Abstract
Background
Multiple myeloma is a malignant tumour of the blood in which abnormal proliferation of plasma cells leads to bone destruction, renal impairment, anaemia, and hypercalcaemia. Renal impairment caused by multiple myeloma is a common and serious condition; however, the prognosis of multiple myeloma at the time of diagnosis remains unclear.
Method
We conducted searches for literature in PubMed, Web of Science, Cochrane, Embase, CNKI, Wanfang, and VIP databases up to 30 April 2023. Progression-free survival and overall survival with and without renal impairment at the time of multiple myeloma diagnosis were compared, and prognostic indicators were analysed.
Results
Six studies were finally included. Among patients with multiple myeloma, 319 had renal impairment, and 1166 had no renal impairment. Compared to the control group, no significant difference was observed in overall or progression-free survival in patients with multiple myeloma complicated with renal impairment.
Conclusion
The limited low-quality evidence available does not support an association between prognosis and multiple myeloma complicated by kidney injury.
Introduction
Multiple myeloma is a haematologic malignant tumour characterised by abnormal hyperplasia of monoclonal plasma cells in the bone marrow [Citation1]. The abnormal proliferation of plasma cells leads to bone destruction, renal impairment, anaemia, and hypercalcaemia. Approximately 588,161 people worldwide are diagnosed with multiple myeloma annually [Citation2,Citation3]. Symptomatic multiple myeloma is usually accompanied by an acute kidney injury caused by various mechanisms. The most common form of monoclonal free light chain nephropathy is light chain tubular nephropathy, caused by precipitation of the free light chain in the distal convoluted tubules with urinary regulatory factors [Citation4]. Excessive production of free light chains can induce renal damage and fibrosis, leading to decreased quality of life and a poor patient prognosis [Citation5]. In recent years, highly truncated haemodialysis has been used to remove the free light chain to avoid direct damage to the kidney and improve the adverse effects of multiple myeloma [Citation6].
Some studies have provided unique insights into the aetiology and treatment of multiple myeloma. New drugs such as proteasome inhibitors and anti-CD38 monoclonal antibodies, used to treat multiple myeloma, can inhibit the increase in monoclonal immunoglobulins to avoid excessive free light chain damage. To reduce the aetiology of kidney damage caused by multiple myeloma [Citation7,Citation8], autologous stem cell transplantation (ASCT) has been the mainstay of multiple myeloma therapy for about 30 years and can be used to specifically improve monoclonal plasma cell production, ameliorating kidney damage in patients with the disease [Citation9].
In a study of 1,038 newly diagnosed patients with multiple myeloma, 25% had some degree of renal insufficiency at the time of diagnosis, and 13% required dialysis. Patients with kidney failure had a median survival of only 21 months, whereas those without kidney failure survived much longer (p < 0.01), indicating that kidney function is an important survival parameter [Citation10]. Royal et al.’s multicentre study of light chain tubular nephropathy showed that renal function was strongly associated with overall survival (OS), with a risk ratio of 1.6 for every 15 mL/min/1.73 m2 decline in estimated glomerular filtration rate below 45 mL/min/1.73 m2 (p < 0.001) [Citation11].
Despite the prevalence of multiple myeloma complicated by kidney injury, a clinical summary of the prognosis of these patients has yet to be performed [Citation12]. The objective of this systematic review was to determine the trends in OS and progression-free survival (PFS) in patients with renal failure at the time of diagnosis of multiple myeloma. We limited our analysis to observational studies on multiple myeloma and thoroughly examined the outcomes of patients with renal failure in these trials. Instead of using a formal definition of renal injury, we explored what renal function threshold each experimenter chose for recruitment and reporting.
Materials and methods
Research methods and strategies
This systematic review evaluated prognostic studies of patients with related multiple myeloma complicated by renal impairment. We searched the PubMed, Embase, Web of Science, Cochrane Library, CNKI, VIP, and Wan Fang databases for all relevant studies from inception until 30 April 2023, without language restrictions. We use the following search string (Multiple Myeloma OR Plasma-Cell Myeloma OR Myeloma OR Myeloma, Plasma) AND (Renal Insufficiency OR Kidney Insufficiency OR Kidney Failure) AND (Progression-Free Survival OR Event-Free Survival OR Survival, Progression-Free) (). This systematic review was recorded in the PROSPERO database (registry number: CRD42023387759).
Table 1. Search strategy.
Inclusion and exclusion criteria
We included studies that met the following criteria: 1) the research was original and included a clear abstract, introduction, methods, results, discussion, and conclusion; 2) study contained a case group and control group, where the case group included patients with multiple myeloma with renal impairment at diagnosis and the control group had no renal impairment; and 3) the findings included progression-free survival or overall survival and mean difference. We excluded literature with the following criteria: 1) other types of research, such as case reports, reviews, and conference abstracts; 2) review articles and meta-analyses; and 3) incomplete data or the full text could not be obtained. Two authors (Yanjie Zhang and Juan Pan) independently screened the selected studies, assessed the quality of inclusion, and extracted the information. Any disagreements were resolved through discussions with a third author (Bo Shen).
Data extraction and quality assessment
Two authors (Yanjie Zhang and Juan Pan) independently extracted the following information from the studies that met the criteria: first author, year of publication, country, type of research, grouping criteria, and the number of people in the disease and control groups, as well as the prognostic parameters and corresponding statistics of the two groups. The Newcastle-Ottawa scale (NOS) scores were used to evaluate the quality of the included studies [Citation13].
Renal function definition and screening procedures
This systematic review followed the PRISMA (2020) [Citation14] recommendations. Thresholds for renal function were determined based on each study rather than a consensus, as the reviewed studies had widely different criteria for the inclusion and reporting of renal function [Citation15]. Therefore, we used the cutoff values for reported renal function in each study rather than using a fixed definition of renal failure.
Data synthesis and analysis
A narrative synthesis was used in this systematic review. The characteristics of each literature as well as the results of OS and PFS were summarized by a table. SPSS version 22.0 (IBM SPSS Inc., Chicago, USA) was used for data and subgroup analysis.
Results
Research selection
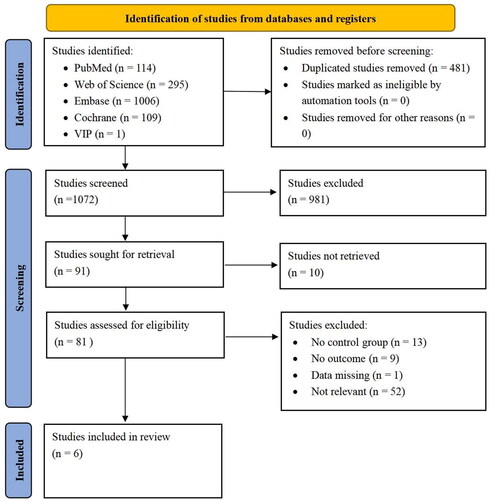
A total of 1553 studies were screened. Six studies were selected based on the inclusion and exclusion criteria ().
Features of the included literature
The features of the included studies are summarised in . The studies were distributed in the United States, United Kingdom, Germany, China, and other countries, and abnormal renal function was clearly defined in each. Due to the different years of the studies, the definitions of renal function and the criteria for grading renal function were updated with guidelines as they changed [Citation16]. Patients in the case group were diagnosed with multiple myeloma with abnormal renal function, whereas those in the control group had normal renal function. Among the included studies, four differed in the treatment regimens for normal and abnormal renal function [Citation17–20]. One study did not focus on treatment modalities [Citation21], but focused on renal function for prognosis; the second had both groups treated with the same regimen [Citation22], and the last two were based on abnormal renal function after drug adjustment, dialysis treatment [Citation17], or drug reduction [Citation18–20]. According to the NOS score, four articles had high quality (>7 stars).
Table 2. Characteristics and quality scores of included studies.
Characteristics of patients
Sirohi et al. [Citation17] reported that the median age of patients with renal insufficiency was 53 years (31–73 years) and that of those in the control group was 52 years (30–74 years) (p = 0.7). The renal insufficiency and control groups included 43 (73%) and 115 (60%) males (p = 0.1), respectively. Raab et al. [Citation18] found that the median age of patients with renal dysfunction was 57 years (39–65 years) and that of patients with normal renal function was 59 years (43–64 years). Notably, there were more males than females in both groups (P = NA). Scheid et al. [Citation20] showed that the median age of all patients included was 57 years (p = 0.64). Zhu et al. [Citation21] found that all patients were 59 years old, and 61.3% were male. Sweiss et al. [Citation22] found that the median age of patients with renal insufficiency was 61 years (38–77 years) and that of those in the control group was 58 years (34–74 years) (P = NS). Further, the renal insufficiency and control groups included 15 males (33%) and 31 females (67%) and 52 males (51%) and 51 females (50%) (P = NS), respectively. Dimopoulos et al. [Citation19] found that the median age of patients with renal insufficiency was 74.5 years old (50–87 years) and that of those in the control group was 68 years (43–82 years) (p = 0.18). Notably, the renal insufficiency and control groups included 4 (33.3%) and 22 (57.9%) males (p = 0.14), respectively.
Characteristics of patient treatment
In the study by Sirohi et al. patients were treated with full sequential therapy, regardless of renal dysfunction status [Citation17]. In the study by Raab et al. all patients received melphalan; as melphalan tends to increase urea nitrogen, a preconditioning regimen was used in the control (200 mg/m2) and dialysis (100 mg/m2) groups. Scheid et al. randomised patients in a 1:1 ratio to administer vincristine, doxorubicin, and dexamethasone induction therapy, high-dose equifarin and ASCT intensive therapy, and thalidomide 50 mg daily maintenance therapy; the other group received bortezomib, doxorubicin, and dexamethasone induction, high-dose melphalan, or ASCT, followed by intravenous maintenance therapy with bortezomib 1.3 mg/m2 twice a week for 2 years. For patients with a creatinine clearance rate (Ccr) of <40 mL/min, high-dose melphalan was administered at 200 or 100 mg/m2.
Zhu et al. did not clearly define the treatment modalities for the two groups. Sweiss et al. studied the effect of melphalan dose on renal function; all patients received melphalan 200 mg/m2 intravenously for 2 days, followed by ASCT 24 h later. Dimopoulos et al. focused on whether renal dysfunction affected lenalidomide dose; therefore, patients with baseline Ccr >50 mL⁄min were administered a daily standard dose of lenalidomide 25 mg orally on days 1 − 21 of the 28-day cycle. Patients with low Ccr were adjusted on day 1 after dialysis: Ccr ≥30 mL⁄min and <50 mL⁄min received lenalidomide 10 mg orally, and lenalidomide 15 mg once every other day was administered to patients with Ccr <30 mL⁄min that were not yet on dialysis. Patients on dialysis received lenalidomide 15 mg orally three times a week after dialysis. Dexamethasone was administered orally at 40 mg on days 1–4 and 15–18 of the first four cycles; thereafter, it was administered only on days 1–4. Lenalidomide was administered every 28 days until disease progression or unacceptable toxicity.
Characteristics of patient outcomes
In a follow-up study of patients with multiple myeloma, Sirohi et al. reported that the median survival was significantly lower in patients with renal impairment than in those with normal renal function (case vs. control, median: 2.5 vs. 4.6 years; p = 0.0025). No significant difference was found in the median event-free survival between the case and control groups (1.2 vs. 2.1 years, p = 0.07) [Citation17].
After an estimated median follow-up period of 35 months, Raab et al. showed no statistically significant differences in event-free survival (23.4 vs. 18.3 months, p = 0.71) and OS (35.6 vs. 52.3 months, p = 0.44) among patients who underwent dialysis and controls after transplantation [Citation18]. Scheid et al. who followed patients for 41.0 months, found that the 3- and 5-year PFS rates were 30 and 20%, respectively, in patients with baseline creatinine ≥2 mg/dL and 46 and 26%, respectively, in patients with baseline creatinine <2 mg/dL (p < 0.001). OS rates were significantly lower in patients with baseline creatinine ≥2 mg/dL (51% at 3 years and 33% at 5 years) compared with patients with baseline creatinine <2 mg/dL (78% at 3 years and 61% at 5 years) (p < 0.001) [Citation20]. The PFS and OS were significantly shorter in the renal insufficiency group than in the control group.
Zhu et al. compared the survival of patients in the renal insufficiency and normal renal function groups. The median follow-up was 24.0 months (2.0 − 114.0 months), and the median PFS was 28.0 months (95% confidence interval (CI): 22.4 − 33.6) in patients with renal impairment and was 29.0 months (95% CI: 25.6 − 32.4 months) in patients with normal renal function. The difference was not statistically significant (p = 0.46) [Citation21]. The median OS of patients with renal impairment was 44.0 months (95% CI: 37.7 − 50.3 months), whereas the expected median OS for the control group was significantly different at 59.2 months (95% CI: 49.7 − 68.7 months) (p = 0.03). The prognosis of the renal insufficiency group was worse than that of the normal renal function group. Sweiss et al. found that the median OS was 35.0 months (2.0 − 132.0 months) in the Ccr <60 mL/min group and 47.0 months (1.0 − 45.0 months) in the Ccr ≥60 mL/min group (P = NS) [Citation22]. The median treatment-free survival was 37.0 and 17.0 months in the renal impairment and normal renal function groups (p = 0.0025), respectively. Dimopoulos et al. found that the median PFS was 9.0 months (3.0 − 14.0) and 8.0 months (4.0 − 11.0) in the renal impairment and normal function groups (p = 0.77), respectively. Further, the median OS was 14.0 months (10.0 − 17.0) and 16.0 months (10.0–21.0) in the renal impairment and normal function groups (p = 0.91), respectively [Citation19].
Discussion
Renal insufficiency is a clinical feature [Citation23], as well as a complication [Citation24] in patients with multiple myeloma. Once multiple myeloma is complicated by kidney damage, not only should the primary disease be treated [Citation3], but when kidney damage reaches a certain extent [Citation25], patients must undergo haemodialysis to better metabolise waste in the body and maintain circulation [Citation26–28]. In addition, kidney transplantation is also sometimes necessary [Citation29]. Kidney damage imposes a physical and mental burden on patients and can lead to a poor quality of life and prognosis [Citation30]. By the time multiple myeloma is diagnosed, kidney damage has often already occurred to such an extent that recovery is difficult [Citation31,Citation32]. Therefore, more attention should be paid to patients with pre-existing kidney damage to avoid incorrect treatment and a worse prognosis and quality of life [Citation33,Citation34].
Based on previous studies [Citation35], this review used a systematic search and clear screening criteria to summarise previous studies related to the prognosis of patients with multiple myeloma complicated by renal impairment and provided certain references for the prognosis and disease management of patients with clinical kidney damage.
The outcomes in the reported literature differ greatly for the patients with multiple myeloma diagnosed with kidney damage and those without kidney damage. Studies comparing the prognosis of patients with kidney damage treated with dialysis with that of patients without kidney damage have found no statistically significant difference in prognosis between the two groups [Citation18]. In addition, some studies have revealed that the primary disease is actively treated regardless of the presence or absence of kidney damage; therefore, no statistically significant difference was observed between the prognosis of patients with kidney damage and the median overall survival of patients without kidney damage [Citation21,Citation22]. Further, new drugs commonly used in recent years can improve kidney damage or have no significant side effects on the kidneys. For example, the use of daratumumab for the treatment of multiple myeloma has raised no new safety concerns [Citation36–39]. Durie et al. showed that bortezomib, in addition to lenalidomide and dexamethasone, significantly improved the PFS and OS in patients with newly diagnosed myeloma [Citation40]. Brian et al. reported that a combination of bortezomib with lenalidomide and dexamethasone induction therapy resulted in a statistically and clinically significant improvement in PFS and better OS. However, no statistically significant difference was observed in the occurrence of kidney injury between the two groups [Citation41]. Notably, new drugs in patients with multiple myeloma can be used without many side effects and can extend PFS and OS [Citation42].
This study had some limitations. First, only six studies were finally included after research screening, implying poor representativeness. Second, as the included studies were observational, most did not provide a sufficient comparative analysis between groups; therefore, specific statistical analysis and statistical indicators could not be used to analyse the results. Third, we could not perform a specific grading analysis of patients with renal impairment owing to the limited data. Notably, the severity of renal function may affect patient outcomes. Finally, due to the wide range of years included in this study, the grading criteria for renal function differed over time, which may lead to slight differences in the degree of renal damage of the patients in the case group, affecting the final judgement result. In the future, more studies are needed to investigate the specific PFS and OS of patients with multiple myeloma complicated by renal impairment at the time of diagnosis, and further statistical analysis is needed to observe the prognosis of patients with renal impairment.
Although patients with multiple myeloma have varying degrees of renal impairment at the time of diagnosis, perhaps because doctors pay sufficient attention to kidney injury and because new drugs have fewer kidney-related side effects, the prognosis of these patients is not worse than that of those without renal impairment. Further studies are needed to provide data on the treatment of patients with kidney injury at the time of diagnosis of multiple myeloma or the importance that physicians attribute to kidney injury to clarify whether the prognosis of patients with multiple myeloma with kidney injury differs significantly from that of patients without kidney injury.
Conclusion
The limited available evidence does not support an association between multiple myeloma complicated by kidney injury and prognosis. Future longitudinal studies are needed to explore whether kidney injury combined with multiple myeloma could affect the prognosis of survival.
Author contributions
Conception and design: Y Zhang, B Shen, T Tung; Collection of data: Y Zhang, J Pan; Data analysis and interpretation: S Peng, H Kang, J Pan; Manuscript writing: Y Zhang; Final approval of manuscript: All authors; all authors agree to be accountable for all aspects of the work.
PRISMA_2020_checklist.docx
Download MS Word (27.8 KB)Acknowledgments
The authors would like to thank all the patients who participated in this study.
Disclosure statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data availability statement
The dataset from which information was collected may be shared by contacting the corresponding author.
Additional information
Funding
References
- Silberstein J, Tuchman S, Grant SJ. What is multiple myeloma? JAMA. 2022;327(5):497. doi:10.1001/jama.2021.25306.
- Cowan AJ, Green DJ, Kwok M, et al. Diagnosis and management of multiple myeloma: a review. JAMA. 2022;327(5):464–477. doi:10.1001/jama.2022.0003.
- van de Donk N, Pawlyn C, Yong KL. Multiple myeloma. Lancet. 2021;397(10272):410–427. doi:10.1016/S0140-6736(21)00135-5.
- Bridoux F, Leung N, Belmouaz M, et al. Management of acute kidney injury in symptomatic multiple myeloma. Kidney Int. 2021;99(3):570–580. doi:10.1016/j.kint.2020.11.010.
- Markovic U, Romano A, Bellofiore C, et al. Role of serum free light chain assay in relapsed/refractory multiple myeloma. A real-life unicentric retrospective study. Cancers (Basel). 2021;13(23):6017. doi:10.3390/cancers13236017.
- Xing Y, Yan J, Yu Z, et al. High-cutoff hemodialysis in multiple myeloma patients with acute kidney injury. Front Oncol. 2022;12:1024133. doi:10.3389/fonc.2022.1024133.
- Bridoux F, Arnulf B, Karlin L, et al. Randomized trial comparing double versus triple bortezomib-based regimen in patients with multiple myeloma and acute kidney injury due to cast nephropathy. J Clin Oncol. 2020;38(23):2647–2657. doi:10.1200/JCO.20.00298.
- Menè P, Moioli A, Stoppacciaro A, et al. Acute kidney injury in monoclonal gammopathies. J Clin Med. 2021;10(17):3871. doi:10.3390/jcm10173871.
- Ntanasis-Stathopoulos I, Gavriatopoulou M, Kastritis E, et al. Multiple myeloma: role of autologous transplantation. Cancer Treat Rev. 2020, 82101929;82:101929. doi:10.1016/j.ctrv.2019.101929.
- Courant M, Orazio S, Monnereau A, et al. Incidence, prognostic impact and clinical outcomes of renal impairment in patients with multiple myeloma: a population-based registry. Nephrol Dial Transplant. 2021;36(3):482–490. doi:10.1093/ndt/gfz211.
- Royal V, Leung N, Troyanov S, et al. Clinicopathologic predictors of renal outcomes in light chain cast nephropathy: a multicenter retrospective study. Blood. 2020;135(21):1833–1846. doi:10.1182/blood.2019003807.
- Chakraborty R, Majhail NS. Treatment and disease-related complications in multiple myeloma: implications for survivorship. Am J Hematol. 2020;95(6):672–690. doi:10.1002/ajh.25764.
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi:10.1007/s10654-010-9491-z.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):89. doi:10.1186/s13643-021-01626-4.
- Mohyuddin GR, Koehn K, Shune L, et al. Renal insufficiency in multiple myeloma: a systematic review and meta-analysis of all randomized trials from 2005-2019. Leuk Lymphoma. 2021;62(6):1386–1395. doi:10.1080/10428194.2020.1867725.
- Ikizler TA, Burrowes JD, Byham-Gray LD, et al. KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am J Kidney Dis. 2020;76(3 Suppl 1):S1–S107. doi:10.1053/j.ajkd.2020.05.006.
- Sirohi B, Powles R, Mehta J, et al. The implication of compromised renal function at presentation in myeloma: similar outcome in patients who receive high-dose therapy: a single-center study of 251 previously untreated patients. Med Oncol. 2001;18(1):39–50. doi:10.1385/mo:18:1:39.
- Raab MS, Breitkreutz I, Hundemer M, et al. The outcome of autologous stem cell transplantation in patients with plasma cell disorders and dialysis-dependent renal failure. Haematologica. 2006;91(11):1555–1558.
- Dimopoulos MA, Christoulas D, Roussou M, et al. Lenalidomide and dexamethasone for the treatment of refractory/relapsed multiple myeloma: dosing of lenalidomide according to renal function and effect on renal impairment. Eur J Haematol. 2010;85(1):1–5. doi:10.1111/j.1600-0609.2010.01432.x.
- Scheid C, Sonneveld P, Schmidt-Wolf IG, et al. Bortezomib before and after autologous stem cell transplantation overcomes the negative prognostic impact of renal impairment in newly diagnosed multiple myeloma: a subgroup analysis from the HOVON-65/GMMG-HD4 trial. Haematologica. 2014;99(1):148–154. doi:10.3324/haematol.2013.087585.
- Zhu W, Lu J, Lu J, et al. [Clinical analysis of newly diagnosed multiple myeloma patients with renal dysfunction]. Zhonghua Yi Xue Za Zhi. 2015;95(10):741–744.
- Sweiss K, Patel S, Culos K, et al. Melphalan 200 mg/m 2 in patients with renal impairment is associated with increased short-term toxicity but improved response and longer treatment-free survival. Bone Marrow Transplant. 2016;51(10):1337–1341. doi:10.1038/bmt.2016.136.
- Gaut JP, Liapis H. Acute kidney injury pathology and pathophysiology: a retrospective review. Clin Kidney J. 2021;14(2):526–536. doi:10.1093/ckj/sfaa142.
- Banaszkiewicz M, Małyszko J, Vesole DH, et al. New biomarkers of ferric management in multiple myeloma and kidney disease-associated anemia. J Clin Med. 2019;8(11):1828. doi:10.3390/jcm8111828.
- Fabbrini P, Finkel K, Gallieni M, et al. Light chains removal by extracorporeal techniques in acute kidney injury due to multiple myeloma: a position statement of the Onconephrology Work Group of the Italian Society of Nephrology. J Nephrol. 2016;29(6):735–746. doi:10.1007/s40620-016-0347-9.
- Petrucci I, Clementi A, Sessa C, et al. Ultrasound and color Doppler applications in chronic kidney disease. J Nephrol. 2018;31(6):863–879. doi:10.1007/s40620-018-0531-1.
- Amaador K, Peeters H, Minnema MC, et al. Monoclonal gammopathy of renal significance (MGRS) histopathologic classification, diagnostic workup, and therapeutic options. Neth J Med. 2019;77(7):243–254.
- Heybeli C, Bentall AJ, Alexander MP, et al. Kidney transplant outcomes of patients with multiple myeloma. Kidney Int Rep. 2022;7(4):752–762. doi:10.1016/j.ekir.2022.01.003.
- Dykes K, Desale S, Javaid B, et al. A new reality for multiple myeloma renal failure: US data report on kidney transplant outcomes. Clin Lymphoma Myeloma Leuk. 2022;22(5):e314–e320. doi:10.1016/j.clml.2021.11.002.
- Guzdar A, Costello C. Supportive care in multiple myeloma. Curr Hematol Malig Rep. 2020;15(2):56–61. doi:10.1007/s11899-020-00570-9.
- Grzasko N, Morawska M, Hus M. Optimizing the treatment of patients with multiple myeloma and renal impairment. Clin Lymphoma Myeloma Leuk. 2015;15(4):187–198. doi:10.1016/j.clml.2014.09.012.
- Leung N, Nasr SH. Myeloma-related kidney disease. Adv Chronic Kidney Dis. 2014;21(1):36–47. doi:10.1053/j.ackd.2013.08.009.
- Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-548–e548. doi:10.1016/S1470-2045(14)70442-5.
- Dimopoulos MA, Sonneveld P, Leung N, et al. International Myeloma Working Group recommendations for the diagnosis and management of myeloma-related renal impairment. J Clin Oncol. 2016;34(13):1544–1557. doi:10.1200/JCO.2015.65.0044.
- Muka T, Glisic M, Milic J, et al. A 24-step guide on how to design, conduct, and successfully publish a systematic review and meta-analysis in medical research. Eur J Epidemiol. 2020;35(1):49–60. doi:10.1007/s10654-019-00576-5.
- Mateos MV, Sonneveld P, Hungria V, et al. Daratumumab, bortezomib, and dexamethasone versus bortezomib and dexamethasone in patients with previously treated multiple myeloma: three-year follow-up of CASTOR. Clin Lymphoma Myeloma Leuk. 2020;20(8):509–518. doi:10.1016/j.clml.2019.09.623.
- Lu J, Fu W, Li W, et al. Daratumumab, bortezomib, and dexamethasone versus bortezomib and dexamethasone in Chinese patients with relapsed or refractory multiple myeloma: phase 3 LEPUS (MMY3009) study. Clin Lymphoma Myeloma Leuk. 2021;23(1):e699–e709. doi:10.1016/j.clml.2021.04.012.
- Avet-Loiseau H, San-Miguel J, Casneuf T, et al. Evaluation of sustained minimal residual disease negativity with daratumumab-combination regimens in relapsed and/or refractory multiple myeloma: analysis of POLLUX and CASTOR. J Clin Oncol. 2021;39(10):1139–1149. doi:10.1200/JCO.20.01814.
- Zheng Y, Shen H, Xu L, et al. Monoclonal antibodies versus histone deacetylase inhibitors in combination with bortezomib or lenalidomide plus dexamethasone for the treatment of relapsed or refractory multiple myeloma: an indirect-comparison meta-analysis of randomized controlled trials. J Immunol Res. 2018;2018:7646913. doi:10.1155/2018/7646913.
- Durie BGM, Hoering A, Abidi MH, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet. 2017;389(10068):519–527. doi:10.1016/S0140-6736(16)31594-X.
- Durie BGM, Hoering A, Sexton R, et al. Longer term follow-up of the randomized phase III trial SWOG S0777: bortezomib, lenalidomide and dexamethasone vs. lenalidomide and dexamethasone in patients (Pts) with previously untreated multiple myeloma without an intent for immediate autologous stem cell transplant (ASCT). Blood Cancer J. 2020;10(5):53. doi:10.1038/s41408-020-0311-8.
- McCaughan GJ, Gandolfi S, Moore JJ, et al. Lenalidomide, bortezomib and dexamethasone induction therapy for the treatment of newly diagnosed multiple myeloma: a practical review. Br J Haematol. 2022;199(2):190–204. doi:10.1111/bjh.18295.