Abstract
Background
Cushing’s syndrome (CS) is associated with increased risk for heart failure, which often initially manifests as left ventricular diastolic dysfunction (LVDD). In this study, we aimed to explore the potential risk factors of LVDD in CS by incorporating body composition parameters.
Methods
A retrospective study was conducted on patients diagnosed with endogenous CS no less than 18 years old. The control group consisted of healthy individuals who were matched to CS patients in terms of gender, age, and BMI. LIFEx software (version 7.3) was applied to measure epicardial adipose tissue volume (EATV) on non-contrast chest CT, as well as abdominal adipose tissue and skeletal muscle mass at the first lumbar vertebral level. Echocardiography was used to evaluate left ventricular (LV) diastolic function. Body compositions and clinical data were examined in relation to early LVDD.
Results
A total of 86 CS patients and 86 healthy controls were enrolled. EATV was significantly higher in CS patients compared to control subjects (150.33 cm3 [125.67, 189.41] vs 90.55 cm3 [66.80, 119.84], p < 0.001). CS patients had noticeably increased visceral fat but decreased skeletal muscle in comparison to their healthy counterparts. Higher prevalence of LVDD was found in CS patients based on LV diastolic function evaluated by E/A ratio (p < 0.001). EATV was proved to be an independent risk factor for LVDD in CS patients (OR = 1.015, 95%CI 1.003–1.026, p = 0.011). If the cut-point of EATV was set as 139.252 cm3 in CS patients, the diagnostic sensitivity and specificity of LVDD were 84.00% and 55.60%, respectively.
Conclusion
CS was associated with marked accumulation of EAT and visceral fat, reduced skeletal muscle mass, and increased prevalence of LVDD. EATV was an independent risk factor for LVDD, suggesting the potential role of EAT in the development of LVDD in CS.
KEY MESSAGES
This study explored the potential risk factors of LVDD in endogenous CS by incorporating body composition parameters. EATV was identified as an independent risk factor for LVDD. Targeted therapeutic interventions to reduce excessive cortisol-induced EAT accumulation may be promising to mitigate the risk of LVDD development in patients with CS.
Introduction
Cushing’s syndrome (CS), an endocrine disorder, is characterized by excessive cortisol exposure from endogenous adrenal secretion or exogenous glucocorticoid therapy [Citation1]. This disease is associated with a variety of cardiovascular risk factors, such as hypertension, insulin resistance, dyslipidemia, and metabolic syndrome, which could contribute to the occurrence of cardiovascular diseases [Citation2]. Moreover, it has been shown that excessive cortisol exposure caused cardiac structural alternations and cardiac dysfunction [Citation3]. Notably, patients affected by CS have higher mortality in comparison with the common population, with cardiovascular complications being the main death cause [Citation2].
Prior studies have indicated an increased risk of heart failure both in endogenous and iatrogenic CS [Citation4,Citation5], possibly due to the myocardial hypertrophy and fibrosis caused by excessive cortisol [Citation6]. Left ventricular diastolic dysfunction (LVDD) has been considered as an underlying pathophysiological process of heart failure, and is linked to heart failure occurrence and reduced survival [Citation7]. In CS patients, the presence of LVDD has been affirmed in cardiac magnetic resonance and echocardiography [Citation3,Citation6,Citation8]. Therefore, it is crucial to identify the risk factors of LVDD in individuals with CS. However, fewer data are accessible on the potential risk factors of LVDD in CS.
Visceral fat accumulation and sarcopenia are known risk factors for cardiovascular diseases [Citation9,Citation10]. Hypercortisolism can cause increase in visceral fat and skeletal muscle loss [Citation11,Citation12]. Both increased visceral adiposity and reduced skeletal muscle mass showed a negative association with left ventricular (LV) diastolic function [Citation13,Citation14]. Similarly, epicardial adipose tissue (EAT), a special visceral fat tissue between the myocardium and visceral pericardium, in contact with the myocardium and the coronary arteries [Citation15], has emerged as an independent cardiovascular risk factor. The relationship between EAT and LV diastolic function has been observed in individuals with metabolic syndrome, type 2 diabetes, and healthy subjects [Citation16–18]. Nonetheless, investigations exploring the effects of hypercortisolism on EAT are scarce [Citation19–21]. The influence of excess cortisol on EAT deposition requires further confirmation. Furthermore, the relative significance of different fat depots and skeletal muscle mass for LV diastolic function remains to be uncovered.
Consequently, this study aimed to investigate the impact of hypercortisolism on various body compositions, with a specific focus on EAT. Secondly, we aimed to elucidate the potential risk factors of LVDD in CS individuals by including body composition parameters.
Method
Participants
In this retrospective study, we investigated the participants diagnosed with endogenous CS no less than 18 years old. All included patients underwent both non-contrast chest CT and echocardiography in Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology between Jan 2016 to July 2023. The included patients were untreated or not cured by transsphenoidal surgery. CS was diagnosed according to the clinical practice criteria [Citation22], including abnormal cortisol rhythms, as well as the absence of cortisol suppression either during the 1 mg overnight dexamethasone suppression test or in the classic low-dose dexamethasone suppression test. Control subjects were selected from health-care center in our hospital, who are 1:1 matched to Cushing’s syndrome patients with comparable gender, body mass index (BMI) and age. The exclusion criteria for all individuals were as follows: (1) Previous or current use of glucocorticoid therapy within 5 years; (2) Severe hepatic, renal, hematological, or other systematic diseases; (3) Left ventricular ejection fraction (LVEF) <50%; (4) Diagnosis of congenital heart diseases, dilated cardiomyopathy, hypertrophic cardiomyopathy, coronary heart diseases, valvular heart diseases, and arrhythmias or history of cardiac surgery. The study was approved by the Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (approval No. # TJ-IRB20230875).
Clinical and laboratory data
We collected clinical characteristics and laboratory test results for both CS and control groups. Clinical characteristics such as age, height, weight, BMI, systolic blood pressure (SBP), diastolic blood pressure (DBP), and history of diseases, were collected. Laboratory test results contained fasting blood glucose (FBG), total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), serum uric acid (SUA), electrolytes (potassium and sodium), serum creatinine (Scr), and estimated glomerular filtration rate (eGFR). Besides, in the CS group, we also recorded the duration of hypercortisolism as well as serum cortisol levels at 0 am, 8 am, and 4 pm.
Hypertension was defined as systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg in two consecutive measurements on different days, or receiving antihypertensive medication. The definition of diabetes included fasting blood glucose level ≥7.0 mmol/L, or random blood glucose level ≥11.1 mmol/L at least twice, or the use of hypoglycemic agents.
Measurement of epicardial adipose tissue, abdominal adipose tissue, and skeletal muscle
All participants underwent non-contrast chest computed tomography (CT) scans on a 64-row CT scanner. Parameters were used for scans including 120 kVp, 0.5 sec/rotation, and a slice thickness of 1.25 mm. EAT was defined as adipose tissue between myocardium and visceral pericardium. We used LIFEx software (version 7.3, www.lifexsoft.org) to quantify EAT volume (EATV, cm3) on non-contrast chest CT from the pulmonary trunk bifurcation to the end of the heart cross-section. The EATV was automatically calculated with CT attenuation value threshold between −190 and −30 Hounsfield unit (HU) [Citation23,Citation24]. The 12th thoracic vertebrae were used to identify the first lumbar vertebral (L1) segment. Visceral fat area (VFA) and subcutaneous fat area (SFA) were also measured by LIFEx software at the L1 level on chest CT with an attenuation range of −190 to −30 HU, expressed in square centimeters [Citation25,Citation26]. Skeletal muscle area (SMA) was measured at the same level with HU ranges corresponding to muscle tissue of −29 to 150 HU [Citation27]. Skeletal muscle index (SMI) was calculated using skeletal muscle area divided by the square of the height: SMA (cm2)/height (m2). The measurement of body composition on non-contrast chest CT was displayed in Supplementary Figure 1.
Echocardiographic examination
All subjects had undergone transthoracic echocardiography performed by a specialized sound operator using GE Vivid E9 ultrasound system (GE Vingmed Ultrasound, Horten, Norway) equipped with a 1.7–3.3 MHz transducer according to American Society of Echocardiography (ASE) [Citation28]. Measurements were obtained in accordance with established protocols. In the parasternal long-axis view, left ventricular end-diastolic diameter (LVEDD), interventricular septal thickness (IVST), left ventricular posterior wall thickness in diastole (LVPWT), and left atrial diameter (LAD) were assessed. LVEF was determined using Biplane Simpson’s method. Additionally, pulsed-wave Doppler was utilized in the four-chamber apical view to measure the peak velocity of early (E) and late (A) atrial inflow. Subsequently, the E/A ratio was obtained, which served as an indicator for evaluating LV diastolic function, as previously described [Citation29]. When the E/A ratio was less than 1, it suggested impaired myocardial diastolic function [Citation29].
Statistical analysis
The normality of continuous variables was examined by the Kolmogorov-Smirnov test (n ≥ 50) and Shapiro-Wilk (n < 50). Normally distributed continuous variables were presented as means ± standard deviation (SD), while non-normally distributed variables were shown as median (interquartile range). To compare differences between the two groups, the t-test and Mann-Whitney U test were used for normally and non-normally distributed data, respectively. Categorical data were described as percentages and numbers, and the comparisons were made using Chi-square test. In the CS group, correlations between the clinical indicators, body composition parameters, echocardiographic parameters and E/A ratio were assessed by Pearson or Spearman’s correlation analysis. The significant variables (p < 0.05) in univariate linear correlation analysis were included in a stepwise multiple regression analysis model to examine the association of clinical indices and body composition parameters with the E/A ratio. The relationship between visceral fat and the E/A ratio was further examined through partial correlation analysis. Univariable binary logistic regression models were made to evaluate the correlations between variables and LVDD in CS subjects. Then a binary multivariable logistic regression (forward selection: likelihood ratio) was utilized to determine independent risk factors of LVDD by including significant variables with a P-value less than 0.1. The receiver operating characteristic (ROC) analysis was used to evaluate the effect of EATV on LVDD in CS. The calculation of ROC curve cutoff values was based on the Youden’s index. All data analyses were conducted using SPSS version 27.0 software. GraphPad Prism version 9.5.1 software (La Jolla, San Diego, CA, USA) was used for data visualization. Statistical significance was considered when the two-sided P-value was less than 0.05.
Results
Baseline characteristics of the Cushing’s syndrome and control subjects
The clinical characteristics, body composition characteristics, and echocardiographic evaluation of the CS and control subjects were summarized in . A total of 86 CS patients and age-, gender-, and BMI-matched 86 healthy controls were included in the analyses. In the CS group, 48 patients were identified as pituitary adenomas, 24 suffered from primary adrenal diseases, while 14 had ectopic ACTH secretion. Compared with control group, CS patients exhibited significantly higher blood pressure, serum sodium levels, elevated fasting blood glucose, lower serum potassium levels, and more severe lipid metabolism disorders (all p < 0.001). The higher prevalence of hypertension and diabetes were also observed in CS patients (both p < 0.001). Regarding body composition parameters, EATV and VFA were considerably increased but SMA and SMI were markedly decreased in CS patients (all p < 0.001). There were no significant differences found between two groups for SUA, Scr, eGFR, and SFA. In the echocardiographic examination, the IVST, LAD, LVPWT and A velocity were higher in CS subjects than controls, whereas E velocity and E/A ratio were remarkably lower (all p < 0.05). However, the LVEDD and LVEF in CS patients were not different from controls. Body compositions and left ventricular diastolic function of the CS group and the control group were shown in .
Figure 1. Body compositions and left ventricular diastolic function in Cushing’s syndrome and control group. Markedly increased epicardial fat volume (A) and visceral fat area (B) were observed, along with decreased skeletal muscle area (C) and skeletal muscle index (D) in CS patients. In terms of left ventricular diastolic function, CS patients exhibited a lower E/A ratio (E) and a higher prevalence of LVDD (F). CS Cushing’s syndrome, EATV epicardial adipose tissue volume, VFA visceral fat area, SMA skeletal muscle area, SMI skeletal muscle index, LVDD left ventricular diastolic dysfunction. ***P less than 0.001.
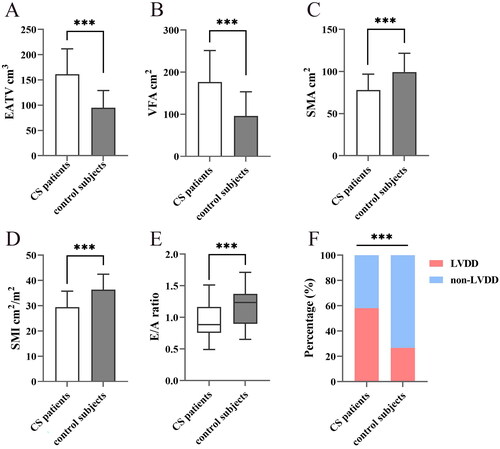
Table 1. Characteristics of Cushing’s syndrome patients and control subjects.
Comparisons between Cushing’s syndrome subjects with or without left ventricular diastolic dysfunction
The comparisons between CS patients with LVDD and CS patients without LVDD were presented in . CS subjects with LVDD tended to be older (p = 0.024), and had higher Scr level (p = 0.020), elevated cortisol level at 4 pm (p = 0.043), but lower HDL-C (p = 0.027) and eGFR (p = 0.019). Moreover, the CS subjects with LVDD had marked increase in EATV (p = 0.002), VFA (p = 0.009) and A velocity (p = 0.001), while E velocity (p < 0.001) and E/A ratio (p < 0.001) were lower compared to the CS patients without LVDD. There were no differences observed in SFA, SMA, SMI, BMI, duration, blood pressure, TC, TG, LDL-C, FBG, hypertension, diabetes, SUA, potassium, sodium, 8 am serum cortisol, 0 am serum cortisol, and other echocardiographic parameters between the two groups.
Table 2. Comparisons of characteristics between Cushing’s syndrome subjects with or without left ventricular diastolic dysfunction.
Relationship of other parameters with E/A ratio in Cushing’s syndrome subjects
The E/A ratio exhibited significant associations with some variables, including disease duration, age, Scr, hypertension, HDL-C, VFA, eGFR, and EATV, 4 pm serum cortisol (Supplementary Table 1). A stepwise multiple linear regression analysis () demonstrated that EATV, eGFR, and HDL-C independently correlated with the E/A ratio. After adjusting for disease duration, age, Scr, hypertension, HDL-C, eGFR, and serum cortisol levels at 4 pm, partial correlation analysis revealed no association between VFA and the E/A ratio (r = −0.159, p = 0.161). Correlation analysis of body compositions, biochemical indicators and E/A ratio was presented in .
Figure 2. Correlation analysis of body compositions, biochemical indicators and E/A ratio. A negative correlation was found between the E/A ratio and EATV and VFA. There was no correlation between SMA, SMI, and the E/A ratio. HDL-C and eGFR were positively correlated with the E/A ratio. EATV epicardial adipose tissue volume, VFA visceral fat area, SMA skeletal muscle area, SMI skeletal muscle index, HDL-C high-density lipoprotein cholesterol, eGFR estimated glomerular filtration rate.
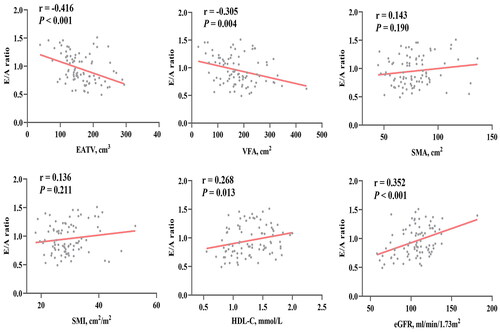
Table 3. Potential factors associated with E/A ratio in Cushing’s syndrome subjects in multiple regression analysis.
Risk factors of left ventricular diastolic dysfunction in Cushing’s syndrome subjects
Logistic regression analysis was conducted to identify risk factors of LVDD in CS subjects, as described in . The age, HDL-C, Scr, eGFR, EATV, and VFA were significantly associated with LVDD in CS in univariate logistic regression analysis (Supplementary Table 2). Notably, increased EATV was independently related to increased risk of LVDD in CS, whereas HDL-C and eGFR correlated with decreased risk. ROC analysis () demonstrated that EATV had a significant association with LVDD in CS, with an area under the curve (AUC) of 0.698 (95%CI 0.581–0.814, p = 0.002). When employing the cutoff of 139.252 cm3 EATV for assessment LVDD in CS, the sensitivity was 84.00%, and specificity 55.60%, positive likelihood ratio 1.89, and negative likelihood ratio 0.29.
Figure 3. Comparative analysis of epicardial adipose tissue volume in Cushing’s syndrome subjects with and without left ventricular diastolic dysfunction and ROC curve analysis. A. Significantly increased EATV in CS patients with LVDD. B. ROC analysis revealed a significant correlation between EATV and LVDD (AUC 0.698, 95%CI 0.581–0.814, p = 0.002). CS Cushing’s syndrome, EATV epicardial adipose tissue volume, LVDD left ventricular diastolic dysfunction, ROC receiver operating characteristic. **P less than 0.01.
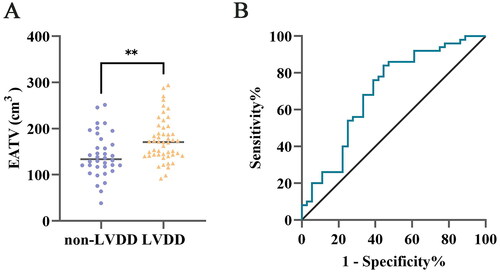
Table 4. Univariate and multivariate logistic regression analysis for the association of left ventricular diastolic dysfunction with various factors in Cushing’s syndrome subjects.
Correlation analysis of serum cortisol levels and EATV, VFA, SFA, and SMA was exhibited in Supplementary Table 3. Additionally, the comparisons of characteristics between ACTH-dependent and ACTH-independent CS patients were shown in Supplementary Table 4.
Discussion
In this retrospective study, we investigated the consequence of cortisol excess on body composition and explored possible risk factors of LVDD in CS. Our findings revealed that CS subjects presented sarcopenic obesity, increased EAT depots, and a higher prevalence of LVDD compared to age-, gender-, and BMI-matched controls. Additionally, following adjustments for confounding variables, EATV was found to be associated with the E/A ratio and identified as an independent risk factor for the presence of LVDD in CS patients, while visceral fat was not independently correlated with the E/A ratio or LVDD. We also failed to discover any correlation between low skeletal muscle mass and E/A ratio and LVDD. These results underscored the potentially pivotal role of excessive cortisol-induced EAT deposition in the development of LVDD in CS patients. It was worth noting that LVDD in CS was also significantly related to HLD-C and eGFR.
Obesity caused by CS is characterized by the predominant accumulation of fat in the abdominal region, particularly visceral fat [Citation2]. Quantitative data have shown that CS patients presented centripetal obesity and sarcopenia, manifesting as accumulation of visceral fat and skeletal muscle loss [Citation11,Citation30]. Our findings aligned with previous studies, demonstrating that CS patients showed increased visceral fat area (VFA) and decreased skeletal muscle area (SMA) at the L1 level compared to the control group [Citation11,Citation30]. Moreover, as a peculiar visceral adipose tissue, EAT has been observed to be overtly accumulated in treatment-naïve endogenous CS patients. Notably, our study included a larger sample size in comparison to previous studies [Citation19–21]. The accumulation of visceral fat and EAT both contribute to elevated cardiovascular disease risk in CS patients, which may stem from dysfunction of adipocytes, inflammation, imbalance in adipokines, abnormalities in lipid metabolism, and insulin resistance [Citation31,Citation32]. Likewise, similar to adipose tissues, the reduction in muscle mass has been linked to cardiovascular diseases [Citation33]. This relationship may be explained by factors, such as insulin resistance, enhanced oxidative stress, chronic inflammation, and impaired endothelial function [Citation34].
CS patients are susceptible to changes in cardiac structure and development of cardiac dysfunction [Citation3]. Impaired diastolic function has been suggested to be associated with progression of heart failure and increased mortality rates [Citation35,Citation36]. Yet, there is a lack of effective treatments for diastolic dysfunction, making it essential to identify potential risk factors so as to intervene in advance. In this study, we first incorporated body composition parameters and clinical indicators to determine risk factors of LVDD in CS. Muiesan et al. found CS subjects had a reduced E/A ratio and decreased E velocity compared to control group [Citation8]. They also noted that the E/A ratio was considerably linked to CS, hypertension and disease duration. These findings are consistent with our study, confirming the presence of LVDD in CS patients. Correlation analyses revealed significant association between the E/A ratio and some variables including age, BMI, Scr, hypertension, HDL-C, VFA, eGFR, and EATV. However, only HDL-C, eGFR, and EATV remained independently correlated with the E/A ratio in multivariate stepwise regression analysis, while disease duration and hypertension were not retained. Logistic regression analysis further identified EATV as an independent risk factor for LVDD. Conversely, there was no independent association of visceral fat with the E/A ratio or LVDD. Collectively, these results suggest that EAT might play a more important role in pathogenesis of LVDD in CS, although both visceral fat and EAT can affect diastolic function by secreting adipokines, as described in previous research [Citation37,Citation38].
In recent studies, the accumulation of EAT has been linked to diastolic dysfunction in some clinical conditions. Fontes-Carvalho et al. have observed an association between total EAT volume and diastolic dysfunction in patients after myocardial infarction [Citation37]. Lin et al. have demonstrated that accumulation EAT was an independent risk factor for LVDD in patients undergoing peritoneal dialysis [Citation39]. EAT can exert its impact on diastolic function through multiple mechanisms under pathological conditions. Firstly, enlarged EAT can cause compression on the heart, resulting in restricted cardiac expansion and consequently affecting diastolic function [Citation40]. Physiologically, EAT exerts cardioprotective effects by secreting a variety of bioactive factors. Nonetheless, abnormally increased EAT produces more pro-inflammatory factors and deleterious adipokines to impair the myocardium function and coronary arteries directly, thus promoting the occurrence of cardiovascular diseases [Citation41]. EAT may contribute to diastolic dysfunction by affecting coronary flow reserve [Citation42]. Furthermore, excessive free fatty acids released by EAT could trigger cardiomyocyte dysfunction and apoptosis, leading to cardiac dysfunction [Citation41,Citation43]. CS patients in remission for at least two years displayed notable accumulation of EAT [Citation19], indicating CS subjects remain at a high risk of developing LVDD even receiving treatment. It has been confirmed that EAT can be modulated by some agents, such as glucagon-like peptide 1 receptor agonists (GLP-1RAs), sodium-glucose cotransporter-2 inhibitors (SGLT2i), pioglitazone and statins [Citation18,Citation44–46]. Pioglitazone, for instance, has been shown to reduce EAT volume and improve diastolic function in patients with type 2 diabetes [Citation18]. The reduction of EAT resulting from SGLT2i may also contribute to the improvement of diastolic function [Citation41]. These approaches hold promise for reducing the risk of developing LVDD and other cardiovascular diseases in CS patients who have already received treatment. Targeted therapy for EAT is also beneficial for these individuals with EAT accumulation as a result of long-term steroid therapy [Citation47].
Previous studies have reported the relationship of low skeletal muscle mass and LVDD. Low skeletal muscle mass may induce insulin resistance, inflammation, and endothelial dysfunction, which ultimately alter myocardial structure and function leading to LVDD occurrence [Citation48–50]. In Korean adults, it has been reported that low skeletal muscle mass was involved in the development of LVDD [Citation14,Citation51]. SMI positively correlated with the E/A ratio, while lower SMI was linked to impaired diastolic function [Citation14,Citation51]. However, our study did not find any association of decreased skeletal muscle mass with LVDD in CS patients. Neither SMA nor SMI was related to LV diastolic function, probably partly attributed to limited sample size compared to previous results. Another probable reason may be the relatively short disease history of sarcopenia in CS patients, as they often resort to medical intervention for typical clinical manifestations. Further investigations involving larger sample sizes are warranted to explore the relationship of reduced skeletal muscle mass with LVDD in CS individuals.
We also observed an association between diastolic dysfunction and eGFR as well as HDL-C levels. Abnormal kidney function displayed a connection with the risk of developing LVDD [Citation52]. Borrelli et al. found that lower eGFR showed correlation with the possibility of occurring LVDD in non-dialysis chronic kidney disease patients [Citation53]. Sun et al. observed that HDL-C to hsCRP ratio was a protective factor for LVDD in patients without significant coronary atherosclerosis [Citation54]. Conversely, impaired diastolic function in individuals with essential hypertension was associated with low levels of HDL-C [Citation55]. The anti-inflammatory properties of HDL-C may explain its role as a protective marker against LVDD [Citation56]. In our study, multivariable logistic regression analysis revealed that both eGFR and HDL-C were protective factors for LVDD in CS subjects, consistently mirroring findings in other populations. These results underlined the heightened risk of developing LVDD in CS patients with renal dysfunction or reduced HDL-C levels. Thus, it is crucial to closely monitor LV diastolic function in CS patients suffering from renal dysfunction and/or diminished HDL-C levels.
There were some limitations in our study. Firstly, despite the exclusion of patients with severe diastolic and systolic dysfunction, hypertrophic cardiomyopathy, arrhythmias, coronary artery atherosclerotic heart disease, and other related conditions, it was important to note that the evaluation of LVDD was deemed relatively simple using the E/A ratio. Additional echocardiographic parameters associated with diastolic function need to be utilized for the assessment of diastolic function, including mitral annular lateral or septal velocity (e’), E/e’ ratio, and left atrial maximum volume index, etc. Secondly, the sample size in our study was relatively small. Future studies with larger sample sizes are required to validate our results and explore the impact of excess cortisol on EAT deposition, as well as identify additional risk factors for the development of LVDD in CS patients.
Conclusion
In conclusion, we observed significant increase in EATV and visceral fat, along with a decrease in skeletal muscle mass, in CS patients compared with age-, gender-, BMI-matched healthy control participants. Our findings revealed that EATV independently correlated with the presence of LVDD in CS subjects. These results suggested that reducing EAT accumulation may be promising to mitigate the risk of LVDD development in patients with CS.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki and approved by the Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (approval No. # TJ-IRB20230875).
Consent to participate
Informed consent was waived by our institutional review board because of the retrospective nature of our study.
Authors contributions
QR: the study design, manuscript drafting and data collection. YS, JL, KW: Assistance in data collection and processing. Z-LL, YY, M-XZ, GY, X-FY, W-TH: the study design, manuscript revising and the approval of the manuscript. All authors contributed to the article and approved the submitted version.
Supplemental Material
Download MS Word (347.6 KB)Acknowledgements
The authors would like to thank Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology for providing the data.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data produced or analyzed during this study are incorporated in this article or displayed in supplementary material.
Additional information
Funding
References
- Gadelha M, Gatto F, Wildemberg LE, et al. Cushing’s syndrome. Lancet. 2023;402(10418):2237–2252. doi: 10.1016/S0140-6736(23)01961-X.
- Pivonello R, Isidori AM, De Martino MC, et al. Complications of Cushing’s syndrome: state of the art. Lancet Diabetes Endocrinol. 2016;4(7):611–629. doi: 10.1016/S2213-8587(16)00086-3.
- Kanzaki A, Kadoya M, Katayama S, et al. Cardiac hypertrophy and related dysfunctions in Cushing syndrome patients-literature review. J Clin Med. 2022;11(23):7035. doi: 10.3390/jcm11237035.
- Dekkers OM, Horváth-Puhó E, Jørgensen JOL, et al. Multisystem morbidity and mortality in Cushing’s syndrome: a cohort study. J Clin Endocrinol Metab. 2013;98(6):2277–2284. doi: 10.1210/jc.2012-3582.
- Fardet L, Petersen I, Nazareth I. Risk of cardiovascular events in people prescribed glucocorticoids with iatrogenic Cushing’s syndrome: cohort study. BMJ. 2012;345:e4928. doi: 10.1136/bmj.e4928.
- Moustaki M, Markousis-Mavrogenis G, Vryonidou A, et al. Cardiac disease in Cushing’s syndrome. Emphasis on the role of cardiovascular magnetic resonance imaging. Endocrine. 2023;83(3):548–558. doi: 10.1007/s12020-023-03623-0.
- Kosmala W, Marwick TH. Asymptomatic left ventricular diastolic dysfunction: predicting progression to symptomatic heart failure. JACC Cardiovasc Imaging. 2020;13(1 Pt 2):215–227. doi: 10.1016/j.jcmg.2018.10.039.
- Muiesan ML, Lupia M, Salvetti M, et al. Left ventricular structural and functional characteristics in Cushing’s syndrome. J Am Coll Cardiol. 2003;41(12):2275–2279. doi: 10.1016/s0735-1097(03)00493-5.
- Chin SO, Rhee SY, Chon S, et al. Sarcopenia is independently associated with cardiovascular disease in older Korean adults: the Korea National Health and Nutrition Examination Survey (KNHANES) from 2009. PLoS One. 2013;8(3):e60119. doi: 10.1371/journal.pone.0060119.
- Fang H, Berg E, Cheng X, et al. How to best assess abdominal obesity. Curr Opin Clin Nutr Metab Care. 2018;21(5):360–365. doi: 10.1097/MCO.0000000000000485.
- Delivanis DA, Iñiguez-Ariza NM, Zeb MH, et al. Impact of hypercortisolism on skeletal muscle mass and adipose tissue mass in patients with adrenal adenomas. Clin Endocrinol (Oxf). 2018;88(2):209–216. doi: 10.1111/cen.13512.
- Schakman O, Kalista S, Barbé C, et al. Glucocorticoid-induced skeletal muscle atrophy. Int J Biochem Cell Biol. 2013;45(10):2163–2172. doi: 10.1016/j.biocel.2013.05.036.
- Canepa M, Strait JB, Milaneschi Y, et al. The relationship between visceral adiposity and left ventricular diastolic function: results from the Baltimore Longitudinal Study of Aging. Nutr Metab Cardiovasc Dis. 2013;23(12):1263–1270. doi: 10.1016/j.numecd.2013.04.003.
- Ko B-J, Chang Y, Kang JG, et al. Low relative muscle mass and left ventricular diastolic dysfunction in middle-aged adults. Int J Cardiol. 2018;255:118–123. doi: 10.1016/j.ijcard.2017.07.089.
- Villasante Fricke AC, Iacobellis G. Epicardial adipose tissue: clinical biomarker of cardio-metabolic risk. Int J Mol Sci. 2019;20(23):5989. doi: 10.3390/ijms20235989.
- de Wit-Verheggen VHW, Altintas S, Spee RJM, et al. Pericardial fat and its influence on cardiac diastolic function. Cardiovasc Diabetol. 2020;19(1):129. doi: 10.1186/s12933-020-01097-2.
- Park HE, Choi SY, Kim M. Association of epicardial fat with left ventricular diastolic function in subjects with metabolic syndrome: assessment using 2-dimensional echocardiography. BMC Cardiovasc Disord. 2014;14(1):3. doi: 10.1186/1471-2261-14-3.
- Moody AJ, Molina-Wilkins M, Clarke GD, et al. Pioglitazone reduces epicardial fat and improves diastolic function in patients with type 2 diabetes. Diabetes Obes Metab. 2023;25(2):426–434. doi: 10.1111/dom.14885.
- Maurice F, Gaborit B, Vincentelli C, et al. Cushing syndrome is associated with subclinical LV dysfunction and increased epicardial adipose tissue. J Am Coll Cardiol. 2018;72(18):2276–2277. doi: 10.1016/j.jacc.2018.07.096.
- Wolf P, Marty B, Bouazizi K, et al. Epicardial and pericardial adiposity without myocardial steatosis in Cushing syndrome. J Clin Endocrinol Metab. 2021;106(12):3505–3514. doi: 10.1210/clinem/dgab556.
- Wang M, Qin L, Bao W, et al. Epicardial and pericoronary adipose tissue and coronary plaque burden in patients with Cushing’s syndrome: a propensity score-matched study. J Endocrinol Invest. 2024;47(8):1995–2005. doi: 10.1007/s40618-023-02295-x.
- Fleseriu M, Auchus R, Bancos I, et al. Consensus on diagnosis and management of Cushing’s disease: a guideline update. Lancet Diabetes Endocrinol. 2021;9(12):847–875. doi: 10.1016/S2213-8587(21)00235-7.
- Grodecki K, Lin A, Razipour A, et al. Epicardial adipose tissue is associated with extent of pneumonia and adverse outcomes in patients with COVID-19. Metabolism. 2021;115:154436. doi: 10.1016/j.metabol.2020.154436.
- Yin L, Yan C, Yang C, et al. Measurement of epicardial adipose tissue using non-contrast routine chest-CT: a consideration of threshold adjustment for fatty attenuation. BMC Med Imaging. 2022;22(1):114. doi: 10.1186/s12880-022-00840-3.
- Derstine BA, Holcombe SA, Ross BE, et al. Healthy US population reference values for CT visceral fat measurements and the impact of IV contrast, HU range, and spinal levels. Sci Rep. 2022;12(1):2374. doi: 10.1038/s41598-022-06232-5.
- Liu D, Garrett JW, Lee MH, et al. Fully automated CT-based adiposity assessment: comparison of the L1 and L3 vertebral levels for opportunistic prediction. Abdom Radiol (NY). 2023;48(2):787–795. doi: 10.1007/s00261-022-03728-6.
- Liu S, Han X, Li J, et al. Feasibility of using chest computed tomography (CT) imaging at the first lumbar vertebra (L1) level to assess skeletal muscle mass: a retrospective study. PeerJ. 2023;11:e16652. doi: 10.7717/peerj.16652.
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16(3):233–270. doi: 10.1093/ehjci/jev014.
- Oh JK, Hatle L, Tajik AJ, et al. Diastolic heart failure can be diagnosed by comprehensive two-dimensional and Doppler echocardiography. J Am Coll Cardiol. 2006;47(3):500–506. doi: 10.1016/j.jacc.2005.09.032.
- Geer EB, Shen W, Gallagher D, et al. MRI assessment of lean and adipose tissue distribution in female patients with Cushing’s disease. Clin Endocrinol (Oxf). 2010;73(4):469–475. doi: 10.1111/j.1365-2265.2010.03829.x.
- Gruzdeva O, Borodkina D, Uchasova E, et al. Localization of fat depots and cardiovascular risk. Lipids Health Dis. 2018;17(1):218. doi: 10.1186/s12944-018-0856-8.
- Neeland IJ, Ross R, Després J-P, et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol. 2019;7(9):715–725. doi: 10.1016/S2213-8587(19)30084-1.
- Damluji AA, Alfaraidhy M, AlHajri N, et al. Sarcopenia and cardiovascular diseases. Circulation. 2023;147(20):1534–1553. doi: 10.1161/CIRCULATIONAHA.123.064071.
- Yuan S, Larsson SC. Epidemiology of sarcopenia: prevalence, risk factors, and consequences. Metabolism. 2023;144:155533. doi: 10.1016/j.metabol.2023.155533.
- Nagueh SF. Left ventricular diastolic function: understanding pathophysiology, diagnosis, and prognosis with echocardiography. JACC Cardiovasc Imaging. 2020;13(1 Pt 2):228–244. doi: 10.1016/j.jcmg.2018.10.038.
- Redfield MM, Jacobsen SJ, Burnett JC, et al. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289(2):194–202. doi: 10.1001/jama.289.2.194.
- Fontes-Carvalho R, Fontes-Oliveira M, Sampaio F, et al. Influence of epicardial and visceral fat on left ventricular diastolic and systolic functions in patients after myocardial infarction. Am J Cardiol. 2014;114(11):1663–1669. doi: 10.1016/j.amjcard.2014.08.037.
- Sawada N, Daimon M, Kawata T, et al. The significance of the effect of visceral adiposity on left ventricular diastolic function in the general population. Sci Rep. 2019;9(1):4435. doi: 10.1038/s41598-018-37137-x.
- Lin HH, Lee JK, Yang CY, et al. Accumulation of epicardial fat rather than visceral fat is an independent risk factor for left ventricular diastolic dysfunction in patients undergoing peritoneal dialysis. Cardiovasc Diabetol. 2013;12(1):127. doi: 10.1186/1475-2840-12-127.
- Nerlekar N, Muthalaly RG, Wong N, et al. Association of volumetric epicardial adipose tissue quantification and cardiac structure and function. J Am Heart Assoc. 2018;7(23):e009975. doi: 10.1161/JAHA.118.009975.
- Iacobellis G. Epicardial adipose tissue in contemporary cardiology. Nat Rev Cardiol. 2022;19(9):593–606. doi: 10.1038/s41569-022-00679-9.
- Nakanishi K, Fukuda S, Tanaka A, et al. Relationships between periventricular epicardial adipose tissue accumulation, coronary microcirculation, and left ventricular diastolic dysfunction. Can J Cardiol. 2017;33(11):1489–1497. doi: 10.1016/j.cjca.2017.08.001.
- Wu C-K, Tsai H-Y, Su M-YM, et al. Evolutional change in epicardial fat and its correlation with myocardial diffuse fibrosis in heart failure patients. J Clin Lipidol. 2017;11(6):1421–1431. doi: 10.1016/j.jacl.2017.08.018.
- Park J-H, Park YS, Kim YJ, et al. Effects of statins on the epicardial fat thickness in patients with coronary artery stenosis underwent percutaneous coronary intervention: comparison of atorvastatin with simvastatin/ezetimibe. J Cardiovasc Ultrasound. 2010;18(4):121–126. doi: 10.4250/jcu.2010.18.4.121.
- Morano S, Romagnoli E, Filardi T, et al. Short-term effects of glucagon-like peptide 1 (GLP-1) receptor agonists on fat distribution in patients with type 2 diabetes mellitus: an ultrasonography study. Acta Diabetol. 2015;52(4):727–732. doi: 10.1007/s00592-014-0710-z.
- Sato T, Aizawa Y, Yuasa S, et al. The effect of dapagliflozin treatment on epicardial adipose tissue volume. Cardiovasc Diabetol. 2018;17(1):6. doi: 10.1186/s12933-017-0658-8.
- Kitterer D, Latus J, Henes J, et al. Impact of long-term steroid therapy on epicardial and pericardial fat deposition: a cardiac MRI study. Cardiovasc Diabetol. 2015;14(1):130. doi: 10.1186/s12933-015-0289-x.
- Kalyani RR, Corriere M, Ferrucci L. Age-related and disease-related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014;2(10):819–829. doi: 10.1016/S2213-8587(14)70034-8.
- Masiha S, Sundström J, Lind L. Inflammatory markers are associated with left ventricular hypertrophy and diastolic dysfunction in a population-based sample of elderly men and women. J Hum Hypertens. 2013;27(1):13–17. doi: 10.1038/jhh.2011.113.
- Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62(4):263–271. doi: 10.1016/j.jacc.2013.02.092.
- Jung M-H, Ihm S-H, Park SM, et al. Effects of sarcopenia, body mass indices, and sarcopenic obesity on diastolic function and exercise capacity in Koreans. Metabolism. 2019;97:18–24. doi: 10.1016/j.metabol.2019.05.007.
- Miyajima Y, Toyama T, Mori M, et al. Relationships between kidney dysfunction and left ventricular diastolic dysfunction: a hospital-based retrospective study. J Nephrol. 2021;34(3):773–780. doi: 10.1007/s40620-020-00940-9.
- Borrelli S, De Nicola L, Garofalo C, et al. Prevalence and renal prognosis of left ventricular diastolic dysfunction in non-dialysis chronic kidney disease patients with preserved systolic function. J Hypertens. 2022;40(4):723–731. doi: 10.1097/HJH.0000000000003069.
- Sun L, Liu X, Li W, et al. HDL-C to hsCRP ratio is associated with left ventricular diastolic function in absence of significant coronary atherosclerosis. Lipids Health Dis. 2019;18(1):219. doi: 10.1186/s12944-019-1157-6.
- Horio T, Miyazato J, Kamide K, et al. Influence of low high-density lipoprotein cholesterol on left ventricular hypertrophy and diastolic function in essential hypertension. Am J Hypertens. 2003;16(11 Pt 1):938–944. doi: 10.1016/s0895-7061(03)01015-x.
- Thacker SG, Zarzour A, Chen Y, et al. High-density lipoprotein reduces inflammation from cholesterol crystals by inhibiting inflammasome activation. Immunology. 2016;149(3):306–319. doi: 10.1111/imm.12638.
