Abstract
Background
Achieving disease control is the goal of asthma management. Serum or sputum eosinophil counts have been known traditional means of assessing eosinophilic airway inflammation in asthma, which is vital in predicting response to corticosteroid therapy which ultimately promotes control of the disease. Evidence suggests that fraction of exhaled nitric oxide (FeNO) may be a more useful non-invasive surrogate biomarker for the assessment of eosinophilic airway inflammation and could help with the timely adjustment of inhaled corticosteroid therapy in the uncontrolled asthma patient. The relationship between FeNO and other markers of airway inflammation has been variable in literature, with limited data in sub-Saharan Africa where FeNO testing is very sparse. We sought to define the relationship between FeNO levels, serum eosinophil counts, spirometry measures and symptom control among asthma patients.
Materials and methods
The study was conducted at the Asthma Clinic of a large tertiary hospital. This study included 82 patients with physician-diagnosed asthma being regularly managed at the clinic. All participants were taken through the asthma control test (ACT), had FeNO and spirometry measurements taken according to the American Thoracic Society (ATS) guidelines. Blood samples were obtained from all participants for serum eosinophil counts. Correlation coefficient was used to ascertain the relationship between FeNO levels and serum eosinophil counts, ACT scores, and spirometry measurements. Logistic regression was used to examine the association between high FeNO and abnormal FEV1 percentage predicted (<80%) with adjustments for age, sex, and BMI.
Results
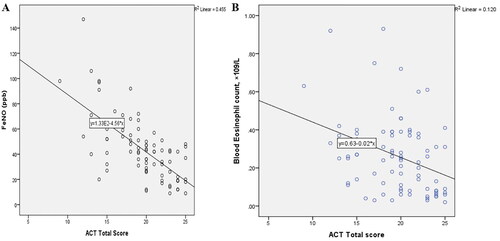
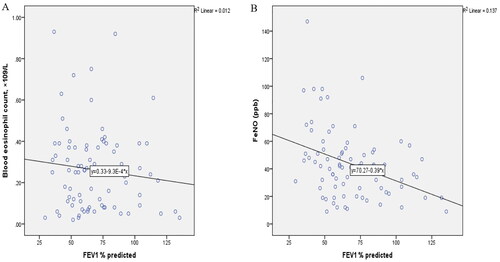
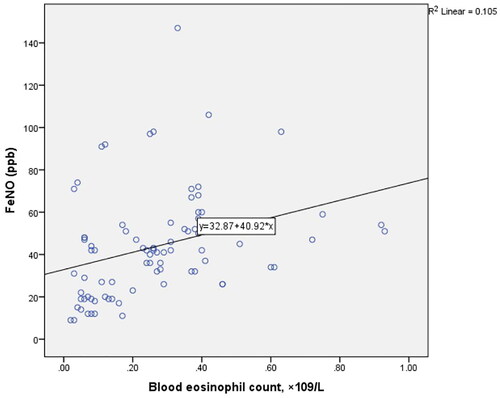
A total of 82 patients with asthma were included in the study, with higher prevalence of females (72%). Majority (40.2%) of the patients were found in the 60 and above age category. The median FeNO level and ACT score was 42.00 (26.00–52.50) parts per billion (ppb) and 20.0 (18–23) respectively. The median serum eosinophil counts was 0.25(0.90–0.38) × 109/L. The median FeNO levels were significantly higher in patients with partly and very poorly controlled asthma than in the well-controlled group (p < 0.001). A total of 47(57%) of the patients were classified as having well controlled asthma and 35 (42%) uncontrolled. FeNO correlated with serum eosinophil counts (r = 0.450, p < 0.001), ACT (r = −0.648, p < 0.001), and FEV1 percentage predicted (r = −0.353, p = 0.001). High FeNO (>50 ppb) was associated with an over fivefold increased risk of having an abnormal FEV1 percentage predicted.
Conclusion
FeNO levels significantly correlated with the ACT scores, serum eosinophil counts and FEV1% predicted among the asthma patients who were on inhaled corticosteroid therapy. High FeNO was significantly associated with abnormal FEV1 percentage predicted. We suggest that the point of care assessment of FeNO is a reliable marker of eosinophilic inflammation in our cohort of patients and together with ‘ACT scores’ in our asthma clinics could increase asthma control rates.
Background
Asthma is a common chronic inflammatory airway disease with varied phenotypic expressions (heterogeneous) and characterized by recurrent episodes of wheezing, shortness of breath, chest tightness, and cough. [Citation1]. Globally, it is estimated that more than 339 million people suffer from asthma, with approximately 250,000 deaths annually [Citation2]. Most asthma-related deaths occur in low- and lower-middle-income countries, which reflects the poor levels of asthma control in these regions [Citation2,Citation3]. Asthma ranks high among diseases associated with significant morbidity worldwide with an age-standardized disability adjusted life years (DALYs) rate of 273/100,000 [Citation4,Citation5]. Achieving asthma control is therefore key in reducing morbidity and mortality associated with the disease. However, asthma control has been suboptimal in clinical practice, resulting in a significant impact on health resources and the health status of patients [Citation5–7].
With regards to the pathophysiology of asthma, eosinophilic airway inflammation induced by T helper 2 (TH2)-mediated immune response to allergic sensitization is a pivotal feature occurring in a significant proportion of asthma patients [Citation8,Citation9]. Bousquet et al. [Citation9] found elevated eosinophil levels in the peripheral blood and bronchoalveolar lavage of patients with asthma which also significantly correlated with the severity of the disease. Other studies have consistently proven that eosinophilic airway inflammation occurs in asthma and responds to corticosteroid therapy which translates into improved asthma symptoms with about 60% reduction in acute exacerbations [Citation10–12]. Inhaled corticosteroids has therefore become the mainstay of asthma treatment [Citation4,Citation13]. Although symptom-based questionnaires and lung function measurements are important tools in assessing disease control in asthma, knowledge of the level of inflammation and tailoring treatment regimen particularly corticosteroid therapy is key if improvement in asthma control rates are to be realized globally, particularly in Africa.
Since uncontrolled airway inflammation is linked with recurrent asthma symptoms, frequent exacerbations, increased hospitalization, OPD and ER attendance, it is imperative that asthma patients with eosinophilic inflammation are identified and monitored to facilitate steroid dose adjustment for better disease outcomes [Citation14,Citation15].
Traditionally, blood and sputum eosinophil counts have been used as effective biomarkers for assessing the level of airway inflammation being described as modifiable risk factors in asthma management [Citation15]. Significant correlation has been found between these two biomarkers [Citation9]. The setback has been the ease of obtaining the above biomarkers, particularly sputum for cell counts, as well as the lack of timely availability of results in clinical practice [Citation16].
The evidence of an increase in the level of endogenous nitric oxide produced in response to eosinophilic airway inflammation led to the discovery that measurement of the fraction of nitric oxide in the exhaled breath can reliably predict airway inflammation in asthma [Citation17,Citation18]. Evidence has it that an increase in FeNO level is shown to be associated with deteriorating asthma control and levels decrease with corticosteroid therapy [Citation18], The fraction of exhaled nitric oxide (FeNO) is therefore considered an indirect marker of airway hyper-responsiveness, eosinophilic inflammation and exhibits significant association with disease severity. This formed the basis for its role in the diagnosis and management of asthma [Citation19,Citation20]. Although FeNO is non-invasive, less expensive and could offer results faster and possibly at the point of care, there are varying reports on how well it correlates with the traditional biomakers [Citation21,Citation22]. Asthma patients can assess their symptom control objectively via the validated asthma control test (ACT) which is expected to be high if background inflammation is controlled but correlation between FeNO and ACT scores has also been variable [Citation23,Citation24].
In our setting, peripheral eosinophil count is the only available test for assessing airway inflammation among suspected and diagnosed asthma patients. Although helpful, it is costly and not readily available to the clinician until the next lengthy clinic appointment to make treatment adjustments. There is anecdotal evidence of poor asthma control seen in the frequent attendance to OPDs and ERs with acute exacerbations, high usage of oral and inhaled short-acting beta-agonists, minimal use of ICS and an age adjusted death rate of 13.95/100,000 in the country. We proposed that FeNO testing which is quick and a less expensive marker of airway inflammation could be beneficial for asthma care in our resource constrained environment.
However, majority of studies assessing correlation between FeNO and serum eosinophila were conducted outside sub-Saharan Africa, with scanty data on the subject in our region. This study therefore sought to establish what the link may be in our population of asthma patients; hence determine its future role in our practice.
Materials and methods
Study design and measurements
This was a cross-sectional study conducted at the outpatient department of a tertiary facility, specifically the asthma clinic. The hospital is the largest tertiary facility in the country and receives referrals from regional and district hospitals as well as from neighbouring countries in the sub-region. The asthma clinic, run by respiratory physicians and medical residents, sees about 20–25 referrals per month. The study involved adult asthma patients (18 years and above). Eligible participants were patients with physician-diagnosed asthma, who were regular attendants of the asthma clinic (≥6 months), on inhaled corticosteroids and consented to be part of the study. Written informed consent was obtained from all participants. Systematic sampling approach using the asthma clinic appointment list. The first patient on the list is selected and thereafter every third .patient selected. If a patient refuses to consent the next on the list is selected. Patients with a history of chronic lung disease other than asthma or in co-existence with asthma, a recent acute exacerbation (within the last 72 h), or diagnosed with an acute upper and/or lower respiratory tract infection within 2 weeks prior to enrolment were excluded [Citation25]. Patients with coronary artery disease, congestive heart failure, corpulmonale, and pregnant women were also excluded from the study. A structured questionnaire was used to gather data on anthropometry and clinical characteristics of participants such as treatment regimen on and the dosage, symptom score on ACT questionnaire and smoking status. Weight and height were measured using the SECA 877 scale and SECA 217 stadiometer, respectively and the body mass index (BMI) computed (kg/m2). Blood samples were collected from all participants into EDTA tubes for serum eosinophil counts using the XS-500i (Sysmex hematology analyzer) within 2 h of sample collection.
Spirometry testing
Spirometry was performed for all study participants by trained technicians using the Vitalograph spirometry machine and in conformity with the ATS guidelines [Citation26]. Spirometry was performed after the FeNO test and blood sample collection. The obtained indices were the best measured values of FEV1, FVC, and the FEV1/FVC ratio. The predicted values of the above spirometry indices was generated by the vitalograph based on participants age, sex, height, and ethnic group [Citation26]. Abnormal FEV1 percentage predicted was defined as FEV1 less than 80% of the predicted value.
Fraction of exhaled nitric oxide
The fraction of exhaled nitric oxide was measured as recommended by the ATS guidelines [Citation27] by trained technicians using NO breath (Bedfont Scientific Ltd., Maidstone, UK). The device uses electrochemical sensor technology to detect NO in parts per billion (ppb). The measurements were performed in triplicate, and the results are reported as the mean of three measurements. All study participants abstained from nitrate-containing food green-leaved vegetables such as lettuce and spinach, coffee for 2 h, and alcohol for 12 h before testing. To ensure patients have abstained from the above mentioned, some participants had to return the next day for the FENO test. The FeNO values were categorized into: low (<25 ppb), intermediate (25–50 ppb), and high (>50 ppb) following the American Thoracic Society (ATS) categorization [Citation27].
Asthma control test
An asthma control test questionnaire (ACT) was used to assess patients symptoms in the last 4 weeks and control determined based on their scores. Patients were asked to rate the following items: daily activity limitations, shortness of breath, nocturnal awakening, use of rescue medications, and how well their asthma is controlled [Citation28].
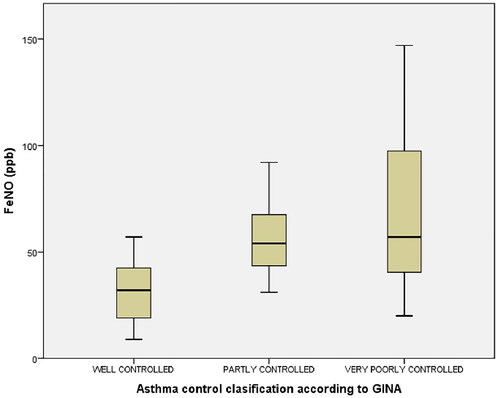
The ACT scores ranged from 5 to 25. Scores of 20–25 were classified as well-controlled asthma, 16–19 as partly controlled asthma, and 5–15 as very poorly controlled asthma.
Statistical methods
Statistical Package for the Social Sciences (SPSS) version 25 (SPSS, Chicago, IL) was used for statistical analyses. Normally distributed data are presented as mean ± standard deviation, and non-normally distributed data are presented as median (interquartile range). Chi-square statistics were used to test the relationships between categorical variables. The analysis of variance was used to compare the means between the three groups (low FeNO, intermediate FeNO and high FeNO), followed by the Tukey’s post hoc analysis. Differences in medians between more than two groups were compared using the Kruskal–Wallis test. The correlation between continuous variables was determined using Spearman’s rank correlation coefficient. A logistic regression model was used to determine a possible association between FeNO and abnormal FEV1 percentage predicted (defined as FEV1<80% predicted) in asthma patients, with adjustments for confounders (age, sex, BMI). Statistical significance was set at a p value <0.05.
Results
General characteristics
A total of 82 adult asthma patients were involved in the study. Fron , the mean age of the patients was 52.72 ± 13.52 years with 72% being female. About 40% of the participants were 60 years and above. The mean BMI was 28.87 ± 5.27 kg/m2 and about 77% of the patients were either overweight or obese. Patients with smoking history (current or past) was 5%. From the analysis, approximately 5(18%) reported asthma diagnosis since childhood. The proportion of patients who reported asthma diagnosis during adolescence and adulthood was 39 (47.6%) and 28 (34.1%) respectively. While 4(4.9%) of the patients reported duration of asthma to be <1 year, 72(87.8%) reported disease duration for more than 10 years.
Table 1. Clinical characteristics of the study participants based on ATS categorization of FeNO.
The median ACT score was 20.0 (18-23). From , 47(57%) of the patients were classified as having well controlled asthma and 35(42%) uncontrolled. All patients were on inhaled corticosteroid therapy with majority using combined inhalers, predominantly Salmeterol and Fluticasone combined inhalers.
From the analysis, the median FeNO level was 42.00 (26.00–52.50) ppb. shows that the proportion of patients with intermediate to high FeNO values was 63(77%). A significant association was observed between FeNO and the levels of asthma control based on ACT scores (p < 0.001). There was significant association between the type of treatment and measured FeNO values (p = 0.243). From the results, the median blood eosinophil count in the well controlled, partially controlled and poorly controlled asthma patients were 0.17 (0.07–0.36) × 109/L, 0.28 (0.12–0.39) × 109/L and 0.30 (0.12–0.39) × 109/L respectively; p < 0.001.
Association between serum eosinophil counts, FeNO and spirometry results of participants
From , the mean serum eosinophil counts was 0.25(0.90–0.38) and that of FEV1% predicted was 68.34 ± 23.92. A significant association was observed between serum eosinophil count and FeNO levels (p < 0.001). The association between FEV1% predicated of the participants and the measured FeNO values was also significant (p = 0.010).
Table 2. Blood eosinophils and spirometry results.
Levels of FeNO in the study participants based on ACT score classification
The median FeNO levels were significantly higher in patients with partly and very poorly controlled asthma (p < 0.001). The median FeNO levels in the well-controlled, partly controlled, and very poorly controlled groups were 32 (19–42.5) ppb, 54 (43.5–67.5) ppb, and 57 (40.5–97.5) ppb respectively. The post hoc test showed that there was no significant difference in the median FeNO between partly controlled asthma patients and very poorly controlled asthma patients (p = 1.00) ().
Correlation between markers of inflammation (FeNO and peripheral blood eosinophil counts) and ACT scores
There was a significant negative correlation between FeNO and the ACT total scores in the participants (r = −0.648, p < 0.001) (). The study also showed a significant negative correlation between blood eosinophil counts and ACT scores (r = −0.339, p = 0.002) ().
Correlation between markers of inflammation (FeNO and serum eosinophils) and FEV1% predicted
There was no correlation between blood eosinophil count and FEV1% predicted in the study participants (r = −0.082, p = 0.462) (). FeNO however, significantly correlated with FEV1% predicted values of the participants (r = −0.353, p = 0.001) ().
Correlation between fraction of exhaled nitric oxide and blood eosinophil count in Ghanaians with asthma
Correlation analysis showed that FeNO levels were significantly associated with blood eosinophil count (r = 0.450, p < 0.001) ().
Logistic regression models for FeNO and abnormal FEV1% predicted in Ghanaians with asthma
In a logistic regression model, high FeNO was significantly associated with abnormal FEV1% predicted. In the unadjusted model, participants with high FeNO (>50 ppb) were about 5 times more likely to have an abnormal FEV1% predicted. In the adjusted model, high FeNO (>50 ppb) was associated with an over fivefold increased risk of abnormal FEV1% predicted, compared to participants with low FeNO (<25 ppb) (model 2 ).
Table 3. Logistic regression models abnormal FEV1 in Ghanaians with asthma.
Discussion
Chronic airway inflammation is a cardinal feature of bronchial asthma that contributes to expiratory airflow limitation and can be fatal if not well-controlled [Citation1]. Eosinophilic airway inflammation is present in approximately 50% of asthma cases, and reduction in airway inflammation with inhaled corticosteroids (ICS) has been associated with improved patient outcomes which manifests as reduced day or night time symptoms, decreased frequency of exacerbations, and overall improved quality of life [Citation1]. Assessing airway inflammation in asthma is therefore not only an aid to establishing diagnosis and staging disease severity but very useful in evaluating treatment response to corticosteroids or biologics, in the case of severe asthma [Citation29].
Serum and sputum eosinophilia remain common effective tools used to determine eosinophilic airway inflammation (type 2 inflammation) in patients with asthma but the latter is not widely available although known to be the most sensitive marker [Citation9,Citation10]. Nitric oxide was first reported to be increased in the exhaled breath of asthma patients compared to controls at the Karolinska Institute in Sweden in 1993 [Citation30]. Eosinophils are a major subset of airway inflammatory cells that produce NO in expired breath by the expression of inducible nitric oxide synthase [Citation30]. Subsequently, other studies have shown that elevated FeNO is associated with eosinophilic infiltration of the airways [Citation31–33]. Unlike the serum and sputum eosinophil assays, FeNO is non-invasive with rapid results at the point of care necessary for timely adjustment of ICS therapy and its usefulness in asthma care has been brought to the fore in current guidelines [Citation34].
Patient-reported outcome measures such as the ACT tool are clinically relevant tools that strongly predict risk of future exacerbations, which are endpoints of poor disease control [Citation35]. Asthma control test (ACT) is a validated numerical asthma control tool that has demonstrated responsiveness to changes in disease control and lung function [Citation23]. Asthma control is often assessed in the clinic with these symptom-based questionnaires and it is imperative that other methods of assessing disease control such as markers of airway inflammation, show significant association with such scores. Based on the ACT tool, the current study has shown that a significant proportion (43%) of the asthma patients were not controlled although majority were on combined (ICS/long-acting beta-agonist) inhaler therapy. We believe that the uncontrolled asthma rates could be much worse in the real sense as our the study was conducted in a tertiary facility where control rates are likely to be better comparatively. This was however, similar to asthma control rates of 44.3% and 44.4% in South Africa and Uganda respectively as reported in a systematic review of asthma in children between 6 and 18 years [Citation36,Citation37] that of Nigeria in the same study was lower at 30.9%.
The current study has shown that both markers of eosinophilc airway inflammation (serum eosinophil counts and FeNO) had a significant negative correlation with ACT scores [Citation23,Citation38]. An increase in FeNO level is shown to be associated with deteriorating asthma control and levels decrease with corticosteroid therapy [Citation18]. This association confirms that in practice, control of eosinophilic airway inflammation should reflect in better symptom score and quality of life of the asthma patient as shown in this study. In support of this finding, addition of FeNO assessment among children in an asthma clinic significantly improved their ACT scores [Citation39]. On the contrary, a study by Szefler et al. in adults, found no significant impact of FeNO monitoring on the ACT scores in adolescents and adults with asthma [Citation40].
Although FeNO and serum eosinophils are established markers of airway inflammation, they appear to act via different inflammatory pathways, which could explain the varying or weak direct correlation reported in the literature [Citation41,Citation42]. Fraction of exhaled Nitric Oxide is mediated by cytokines IL-14 and IL-13 (type 2 inflammation) whiles peripheral eosinophilia is mediated via IL-5. We however observed a strong positive correlation between these two biomarkers (r = 0.450, p < 0.001). Gao et al. [Citation33] reported that both FeNO and serum eosinophils accurately and independently predicted sputum eosinophilia in patients with uncontrolled asthma, but the two showed no direct correlation. There is evidence that the simultaneous rise in both FeNO levels and serum eosinophil count is associated with a higher likelihood of acute asthma events rather than a rise in either of them [Citation32]. This finding suggests that FeNO values, in combination with serum eosinophilia, could play a role in assessing the future risk of adverse events among asthma patients.
Impaired FEV1 particularly <60% predicted, has been shown to be an independent risk factor for adverse future events among asthma patients on treatment and is considered important in assessing asthma control during clinic visits [Citation2,Citation43]. The direct relationship between airway inflammation and FEV1 is not well understood, with varying study reports [Citation42,Citation44]. Analysis of the participants with abnormal FEV1% predicted showed that high FeNO (>50 ppb) was significantly associated with abnormal FEV1% predicted and exhibited over a fivefold risk of having abnormal FEV1 compared to those with low FeNO (<25 ppb).
The lengthy interval between clinic appointments and the cost of laboratory requests for serum eosinophilia in our setting lead to real challenges in the periodic assessment of airway inflammation at the point of care. The results of this study have not only highlighted the benefits of FeNO as an indirect biomarker of eosinophilic airway inflammation, but promises to be a faster means of evaluating the control of airway inflammation in the clinic, and could allow for timely and cost-effective ICS treatment review in our setting.
Conclusions
Majority of the patients with well controlled asthma were noted in the low FeNO group. Since FeNO correlated significantly with ACT scores, serum eosinophil count, as well as FEV1% predicted. We suggest that the assessment of FeNO together with ‘ACT scores’ or ‘usual care’ in our asthma clinics could further improve asthma control rates.
Institutional review board statement
The study was conducted in accordance with the guidelines of the Declaration of Helsinki and was approved by the Scientific and Technical Committee/Institutional Review Board of Korle-Bu Teaching Hospital (STC/IRB/00096/2019).
Consent for publication
Not applicable.
Informed consent statement
Informed consent was obtained from all the subjects involved in the study.
Availability of data and material
The dataset analyzed during this study is available from the corresponding author upon reasonable request.
Authors contributions
J.S.A-M and C.A-B conceived of the study. P.D, G.B.D, and R.A recruited the patients and performed statistical analysis. J.S.A-M, C.A-B, and C.F.H-B were the major contributors to the study design, data analysis, discussion, and manuscript preparation. Y.K-M, M.N, and B.D were involved in the interpretation and discussion of data. All the authors have read and approved the final manuscript.
| Abbreviations | ||
| ACT | = | Asthma control test |
| BMI | = | Body mass index |
| FeNO | = | Fractional exhaled nitric oxide |
| FEV1 | = | Forced expiratory volume in 1 s |
| FVC | = | Forced vital capacity |
| GINA | = | Global Initiative for Asthma |
Acknowledgments
The authors wish to acknowledge all the participants in the study.
Disclosure statement
The authors declare no competing interests.
Additional information
Funding
References
- Global Strategy for Asthma Management and Prevention (GINA) 2018. Global Strategy for Asthma Management and Prevention (2017 update). Vol. 135; 2018. Retrieved from www.ginasthma.org.
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention at Online Appendix. Vol. 89; 2016.
- Soto-Martínez ME, Soto-Quiros ME, Custovic A. Childhood asthma: low and middle-income countries perspective. Acta Med Acad. 2020;49(2):181–190. doi: 10.5644/ama2006-124.296.
- Global initiative for Asthma. Global strategy for asthma management and prevention; 2023. Available from:www.ginaasthma.org.
- Wang Z, Li Y, Gao Y, et al. Global, regional, and national burden of asthma and its attributable risk factors from 1990 to 2019: a systematic analysis for the Global Burden of Disease Study 2019. Respir Res. 2023;24(1):169. doi: 10.1186/s12931-023-02475-6.
- Demoly P, Annunziata K, Gubba E, et al. Repeated cross-sectional survey of patient-reported asthma control in Europe in the past 5 years. Eur Respir Rev. 2012;21(123):66–74. doi: 10.1183/09059180.00008111.
- Rabe KF, Vermeire PA, Soriano JB, et al. Clinical management of asthma in 1999: the Asthma Insights and Reality in Europe (AIRE) study. Eur Respir J. 2000;16(5):802–807. doi: 10.1183/09031936.00.16580200.
- George L, Brightling CE. Eosinophilic airway inflammation: role in asthma and chronic obstructive pulmonary disease. Ther Adv Chronic Dis. 2016;7(1):34–51. doi: 10.1177/2040622315609251.
- Bousquet J, Chanez P, Lacoste JY, et al. Eosinophilic inflammation in asthma. N Engl J Med. 1990;323(15):1033–1039. doi: 10.1056/NEJM199010113231505.
- Green RH, Brightling CE, McKenna S, et al. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet. 2002;360(9347):1715–1721. doi: 10.1016/S0140-6736(02)11679-5.
- Jayaram L, Pizzichini MM, Cook RJ, et al. Determining asthma treatment by monitoring sputum cell counts: effect on exacerbation. Eur Respir J. 2006;27(3):483–494. doi: 10.1183/09031936.06.00137704.
- Ulrik CS, Lange P, Hilberg O. Fractional exhaled nitric oxide as a determinant for the clinical course of asthma: a systematic review. Eur Clin Respir J. 2021;8(1):1891725. doi: 10.1080/20018525.2021.1891725.
- Louis R, Schleich F, Barnes PJ. Corticosteroids: still at the frontline in asthma treatment? Clin Chest Med. 2012;33(3):531–541. doi: 10.1016/j.ccm.2012.05.004.
- Schleich FN, Chevremont A, Paulus V, et al. Importance of concomitant local and systemic eosinophilia in uncontrolled asthma. Eur Respir J. 2014;44(1):97–108. doi: 10.1183/09031936.00201813.
- Mallah N, Rodriguez-Segade S, Gonzalez-Barcala F-J, Takkouche B. Blood eosinophil count as predictor of asthma exacerbation: a meta-analysis. Pediatr Allergy Immunol. 2021;32(3):465–478.
- ten Brinke A, de Lange C, Zwinderman AH, et al. Sputum induction in severe asthma by a standardized protocol: predictors of excessive bronchoconstriction. Am J Respir Crit Care Med. 2001;164(5):749–753. doi: 10.1164/ajrccm.164.5.2009035.
- Gustafsson LE, Leone AM, Persson MG, et al. Endogenous nitric oxide is present in the exhaled air of rabbits, guinea pigs and humans. Biochem Biophys Res Commun. 1991;181(2):852–857. doi: 10.1016/0006-291x(91)91268-h.
- Neelamegan R, Saka V, Tamilarasu K, et al. Clinical utility of fractional exhaled nitric oxide (FeNO) as a biomarker to predict severity of disease and response to inhaled corticosteroid (ICS) in asthma patients. J Clin Diagn Res. 2016;10(12):1.
- Menzies-Gow A, Mansur AH, Brightling CE. Clinical utility of fractional exhaled nitric oxide in severe asthma management. Eur Respir J. 2020;55(3):1901633. doi: 10.1183/13993003.01633-2019.
- Heffler E, Carpagnano GE, Favero E, et al. Fractional exhaled nitric oxide (FENO) in the management of asthma: a position paper of the Italian Respiratory Society (SIP/IRS) and Italian Society of Allergy, Asthma and Clinical Immunology (SIAAIC). Multidiscip Respir Med. 2020;15(1):36. doi: 10.4081/mrm.2020.36.
- Petsky HL, Cates CJ, Lasserson TJ, et al. A systematic review and meta-analysis: tailoring asthma treatment on eosinophilic markers (exhaled nitric oxide or sputum eosinophils). Thorax. 2012;67(3):199–208. doi: 10.1136/thx.2010.135574.
- Honkoop PJ, Loijmans RJ, Termeer EH, Asthma Control Cost-Utility Randomized Trial Evaluation (ACCURATE) Study Group, et al. Symptom- and fraction of exhaled nitric oxide-driven strategies for asthma control: a cluster-randomized trial in primary care. J Allergy Clin Immunol. 2015;135(3):682–688.e11. doi: 10.1016/j.jaci.2014.07.016.
- Nguyen VN, Chavannes NH. Correlation between fractional exhaled nitric oxide and asthma control test score and spirometry parameters in on-treatment-asthmatics in Ho Chi Minh City. J Thorac Dis. 2020;12(5):2197–2209. doi: 10.21037/jtd.2020.04.01.
- Katoch CDS, Vasan AS, Pathak K. Correlation of fraction of exhaled nitric oxide with asthma control test and asthma severity in diagnosed cases of asthma. Med J Armed Forces India. 2022;78(4):443–447. doi: 10.1016/j.mjafi.2021.01.018.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Bethesda, MD: Global Initiative for Chronic Obstructive Pulmonary Disease; 2010.
- Perzanowski MS, Yoo Y. Exhaled nitric oxide and airway hyperresponsiveness to adenosine 5'-monophosphate and methacholine in children with asthma. Int Arch Allergy Immunol. 2015;166(2):107–113. doi: 10.1159/000375237.
- Dweik RA, Boggs PB, Erzurum SC, American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels (FENO) for Clinical Applications, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FeNO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602–615. doi: 10.1164/rccm.9120-11ST.
- Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113(1):59–65. doi: 10.1016/j.jaci.2003.09.008.
- Majellano EC, Clark VL, Winter NA, et al. Approaches to the assessment of severe asthma: barriers and strategies. J Asthma Allergy. 2019;12:235–251. doi: 10.2147/JAA.S178927.
- Alving K, Weitzberg E, Lundberg JM. Increased amount of nitric oxide in exhaled air of asthmatics. Eur Respir J. 1993;6(9):1368–1370. doi: 10.1183/09031936.93.06091368.
- Wenzel SE, Schwartz LB, Langmack EL, et al. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med. 1999;160(3):1001–1008. doi: 10.1164/ajrccm.160.3.9812110.
- Mogensen I, Alving K, Jacinto T, et al. Simultaneously elevated FeNO and blood eosinophils relate to asthma morbidity in asthmatics from NHANES 2007–12. Clin Exp Allergy. 2018;48(8):935–943. doi: 10.1111/cea.13137.
- Gao J, Chen Z, Jie X, et al. Both fractional exhaled nitric oxide and sputum eosinophil were associated with uncontrolled asthma. J Asthma Allergy. 2018;11:73–79. doi: 10.2147/JAA.S155379.
- Truong-Thanh T, Vo-Thi-Kim A, Vu-Minh T, et al. The beneficial role of FeNO in association with GINA guidelines for titration of inhaled corticosteroids in adult asthma: a randomized study. Adv Med Sci. 2020;65(2):244–251. doi: 10.1016/j.advms.2020.03.001.
- Meltzer EO, Busse WW, Wenzel SE, et al. Use of the asthma control questionnaire to predict future risk of asthma exacerbation. J Allergy Clin Immunol. 2011;127(1):167–172. doi: 10.1016/j.jaci.2010.08.042.
- Mphahlele RE, Kitchin O, Masekela R. Barriers and determinants of asthma control in children and adolescents in Africa: a systematic review. BMJ Open. 2021;11(10):e053100. doi: 10.1136/bmjopen-2021-053100.
- Feng JX, Lin Y, Lin J, et al. Relationship between fractional exhaled nitric oxide level and efficacy of inhaled corticosteroid in asthma–COPD overlap syndrome patients with different disease severity. J Kor Med Sci. 2017;32(3):439–447. doi: 10.3346/jkms.2017.32.3.439.
- Kriti CY, Mohapatra AK, Manu MK, et al. Comparison of fractional exhaled nitric oxide, spirometry, and asthma control test, in predicting asthma exacerbations: a prospective cohort study. Lung India. 2020;37(5):394–399. doi: 10.4103/lungindia.lungindia_546_19.
- Voorend-van Bergen S, Vaessen-Verberne AA, Brackel HJ, et al. Monitoring strategies in children with asthma: a randomised controlled trial. Thorax. 2015;70(6):543–550. doi: 10.1136/thoraxjnl-2014-206161.
- Szefler SJ, Mitchell H, Sorkness CA, et al. Management of asthma based on exhaled nitric oxide in addition to guideline-based treatment for inner-city adolescents and young adults: a randomised controlled trial. Lancet. 2008;372(9643):1065–1072. doi: 10.1016/S0140-6736(08)61448-8.
- Al Ghobain MO, Alsubaie AS, Aljumah WA, et al. The correlation between fractional exhaled nitric oxide (FeNO), blood eosinophil count, immunoglobulin E levels, and spirometric values in patients with asthma. Cureus. 2023;15(2):e35289. doi: 10.7759/cureus.35289.
- Lommatzsch M, Klein M, Stoll P, et al. Type 2 biomarker expression (FeNO and blood eosinophils) is higher in severe adult-onset than in severe early-onset asthma. Allergy. 2021;76(10):3199–3202. doi: 10.1111/all.14997.
- Moeller A, Carlsen KH, Sly PD, ERS Task Force Monitoring Asthma in Children, et al. Monitoring asthma in childhood: lung function, bronchial responsiveness and inflammation. Eur Respir Rev. 2015;24(136):204–215. doi: 10.1183/16000617.00003914.
- Badar A, Salem AM, Bamosa AO, et al. Association between FeNO, total blood IgE, peripheral blood eosinophil and inflammatory cytokines in partly controlled asthma. JAA. 2020;13:533–543. doi: 10.2147/JAA.S274022.