcutaneous sesame oil injections for an equivalent length of time.
Elevated plus maze test
The elevated plus maze consisted of two opposing enclosed arms with 40 cm high opaque walls and two opposing open arms of the same size (48 × 8 cm) [Citation23,Citation24]. The rats were acclimatized for at least 30 min before the test. Each rat was placed in the center of the elevated plus maze heading toward the same open arm and videotaped for 10 min. Avoidance of the open arms was used to indicate heightened levels of anxiety-like behavior. The time spent and number of entries into the open and closed arms were analyzed using SMART software. The maze was cleaned with 75% ethanol between sessions.
Open field test
The apparatus used for the open-field test was a square arena (100 × 100 × 50 cm) [Citation25]. Each rat was placed in the center of the field and its behavior was videotaped for 10 min. The total distance traveled in the field was used to reflect motor function. Avoidance of the central section of the field could partially reflect heightened levels of anxiety-like behaviors. The total distance traveled, time spent, and number of entries into the central area (60 × 60 cm) were analyzed using the SMART software. The apparatus was cleaned with 75% ethanol between sessions.
Quantitative real-time PCR
Total RNA was extracted using the commercial TRIzol reagent following the manufacturer’s protocol. The aqueous phase was transferred to a tube and mixed with isopropanol, after which RNA pellets were obtained by centrifugation and washed with 75% ethanol in RNase-free ddH2O. The pellets were then dissolved in diethyl pyrocarbonate-treated water. To obtain cDNA, 2 μg of RNA was subjected to DNase digestion, and reverse transcription was performed using Hifair μII 1st Strand cDNA Synthesis SuperMix for quantitative polymerase chain reaction (qPCR). Subsequently, PCR amplification was conducted by a mixture containing Hieff® qPCR SYBR® Green Master Mix, forward primer, reverse primer, template cDNA, and sterile ultrapure water. The primer sequences for each gene are listed in Supplement Table 1. The relative mRNA expression levels were quantified using the 2−ΔΔCT method from Livak and Schmittgen [Citation26].
Radioimmunoassay
As previously reported, we used 125I-labelled radioimmunoassay kits (Beijing North Institute of Biological Technology, Beijing, China) to assess hormones levels (LH, T) in the hypothalamus, hippocampus, and serum of rats [Citation22]. Radioimmunoassays were conducted with reagent volume and incubation time according to the manufacturer’s instructions.
Statistical analysis
In clinical studies, all validated questionnaires were scored and analyzed according to published guidelines. Normally distributed continuous variables were expressed as mean ± standard deviation and compared using a two-sided t-test. Abnormally distributed continuous variables are expressed as medians (upper and lower quartiles) and compared using the Wilcoxon rank-sum test. Categorical variables are expressed as the number of cases (percentage) using the contingency table chi-square test. To identify variables related to SAS, SDS, and PSS scores. For categorical variables, the patients with PCOS were grouped and compared using two-sided t-tests. For continuous variables, Spearman’s correlation was used to measure the relationships. Restrictive cubic splines were used to explore the relationship between body mass index (BMI) and negative emotions. To verify this relationship, we grouped patients with PCOS by BMI and used one-way analysis of variance (ANOVA) for comparison, followed by post-hoc tests using the Tukey method. All statistical analyses in the clinical studies were conducted using R software version 4.2.3 (R Foundation, Vienna, Austria. www.r-project.org).
In animal studies, data are expressed as mean ± standard error of the mean. We used the Shapiro–Wilk test to assess the normality of the data distribution. One-way or two-way ANOVA followed by Tukey’s post-hoc test, was used to analyze differences among groups. A Kruskal–Wallis test was performed because the values did not pass the normality test. p < 0.05 was considered statistically significant.
Results
Participants and clinical characteristics
Our study enrolled 608 women with PCOS and 184 healthy women in the final analysis. The basic information is presented in Supplement Table 2. No differences were found in the mean age and height between the PCOS and control groups. Compared to the control group, women in the PCOS group displayed obesity features more frequently, with a larger weight (66.27 ± 13.43 in PCOS, 63.03 ± 11.69 in control, p < 0.001), body mass index (BMI) (25.56 ± 5.02 in PCOS, 24.13 ± 4.28 in control, p < 0.001), waist circumference (85.08 ± 12.28 in PCOS, 81.73 ± 10.22 in control, p < 0.001), hip circumference (101.11 ± 9.76 in PCOS, 98.86 ± 8.8 in control, p < 0.05) and waist-to-hip ratio (0.84 ± 0.07 in PCOS, 0.83 ± 0.06 in control, p < 0.05). In addition, women with PCOS displayed acne [135 (24.3%) in PCOS, 7 (3.8%) in control, p < 0.001] and hirsutism [298 (53.6%) in PCOS, 0 (0%) in control, p < 0.001] more frequently than women without PCOS, although they displayed similar rates of baldness [18 (3.3%) in PCOS, 2 (1.1%) in control, p = 0.191]. Women in the PCOS group had fewer reported regular menstrual cycles [119 (21.6%) in PCOS, 113 (65.7%) in control, p < 0.001].
No difference was observed in serum prolactin or follicle-stimulating hormone (FSH). However, women with PCOS had significantly higher levels of LH [6.54 (3.7, 11.17) in PCOS, 3.84 (2.43, 5.32) in control, p < 0.001], LH/FSH [1.18 (0.67, 1.86) in PCOS, 0.63 (0.42, 0.89) in control, p < 0.001], estradiol [184 (136.75, 240) in PCOS, 160 (116.75, 208.75) in control, p < 0.001], total testosterone [1.06 (0.69, 1.52) in PCOS, 0.69 (0.69, 0.82) in control, p < 0.001], and androstenedione [12.7 (8.77, 16.75) in PCOS, 7.39 (5.32, 10.2) in control, p < 0.001] than control participants.
Women with PCOS scored significantly higher on the self-reported scales for anxiety (SAS; 44.89 ± 7.96 in PCOS, 41.17 ± 7.35 in control, p < 0.001), self-depression (SDS; 47.1 ± 10.54 in PCOS, 43.49 ± 9.27 in control, p < 0.001), and perceived stress (PSS; 24.89 ± 7.57 in PCOS, 23.46 ± 7.27 in control, p < 0.05) than control participants. Taken together, these findings indicate several subjective and objective differences, including negative emotions, in women with PCOS compared to healthy controls.
Variables correlated with negative emotions in PCOS
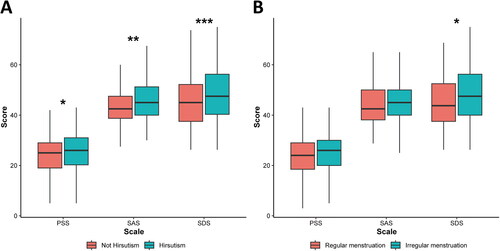
Next, we examined the variables that were correlated with the negative emotional state of women with PCOS. Among the PCOS symptoms, patients with hirsutism had higher SAS (p < 0.001), SDS (p < 0.05), and PSS (p < 0.05) (; Supplement Table 3) scores. Among patients with PCOS, SDS scores were higher (; p < 0.05) in those with irregular menstruation compared to patients with regular menstruation.
Figure 1. Boxplots of emotional scales for PCOS patients with different symptoms. (A) Hirsutism vs. no hirsutism. (B) Menstrual irregularity vs. menstrual regularity. These groups were compared by Wilcoxon rank sum test, *p < 0.05, **p < 0.01,***p < 0.001 as indicated. Borders of box represent 25th and 75th percentiles; internal line within large shaded box represents median; Central dot represents mean; and external whisker lines represent 1.5× interquartile range.
Although the correlation coefficients were low, interesting results were obtained. In patients with PCOS, hormones (LH and LH/FSH) were more strongly correlated with SAS and SDS scores than with body parameters (BMI, waist circumference, hip circumference, and waist-hip ratio) (Supplement Table 4). Moreover, unintuitively, the correlation between body parameters and SAS scores observed in the control group was not observed in the PCOS group (Supplement Table 4).
Relationship between negative emotions with BMI in PCOS
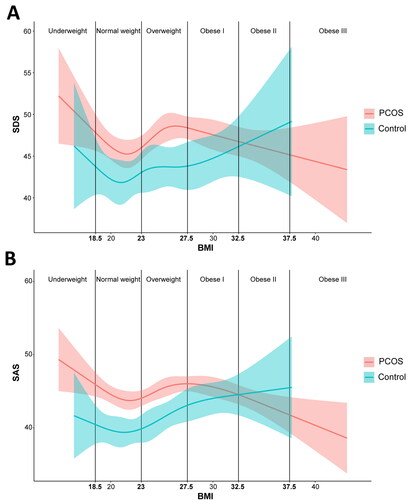
When exploring the factors related to emotional state, we were surprised to find that BMI, waist circumference, and hip circumference showed a linear correlation with SAS only in the control group, but not in the PCOS group, implying a more complex non-linear relationship between body parameters and negative emotions in the PCOS group. Therefore, we used restricted cubic splines to automatically set the node positions to flexibly model the association between BMI and emotions. As shown in , with an increase in BMI, the SDS and SAS scores in control subjects showed a U-shaped curve, whereas the SDS and SAS scores in patients with PCOS showed a N-shaped curve. Particularly, women with PCOS and comorbid obesity tended to show lower levels of negative emotions with increasing BMI.
Figure 2. Restricted cubic spline analysis of the non-linear relationship between BMI and negative emotions for PCOS and control groups. (A) Relationship between BMI and SDS. (B) Relationship between BMI and SAS. The vertical line is the WHO Asian classification standard of BMI. Residuals are represented by shadows.
To further verify this finding, we grouped the patients with PCOS according to the WHO Asian BMI classification standards (Underweight: BMI < 18.5; Normal weight: 18.5 ≤ BMI < 23; Overweight: 23 ≤ BMI < 27.5; Obese I: 27.5 ≤ BMI < 32.5; Obese II: 32.5 ≤ BMI < 37.5; Obese III: BMI ≥ 37.5) [Citation27]. Because the number of people in the Obese III group (n = 14) was too low, we combined the Obese II and Obese III groups (n = 59). As shown in , significant differences were observed in SAS scores. The obese II and III groups showed a significant decrease in SAS compared with the Underweight (p < 0.05), Overweight (p < 0.05), and Obese I groups (p < 0.05). Although no significant difference was found among the groups in the SDS score, the trend of change was consistent with our hypothesis (). To further investigate whether the negative correlation between negative emotions and BMI in obese PCOS individuals is caused by abnormalities in glucose and lipid metabolism, we analyzed the correlation between negative emotions and fasting plasma glucose (FPG), fasting insulin (INS), triglyceride (TG), and high-density lipoprotein (HDL) in obese PCOS individuals. We found that in obese PCOS patients, the correlation between FPG, INS, TG, HDL, and negative emotions was not significant (Supplement Figure 1). However, relatively, lipid metabolism, particularly TG, showed a stronger correlation with negative emotions compared to glucose metabolism.
Figure 3. Boxplots of negative emotions and LH level of PCOS patients in different BMI groups. (A) SAS scores in different BMI groups. (B) SDS score in different BMI groups. (C) LH level in different BMI groups. (D) LH/FSH ratio in different BMI groups. A one-way ANOVA was conducted to compare the differences among these groups, followed by post-hoc tests using the Tukey method, *p < 0.05 as indicated. Borders of box represent 25th and 75th percentiles; internal line within large shaded box represents median; Central dot represents mean; and external whisker lines represent 1.5× interquartile range.
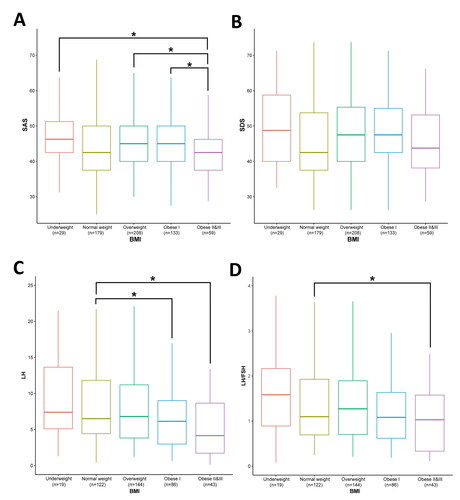
We also found that LH levels were associated with BMI in patients with PCOS. As shown in , the Obese I, Obese II, and Obese III groups showed significant decreases in LH levels compared with the normal weight group (p < 0.05). In addition, the obese II and III groups showed a significant decrease in the LH/FSH ratio compared to the normal-weight group (; p < 0.05).
High-fat diet gained weight in DHEA-induced PCOS rats
The experimental design is shown in Supplement Figure 2A. The results showed that body weight did not change significantly in the DHEA group compared to that in the control group (). Body weights were significantly higher in the 45% and 60% high-fat diet (HFD) group. The estrous cycle stages were identified according to cell type (Supplement Figure 2D). The vaginal smear results showed that the estrous cycle was disrupted by DHEA exposure (Supplement Figure 2E). After intervention with the 45 and 60% high-fat diet, the estrous cycle remained irregular. Normal ovarian morphology was observed in control rats (Supplement Figure 2F). Compared with the control group, rats in the DHEA, DHEA + 45% HFD, and DHEA + 60% HFD groups showed a remarkable cyst-like follicular appearance.
High-fat diet reversed negative emotions in DHEA-induced PCOS rats
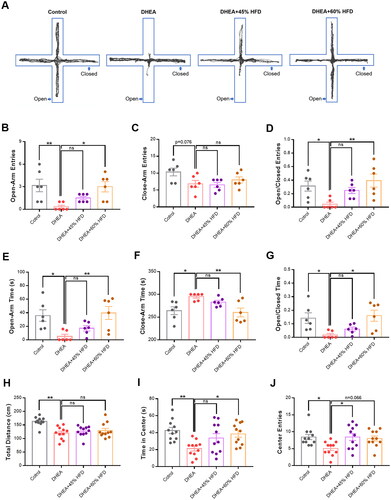
Representative activity traces of rats in the elevated plus maze test are shown in . Rats in the DHEA group exhibited fewer entries into the open arm than those in the control group (). However, compared with the DHEA group, entry into the open arm was significantly increased in the DHEA + 60% HFD group. For the closed-arm entries, the opposite trend was observed. Rats in the DHEA group also spent less time in the open arm than did rats in the control group (). After being fed a 60% HFD, the rats spent significant time in the open arm and less time in the closed arm. In the open field test, the total distance moved was lower in the DHEA group than in the control group (). HFD did not significantly change the total distance moved. Rats in the DHEA group spent less time in the central zone than those in the control group (). However, the 60% HFD decreased the time spent in the center zone. Similarly, DHEA decreased entry into the central zone, whereas a high-fat diet reversed this effect ().
Figure 4. Effect of high-fat diet on anxiety- and depressive-like behaviors in DHEA-induced PCOS rats. (A) Representative activity traces of rats in the elevated plus maze test. (B) Frequency of entries into open arm. (C) Frequency of entries into close arm. (D) Entries into open-to-close arm ratio. (E) Time spent in open arm. (F) Time spent in close arm. (G) Time spent in open-to-close arm ratio. (H) Total distance moved. (I) Time spent in the center zone. (J) Frequency of entries into the center zone. *p < 0.05, **p < 0.01, compared as indicated. ns: not statistically significant.
High-fat diet depressed the mRNA and protein levels of several hormones in PCOS rats
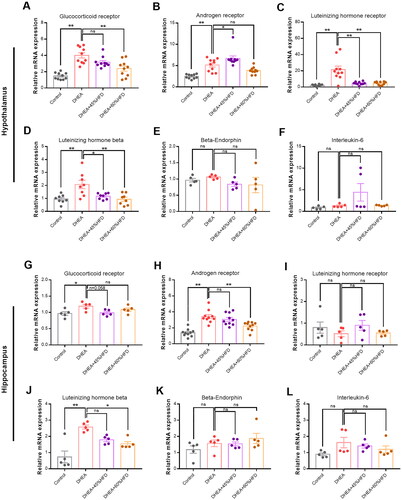
Hypothalamic and hippocampal mRNA levels of the glucocorticoid receptor, androgen receptor, and LHβ were evaluated using qPCR. Compared with the control group, the mRNA levels of the glucocorticoid receptor, androgen receptor, LH receptor, and LHβ in the hypothalamus were significantly decreased by DHEA injection (). However, the decrease in mRNA expression was reversed by a high-fat diet. No significant differences were found between groups in the hypothalamic mRNA expression of beta-endorphin and interleukin-6 (). Compared with the control group, the hippocampal mRNA levels of glucocorticoid receptor, androgen receptor, and LHβ were significantly increased by DHEA injection (). An HFD reversed this abnormal increase in mRNA levels. No significant differences were found among the groups in the hippocampal mRNA expression of the LH receptor, beta-endorphin, and interleukin-6 ().
Figure 5. Effect of high-fat diet on mRNA levels in DHEA-induced PCOS rats. (A–F) The mRNA levels of glucocorticoid receptor, androgen receptor, LH receptor, LHβ, β-endorphin, and interleukin-6 in hypothalamus. (G–L) The mRNA levels of glucocorticoid receptor, androgen receptor, LH receptor, LHβ, β-endorphin, and interleukin-6 in hippocampus. *p < 0.05, **p < 0.01, compared as indicated. ns: not statistically significant.
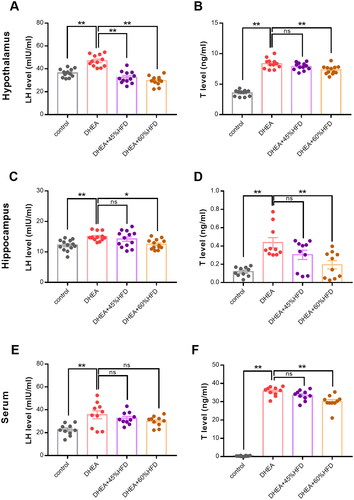
The protein levels of LH and testosterone in the hypothalamus, hippocampus, and serum were measured using a radioimmunoassay. Compared with the control group, the protein levels of LH and testosterone in the hypothalamus dramatically increased after DHEA injection (). This abnormal increase was significantly suppressed by HFD in rats with DHEA-induced PCOS. The protein levels of LH and testosterone in the hippocampus were similar to those in the hypothalamus (). DHEA increased protein levels, which were reversed by high-fat diet. In the serum, DHEA also increased the protein levels of LH and testosterone (). Similarly, high-fat diet suppressed the increased expression of testosterone. However, the expression of LH was not changed by high-fat diet.
Figure 6. Effect of high-fat diet on protein levels in DHEA-induced PCOS rats. (A,B) Hypothalamic protein levels of LH and testosterone. (C,D) Hippocampal protein levels of LH and testosterone. (E,F) Serum protein levels of LH and testosterone. *p < 0.05, **p < 0.01, compared as indicated. ns: not statistically significant.
Discussion
Our study showed that women with PCOS had higher weight, waist circumference, and hip circumference than those without PCOS. This is consistent with previous findings that women with PCOS are more likely to be overweight or have obesity [Citation28]. We also found that women with PCOS had increased depression, anxiety, and perceived stress compared with women who did not report PCOS, which is consistent with previous studies [Citation5,Citation29]. In addition, the prevalence of depression and anxiety in women with PCOS was higher than that in the general infertile population [Citation11]. Higher body weight appears to be associated with increased negative emotions in patients with PCOS [Citation30]. Instead, our results suggest that obesity is associated with lower levels of negative emotions in PCOS. In more specific terms, we categorized PCOS patients with weight beyond the normal range into overweight and different degrees of obesity groups, and found significant differences in the levels of negative emotions among overweight and obese PCOS populations. Additionally, alterations in the hypothalamic-pituitary-gonadal (HPG) axis driven by obesity in women with PCOS may explain this phenomenon.
Hyperandrogenemia and its consequent clinical manifestations (e.g. hirsutism, acne, and irregular menstruation) are widespread in women with PCOS. It is considered one of the chief causes of PCOS [Citation31]. Excess androgen levels have been linked to increased levels of depression and anxiety in women with PCOS [Citation32]. Our results indicated that hirsutism was significantly associated with negative emotions. Consistent with our findings, a previous study showed that hirsutism may lead to high levels of depression and anxiety in women with PCOS [Citation33]. Changes in appearance and body image dissatisfaction may cause profound depression in women with PCOS [Citation34]. In addition, menstrual irregularity, infertility, and acne-prone skin cause significant societal pressure, resulting in a significant decline in their life satisfaction. Menstrual irregularity was associated with SDS scores. A recent study showed that women with PCOS who reported irregular menstrual cycles were more susceptible to depression [Citation35]. Stress and depression are risk factors for irregular menstrual cycles in young women [Citation36]. In PCOS, menstrual irregularities and negative emotions may reinforce one another and aggravate the disorder.
Body parameters including BMI, waist circumference, and hip circumference were associated with negative emotions in our study. Appearance anxiety usually manifests as excessive anxiety about a certain physical appearance, which may partially account for this finding [Citation37]. Women who are overweight or obese are more likely to have psychiatric disorders than those with a normal BMI [Citation38]. A recent study suggested that individuals with higher BMI relied more on self-criticism, which significantly predicted anxiety and depressive symptoms [Citation39]. Increased waist circumference is associated with worsening interpersonal functioning and may therefore increase the risk of depression [Citation40]. Waist and hip circumference were only associated with anxiety and depression in women without PCOS. This is likely because other more serious symptoms, such as infertility and menstrual disorders, affect women with PCOS.
Women with PCOS usually have high serum LH [Citation41]. Circulating LH levels are correlated with depression and anxiety [Citation42]. Low serum testosterone levels have been associated with major depression, and testosterone supplementation may improve depressive symptoms [Citation43,Citation44]. Serum testosterone levels were significantly higher in patients with PCOS [Citation45]. High levels of stress hormones are associated with anxiety- and depression-like behaviors [Citation46]. Corticosterone has been widely used to induce anxiety- and depression-like behaviors in animal models [Citation47,Citation48]. In the present study, LH and testosterone, and glucocorticoid receptor levels were positively associated with negative emotions in patients with PCOS. Cortisol and testosterone play major roles in regulating the associations of most hormones associated with depression and anxiety [Citation49]. Changes in hormonals may lead to anxiety, depression might be a secondary reflection of these changes [Citation50].
In the present study, negative emotions were negatively correlated with BMI in women with PCOS and obesity. The results of this clinical study were confirmed in our animal experiments. We observed that women who were overweight with PCOS were more anxious and depressed with an increase in BMI, which is consistent with the results of previous studies. However, anxiety and depression were negatively correlated with BMI in women with obesity and PCOS. PCOS rats fed a 60% HFD also showed attenuated anxiety-like behaviors. Previous studies have generally suggested that the severity of obesity is directly proportional to or unrelated to the levels of anxiety and depression. Emeksiz et al. found that a higher BMI was linked to increased levels of both depression and anxiety [Citation51]. A study conducted in Turkey revealed that BMI was not significantly correlated with depression or anxiety in women with PCOS [Citation52]. Similarly, another study reported no correlation between weight gain and depression or anxiety in women with PCOS [Citation53]. Certainly, some studies align with our research findings. In a previous study, it was observed that compared to men with normal weight, obese men had a significantly lower probability of experiencing severe depression or suicidal thoughts. This suggests that weight gain may potentially be beneficial in combating adverse emotional states [Citation54]. In another study, higher body weight was found to be linked to fewer pessimistic thoughts [Citation55]. Obesity was found to be inversely associated with anxiety and depression in adolescent girls with PCOS, suggesting that higher weight levels corresponded to lower levels of depression and anxiety [Citation56]. The reasons for these conflicting results are complex. Firstly, our results indicate a positive correlation between weight and negative emotions in the overweight stage, but a negative correlation between weight and negative emotions in the obese stage. Therefore, studies that do not differentiate between being overweight and obese may yield two entirely opposing research outcomes. Secondly, numerous factors influence the correlation between weight and emotions, such as different ages, races, aesthetic preferences, sample sizes, and classifications of obesity, which may lead to varied conclusions. Thirdly, several confounding factors, including severe glucose/lipid metabolism disorders and hormonal disturbances, may play a role in regulating emotions among women with PCOS and concurrent obesity. Based on the results of our study, obesity alone cannot be directly deemed as a cause for improved mood in women with PCOS.
Given the intricate relationship between obesity and glucose-lipid metabolism, we examined the correlation between glucose-lipid metabolism abnormalities and negative emotions in obese PCOS patients. While indicators related to glucose-lipid metabolism in these patients did not exhibit significant correlations with depressive and anxiety stress emotions, heightened triglyceride levels, specifically, appeared to be notably associated with the amelioration of negative emotions. According to studies, a medium-chain triglyceride diet may reduce enzymes involved in glycolysis and oxidative phosphorylation, enhance proteins regulating glucose and glutamate transport, and potentially demonstrate an anti-anxiety effect [Citation57]. This insight offers a plausible rationale for the outcomes of this research. Our observations indicated that a high-fat diet worsened glucose-lipid metabolism irregularities in rats, yet the rodents exhibited an improvement in anxiety and depressive emotions. This indicates that, at least within animal models, emotional enhancements do not strongly correlate with glucose-lipid metabolism abnormalities and may be more linked to sex hormones. The discrepancy between clinical and basic research could be attributed to the limitations of our animal model in accurately mirroring human diseases and the influence of diverse, intricate factors impacting emotions.
The research team led by Elisabet Stener-Victorin has conducted many studies on the weight and anxiety/depression emotions in PCOS. An interesting study by the team suggested that offspring of diet-induced obese PCOS mice exhibit more prominent anxiety-like behaviors [Citation58], and this effect can be transmitted transgenerationally to the third-generation descendants of androgenized lineage [Citation59]. Their research also indicates that PCOS women show greater concern about their weight, suggesting that weight-related anxiety may be one of the psychological symptoms of PCOS [Citation60].
This study had several limitations. First, we only found that obesity was associated with negative emotions in PCOS and no causal relationship could be concluded from the present results. Second, as this was a cross-sectional study, it was impossible to estimate attenuated negative emotions as causes or consequences of obesity. A cohort study based on different BMIs in women with PCOS may help solve this problem. Third, the mechanistic analysis in our study was not sufficiently detailed. Further studies using inhibitors or gene-knockout mice are warranted to explore these potential mechanisms. Moreover, we used 45 and 60% HFD to induce different levels of obesity in the PCOS animal models in this study. However, the amount of fat in the diet does not equate to the degree of obesity. Therefore, it may be preferable to use animals with pre-established varying degrees of obesity.
Conclusion
To our knowledge, this is the first study to investigate the relationship between BMI and negative emotions in patients with PCOS. Results showed that obesity was negatively correlated with negative emotions in patients with PCOS. Obesity may affect the downregulation of LH and testosterone and participate in the regulation of emotions. Increased BMI may be beneficial for patients with PCOS in terms of the psychological aspects. Further studies are required to verify our findings and elucidate the underlying mechanisms.
Authors contributions
Z.H.L., L.D., and L.R. conceived and designed the research. Z.H.L., Y.Y., J.Y.X., Z.L.F., and L.J.Y. performed experiments. Z.Y., L.S., J.P.J., Y.Y., Z.L., Z.H., C.M.S., and J.H. interpreted the results of experiments. Y.Y., J.P.J., Z.L.F., L.J.Y., and J.Y.X. prepared the figures. Z.H.L. and Y.Y. drafted the manuscript. Z.H.L., Y.Y., and L.D. edited and revised the manuscript. All the authors were responsible for reviewing the data. In addition, all the authors confirmed the final manuscript.
| Abbreviations | ||
| A4 | = | androstenedione |
| ANOVA | = | one-way analysis of variance |
| BMI | = | body mass index |
| DHEA | = | dehydroepiandrosterone |
| E2 | = | estrogen |
| FPG | = | fasting plasma glucose |
| FSH | = | follicle-stimulating hormone |
| HDL | = | high-density lipoprotein |
| HFD | = | high-fat diet |
| INS | = | fasting insulin |
| LH | = | luteinizing hormone |
| PCOS | = | polycystic ovary syndrome |
| PRL | = | prolactin |
| qPCR | = | quantitative polymerase chain reaction |
| SAS | = | Self-Rating Anxiety Scale |
| SDS | = | Self-Rating Depressive Scale |
| T | = | testosterone |
| TG | = | triglyceride |
Supplemental Material
Download PDF (463.8 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Hoeger KM, Dokras A, Piltonen T. Update on PCOS: consequences, challenges, and guiding treatment. J Clin Endocrinol Metab. 2021;106(3):1–16. doi: 10.1210/clinem/dgaa839.
- Zhang J, Zhang H, Xin X, et al. Efficacy of flavonoids on animal models of polycystic ovary syndrome: a systematic review and meta-analysis. Nutrients. 2022;14(19):4128. doi: 10.3390/nu14194128.
- Ye Y, Zhou CC, Hu HQ, et al. Underlying mechanisms of acupuncture therapy on polycystic ovary syndrome: evidences from animal and clinical studies. Front Endocrinol. 2022;13:1035929. doi: 10.3389/fendo.2022.1035929.
- Tay CT, Teede HJ, Hill B, et al. Increased prevalence of eating disorders, low self-esteem, and psychological distress in women with polycystic ovary syndrome: a community-based cohort study. Fertil Steril. 2019;112(2):353–361. doi: 10.1016/j.fertnstert.2019.03.027.
- Damone AL, Joham AE, Loxton D, et al. Depression, anxiety and perceived stress in women with and without PCOS: a community-based study. Psychol Med. 2019;49(9):1510–1520. doi: 10.1017/S0033291718002076.
- Himelein MJ, Thatcher SS. Polycystic ovary syndrome and mental health: a review. Obstet Gynecol Surv. 2006;61(11):723–732. doi: 10.1097/01.ogx.0000243772.33357.84.
- Rodriguez-Paris D, Remlinger-Molenda A, Kurzawa R, et al. Psychiatric disorders in women with polycystic ovary syndrome. Psychiatr Pol. 2019;53(4):955–966. doi: 10.12740/PP/OnlineFirst/93105.
- Kourtidou C, Tziomalos K. Pharmacological management of obesity in patients with polycystic ovary syndrome. Biomedicines. 2023;11(2):496. doi: 10.3390/biomedicines11020496.
- Lim SS, Norman RJ, Davies MJ, et al. The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obes Rev. 2013;14(2):95–109. doi: 10.1111/j.1467-789X.2012.01053.x.
- Zhang H, Wang W, Zhao J, et al. Relationship between body composition, insulin resistance, and hormonal profiles in women with polycystic ovary syndrome. Front Endocrinol. 2022;13:1085656. doi: 10.3389/fendo.2022.1085656.
- Cooney LG, Dokras A. Depression and anxiety in polycystic ovary syndrome: etiology and treatment. Curr Psychiatry Rep. 2017;19(11):83. doi: 10.1007/s11920-017-0834-2.
- Zehravi M, Maqbool M, Ara I. Depression and anxiety in women with polycystic ovarian syndrome: a literature survey. Int J Adolesc Med Health. 2021;33(6):367–373. doi: 10.1515/ijamh-2021-0092.
- Dokras A, Sarwer DB, Allison KC, et al. Weight loss and lowering androgens predict improvements in health-related quality of life in women with PCOS. J Clin Endocrinol Metab. 2016;101(8):2966–2974. doi: 10.1210/jc.2016-1896.
- Burnatowska E, Wikarek A, Oboza P, et al. Emotional eating and binge eating disorders and night eating syndrome in polycystic ovary syndrome–a vicious circle of disease: a systematic review. Nutrients. 2023;15(2):295. doi: 10.3390/nu15020295.
- Franks S. Controversy in clinical endocrinology: diagnosis of polycystic ovarian syndrome: in defense of the Rotterdam criteria. J Clin Endocrinol Metab. 2006;91(3):786–789. doi: 10.1210/jc.2005-2501.
- Polak AM, Adamska A, Krentowska A, et al. Body composition, serum concentrations of androgens and insulin resistance in different polycystic ovary syndrome phenotypes. J Clin Med. 2020;9(3):732. doi: 10.3390/jcm9030732.
- Balen AH, Laven JS, Tan SL, et al. Ultrasound assessment of the polycystic ovary: international consensus definitions. Hum Reprod Update. 2003;9(6):505–514. doi: 10.1093/humupd/dmg044.
- Xu H, Shi L, Feng G, et al. An ovarian reserve assessment model based on anti-mullerian hormone levels, follicle-stimulating hormone levels, and age: retrospective cohort study. J Med Internet Res. 2020;22(9):e19096. doi: 10.2196/19096.
- Ruiz-Fernández MD, Ramos-Pichardo JD, Ibáñez-Masero O, et al. Compassion fatigue, burnout, compassion satisfaction and perceived stress in healthcare professionals during the COVID-19 health crisis in Spain. J Clin Nurs. 2020;29(21–22):4321–4330. doi: 10.1111/jocn.15469.
- Dunstan DA, Scott N. Norms for Zung’s Self-rating Anxiety Scale. BMC Psychiatry. 2020;20(1):90. doi: 10.1186/s12888-019-2427-6.
- Jokelainen J, Timonen M, Keinänen-Kiukaanniemi S, et al. Validation of the Zung Self-Rating Depression Scale (SDS) in older adults. Scand J Prim Health Care. 2019;37(3):353–357. doi: 10.1080/02813432.2019.1639923.
- Zhang H, Yi M, Zhang Y, et al. High-fat diets exaggerate endocrine and metabolic phenotypes in a rat model of DHEA-induced PCOS. Reproduction. 2016;151(4):431–441. doi: 10.1530/REP-15-0542.
- Yeom M, Ahn S, Jang SY, et al. Acupuncture attenuates comorbid anxiety- and depressive-like behaviors of atopic dermatitis through modulating neuroadaptation in the brain reward circuit in mice. Biol Res. 2022;55(1):28. doi: 10.1186/s40659-022-00396-0.
- Cavichioli AM, Santos-Silva T, Grace AA, et al. Levetiracetam attenuates adolescent stress-induced behavioral and electrophysiological changes associated with schizophrenia in adult rats. Schizophr Bull. 2022;49(1):68–77. doi: 10.1093/schbul/sbac106.
- Knight P, Chellian R, Wilson R, et al. Sex differences in the elevated plus-maze test and large open field test in adult Wistar rats. Pharmacol Biochem Behav. 2021;204:173168. doi: 10.1016/j.pbb.2021.173168.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262.
- Consultation WHOE. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–163. doi: 10.1016/S0140-6736(03)15268-3.
- Gill L, Coborn JE, Hoovler AR, et al. Polycystic ovary syndrome and obesity: a cross-sectional survey of patients and obstetricians/gynecologists. J Womens Health. 2023;32(6):723–731. doi: 10.1089/jwh.2022.0471.
- Light RS, Chilcot J, McBride E. Psychological distress in women living with polycystic ovary syndrome: the role of illness perceptions. Womens Health Issues. 2021;31(2):177–184. doi: 10.1016/j.whi.2020.11.003.
- Kolhe JV, Chhipa AS, Butani S, et al. PCOS and depression: common links and potential targets. Reprod Sci. 2022;29(11):3106–3123. doi: 10.1007/s43032-021-00765-2.
- Wang J, Wu D, Guo H, et al. Hyperandrogenemia and insulin resistance: the chief culprit of polycystic ovary syndrome. Life Sci. 2019;236:116940. doi: 10.1016/j.lfs.2019.116940.
- Dokras A, Stener-Victorin E, Yildiz BO, et al. Androgen Excess-Polycystic Ovary Syndrome Society: position statement on depression, anxiety, quality of life, and eating disorders in polycystic ovary syndrome. Fertil Steril. 2018;109(5):888–899. doi: 10.1016/j.fertnstert.2018.01.038.
- Clayton WJ, Lipton M, Elford J, et al. A randomized controlled trial of laser treatment among hirsute women with polycystic ovary syndrome. Br J Dermatol. 2005;152(5):986–992. doi: 10.1111/j.1365-2133.2005.06426.x.
- Scaruffi E, Franzoi IG, Civilotti C, et al. Body image, personality profiles and alexithymia in patients with polycystic ovary syndrome (PCOS). J Psychosom Obstet Gynaecol. 2019;40(4):294–303. doi: 10.1080/0167482X.2018.1530210.
- Vishnubhotla DS, Tenali SN, Fernandez M, et al. Evaluation of prevalence of PCOS and associated depression, nutrition, and family history: a questionnaire-based assessment. Indian J Endocrinol Metab. 2022;26(4):341–347. doi: 10.4103/ijem.ijem_467_21.
- Bhardwaj P, Yadav SK, Taneja J. Magnitude and associated factors of menstrual irregularity among young girls: a cross-sectional study during COVID-19 second wave in India. J Family Med Prim Care. 2022;11(12):7769–7775. doi: 10.4103/jfmpc.jfmpc_1201_22.
- Gao J, Feng Y, Xu S, et al. Appearance anxiety and social anxiety: a mediated model of self-compassion. Front Public Health. 2023;11:1105428. doi: 10.3389/fpubh.2023.1105428.
- Zhao G, Ford ES, Dhingra S, et al. Depression and anxiety among US adults: associations with body mass index. Int J Obes. 2009;33(2):257–266. doi: 10.1038/ijo.2008.268.
- Carter A, Hoang N, Gilbert P, et al. Body weight perception outweighs body weight when predicting shame, criticism, depression and anxiety for lower BMI range and higher BMI range adults. J Health Psychol. 2022;27(10):2276–2290. doi: 10.1177/13591053211027641.
- Yusufov M, Kopeski LM, Silverman AL, et al. Associations of body weight and waist circumference with psychopathology, substance use, and well-being in an adult transdiagnostic sample. J Affect Disord. 2021;281:279–288. doi: 10.1016/j.jad.2020.12.029.
- Tata B, Mimouni NEH, Barbotin AL, et al. Elevated prenatal anti-Mullerian hormone reprograms the fetus and induces polycystic ovary syndrome in adulthood. Nat Med. 2018;24(6):834–846. doi: 10.1038/s41591-018-0035-5.
- Ðoković DD, Jović JJ, Ðoković JD, et al. Effects of hormone replacement therapy on depressive and anxiety symptoms after oophorectomy. Med Glas. 2015;12(1):79–85.
- Khera M. Patients with testosterone deficit syndrome and depression. Arch Esp Urol. 2013;66(7):729–736.
- Giltay EJ, Enter D, Zitman FG, et al. Salivary testosterone: associations with depression, anxiety disorders, and antidepressant use in a large cohort study. J Psychosom Res. 2012;72(3):205–213. doi: 10.1016/j.jpsychores.2011.11.014.
- Song Y, Ye W, Ye H, et al. Serum testosterone acts as a prognostic indicator in polycystic ovary syndrome-associated kidney injury. Physiol Rep. 2019;7(16):e14219. doi: 10.14814/phy2.14219.
- Kott JM, Mooney-Leber SM, Shoubah FA, et al. Effectiveness of different corticosterone administration methods to elevate corticosterone serum levels, induce depressive-like behavior, and affect neurogenesis levels in female rats. Neuroscience. 2016;312:201–214. doi: 10.1016/j.neuroscience.2015.11.006.
- Peng B, Xu Q, Liu J, et al. Corticosterone attenuates reward-seeking behavior and increases anxiety via D2 receptor signaling in ventral tegmental area dopamine neurons. J Neurosci. 2021;41(7):1566–1581. doi: 10.1523/JNEUROSCI.2533-20.2020.
- Bai G, Qiao Y, Lo PC, et al. Anti-depressive effects of Jiao-Tai-Wan on CORT-induced depression in mice by inhibiting inflammation and microglia activation. J Ethnopharmacol. 2022;283:114717. doi: 10.1016/j.jep.2021.114717.
- Chronister BN, Gonzalez E, Lopez-Paredes D, et al. Testosterone, estradiol, DHEA and cortisol in relation to anxiety and depression scores in adolescents. J Affect Disord. 2021;294:838–846. doi: 10.1016/j.jad.2021.07.026.
- Balikci A, Erdem M, Keskin U, et al. Depression, anxiety, and anger in patients with polycystic ovary syndrome. Noro Psikiyatr Ars. 2014;51(4):328–333. doi: 10.5152/npa.2014.6898.
- Emeksiz HC, Bideci A, Nalbantoğlu B, et al. Anxiety and depression states of adolescents with polycystic ovary syndrome. Turk J Med Sci. 2018;48(3):531–536. doi: 10.3906/sag-1708-131.
- Öztürk A, Kucur SK, Seven A, et al. Temperament and character differences of patients with polycystic ovary syndrome. J Gynecol Obstet Hum Reprod. 2019;48(4):255–259. doi: 10.1016/j.jogoh.2019.01.006.
- Awoke MA, Earnest A, Joham AE, et al. Weight gain and lifestyle factors in women with and without polycystic ovary syndrome. Hum Reprod. 2021;37(1):129–141. doi: 10.1093/humrep/deab239.
- Goldney RD, Dunn KI, Air TM, et al. Relationships between body mass index, mental health, and suicidal ideation: population perspective using two methods. Aust N Z J Psychiatry. 2009;43(7):652–658. doi: 10.1080/00048670902970825.
- Berlin I, Lavergne F. Relationship between body-mass index and depressive symptoms in patients with major depression. Eur Psychiatry. 2003;18(2):85–88. doi: 10.1016/s0924-9338(03)00007-5.
- Zachurzok A, Pasztak-Opilka A, Gawlik AM. Depression, anxiety and self-esteem in adolescent girls with polycystic ovary syndrome. Ginekol Pol. 2021;92(6):399–405. doi: 10.5603/GP.a2021.0042.
- Hollis F, Mitchell ES, Canto C, et al. Medium chain triglyceride diet reduces anxiety-like behaviors and enhances social competitiveness in rats. Neuropharmacology. 2018;138:245–256. doi: 10.1016/j.neuropharm.2018.06.017.
- Manti M, Fornes R, Qi XJ, et al. Maternal androgen excess and obesity induce sexually dimorphic anxiety-like behavior in the offspring. FASEB J. 2018;32(8):4158–4171. doi: 10.1096/fj.201701263RR.
- Risal S, Manti M, Lu HJ, et al. Prenatal androgen exposure causes a sexually dimorphic transgenerational increase in offspring susceptibility to anxiety disorders. Transl Psychiatry. 2021;11(1):45. doi: 10.1038/s41398-020-01183-9.
- Larsson I, Hulthén L, Landén M, et al. Dietary intake, resting energy expenditure, and eating behavior in women with and without polycystic ovary syndrome. Clin Nutr. 2016;35(1):213–218. doi: 10.1016/j.clnu.2015.02.006.
