Abstract
Clinical islet transplantation trials based on cadaveric allogenic islets have demonstrated that it is indeed possible to restore near‐physiological insulin secretion capacity in a type 1 diabetic patient through transplantation of insulin‐producing cells. In order to develop this form of therapy to become available for the vast majority of patients with diabetes, new sources of transplantable insulin‐producing cells need to be identified. Stem cells provide the best potential to achieve this goal. Controversial results have been presented concerning the existence and nature of pancreatic islet stem or precursor cells. An increasing body of evidence suggests that the pancreatic and hepatic cell types (hepatocytes, islet, acinar and ductal cells) have remarkable plasticity and can de‐ and trans‐differentiate into each other under appropriate conditions. Elucidation of the molecular mechanisms regulating these processes could lead to clinically applicable ways of either inducing pancreatic islet regeneration in situ or to expanding the insulin‐producing cells in vitro for transplantation. The emergence of human embryonic stem cells has led to an active area of research aiming to achieve targeted differentiation of these cells into a safely transplantable beta‐like cell. After initial excitement, it appears that much basic research is still required before this goal could be achieved.
Introduction
Type 1 diabetes results from specific autoimmune‐mediated destruction of the insulin‐producing beta cells within the pancreatic islets of Langerhans. Since the discovery of insulin more than 80 years ago, replacement therapy by injected insulin has been the only option for patients with type 1 diabetes. Although insulin therapy has developed enormously, even the most modern technologies do not allow the maintenance of normoglycemia. As a result of this, hyperglycemia‐related microvascular complications remain a major problem. It is obvious that this problem can only be overcome by a dynamic form of insulin replacement, where minute‐to‐minute changes in glycemia control the delivery of insulin. While there has been some progress in the building of mechanical closed‐loop systems where glucose sensors control an insulin‐delivery device, the technological challenges for long‐term safety and function are formidable. The biological alternative, the re‐introduction of beta cells into the organism, seems more attractive because no mechanical device could ever mimic the fine‐tuned control of nutrient‐induced insulin release by a normal beta cell.
The proof of principle for beta‐cell replacement therapy has been established by clinical islet transplantation trials. Injection of islets isolated from cadaveric organ donor pancreases into the portal vein of a type 1 diabetic patient can result in independence of injected insulin Citation1. However, this most often requires the use of islets from more thanone donor, making it impossible to include more than a few patients in the clinical trials. Moreover, preserved graft function is based on the use of immunosuppressive medication which has to counteract both immunorejection and recurrent beta‐cell autoimmunity. For these reasons, beta‐cell transplantation cannot currently be viewed as a treatment option for a type 1 diabetic patient whodoes not have terminal kidney disease or exceptionally labile disease. In order for beta‐cell replacement therapy to really have a major clinical impact, new sources oftransplantable insulin‐producing cells need to be developed and graft function needs to be maintained without the use of extensive immunosuppression. In this short review,we focus on the stem‐cell‐based possibilities for the generation of insulin‐producing cells for transplantation. The immunological and other aspects of clinical islet transplantation have recently been reviewed elsewhere Citation2.
Key messages
The pancreatic cell mass changes in response to insulin demand through either proliferation of beta cells or neogenesis of islets from precursor cells.
The plasticity of pancreatic cell types is greater than previously thought, indicating that the endocrine precursors may be derived from dedifferentiated acinar or islet cells.
Stem cells could provide a source of normal beta cells for cell replacement therapy of diabetes, but much basic research is still required to reach this goal.
Pancreatic stem cells
The most logical source of islet stem cells would naturally be the pancreas, assuming that such precursor cells could be isolated and grown in large numbers. Pancreas is an endoderm‐derived organ consisting of a large exocrine (acinar) and a small endocrine (islet) compartment. During embryonic development, both endocrine and exocrine cells develop from common stem cells within the early gut endoderm. A specific cell type, characterized by the expression of two transcription factors, Pdx‐1 (human equivalent is Ipf‐1) and neurogenin‐3 (Ngn‐3) represents the precursor of all islet cell types Citation3, Citation4. The pancreatic islet stem cells have been fairly well characterized in the developing pancreas. However, much more controversy remains concerning the possible existence and nature of stem or precursor cells in the adult pancreas. Numerous studies in several rodent species have shown that even the adult pancreas has a considerable capacity to regenerate after subtotal pancreatectomy or injury from ductal occlusion. A general view has been that the islet precursor cells reside within the pancreatic ductal epithelium, and that neogenesis would be an important component in islet regeneration, in addition to replication of endocrine cells Citation5, Citation6. More primitive types of islet‐associated stem cells have also been identified, particularly those characterized by expression of the neural precursor marker nestin Citation7. These concepts were seriously challenged by a transgenic cell lineage tracing analysis in the mouse, which clearly showed that in postnatal life, all new beta cells were derived from pre‐existing insulin‐expressing cells, with no contribution from an insulin‐negative cell pool Citation8. Significant controversy currently exists within the area of potential islet stem/progenitor cells. Nevertheless, several studies clearly suggest that new beta cells could be generated in vitro from cultured pancreatic islet or duct cells.
Beta‐cell generation from pancreatic ductal cells
The hypothesis that new islets could be generated from pancreatic ducts is based on observations of embryonic pancreatic development and pancreatic regeneration which clearly show features suggesting the budding of new islets from ductal epithelium. Ramiya et al. first described the generation of new islets from pancreatic ductal epithelial cells in vitroCitation9. The authors isolated and digested the pancreatic ducts from adult prediabetic NOD (non‐obese diabetic) mice and established long‐term cultures of putative islet‐producing stem cells. These cells formed well organized islet‐like structures characterized by the expression of insulin, glucagon and other islet‐associated markers. Implantation of these in vitro‐derived islets could reverse the hyperglycemia of diabetic mice.
Neogenesis of islets in human adult pancreatic ductal tissue culture has been reported by Bonner‐Weir et al. and our group Citation10, Citation11. This method is based on the culture of mixed cells derived from left‐over fractions after human islet isolation. In the first stage, the cells are expanded in culture, resulting in cell monolayers consisting mainly of cytokeratin 19 (CK19)‐positive ductal epithelial cells, with some mesenchymal cells and a few endocrine cells. The cells are then induced to differentiate by applying serum‐free medium supplemented with nicotinamide and a thin layer of commercial extracellular matrix (Matrigel) on top of the cells to induce the formation of three‐dimensional cysts from which the islet cells bud out. The cultivated human islet buds (CHIBs) contain insulin‐ and glucagon‐positive endocrine cells and some cytokeratin (CK19)‐positive epithelial cells. The level of insulin gene expression in the CHIBs is only about 5% of that seen in freshly isolated pure islets, but they are able to release insulin in response to glucose. By pulse‐chase analysis of bromodeoxyuridine (BrdU)‐labelled cells, we obtained direct evidence that the differentiated endocrine cells were derived from proliferating CK19‐positive cells Citation11.
Studies in rodents have shown that the combination of epidermal growth factor (EGF) and gastrin stimulates the proliferation of pancreatic ductal cells and their differentiation into islets Citation12, Citation13. This same growth factor combination also induced a clear increase in the number of beta cells when applied on cultured human adult islet cells Citation14. Analysis of cell populations and their proliferation in these experiments suggests that the increase in beta‐cell number resulted from the differentiation of islet‐associated duct cells rather than replication of pre‐existing beta cells. An endocrine differentiation program can also be activated in human pancreatic duct cells through the forced expression of neurogenin 3 in these cells Citation15.
All of the studies with cultured human pancreatic cells are hampered by the fact that it is very difficult to obtain 100% pure cell populations – either endocrine, exocrine or ductal – for these studies and it is also becoming apparent that the phenotype of the various cell types is not stable once the cells are placed in culture. Definitive answers to the cell lineage origin of new islet cells would require efficient labelling of specific cell types prior to the tissue culture experiments, and this has so far not been possible.
Islet‐associated tissue stem cells
Nestin, a cytoskeletal protein expressed in neural stem cells, is expressed in adult pancreatic stellate cells and vascular endothelium Citation16, Citation17. Based on the studies of Zulewski et al. Citation7, nestin‐positive cells can be grown out of purified islets and passaged repeatedly in media favouring neural stem cell proliferation. Upon confluence, these multipotential cells differentiated into cells expressing pancreatic exocrine and endocrine genes as well as some hepatic markers. The authors concluded that nestin‐positive islet‐derived progenitor cells may participate in the neogenesis of islet endocrine cells. Clonal nestin‐positive cells isolated from adult mouse pancreas have also been shown to give rise to neural and pancreatic lineages Citation18. Controversial to these studies, a detailed analysis of early human pancreatic development showed that endocrine precursors do not express nestin Citation19. Two transgenic lineage tracing experiments in mice also conclusively showed that pancreatic islet cells are not derived from nestin‐expressing precursors Citation20, Citation21. It is thus clear that during normal development in vivo, the pancreatic endocrine cells do not differentiate from non‐epithelial cells resembling the neuronal stem cells, and the tissue culture observations most likely represent a phenomenon specific for the in vitro conditions.
Plasticity of pancreatic cell types
Adult acinar cells of rodents show remarkable capacity to transdifferentiate into ductal epithelial cells in vivo after pancreatic duct occlusion as well as in vitroCitation22, Citation23. These transdifferentiated cells may also give rise to new islet tissue both in vivoCitation13 and in vitro after stimulation with specific growth factors Citation24. Also islet cells may dedifferentiate into a more primitive proliferative epithelial phenotype. This has been shown for cultured human islets which could be expanded for several passages in vitro as epithelial cells expressing Pdx‐1/Ipf‐1 but not insulin Citation25. We have recently shown that many of the human islet cells start to co‐express ductal cell markers and dedifferentiate into duct‐like epithelial cells soon after being placed in culture. These cells can serve as islet precursors when induced to differentiate. However, if the original endocrine cells are depleted (by negative selection for neuronal cell adhesion molecule/N‐CAM expression) from the cultures at the onset of the experiment, the remaining CK19‐positive duct‐like epithelial cells can proliferate but not differentiate into islet cells Citation26. Gershengorn et al. showed that human islet cells may even undergo epithelial‐mesenchymal transition into fibroblastoid cells which can, after a period of substantial expansion, re‐differentiate into aggregates of epithelial cells with a low level expression of islet endocrine genes Citation27. It is possible that the previously described nestin‐positive precursor cells could represent a similar reversible epithelial‐mesenchymal transition Citation28, Citation29.
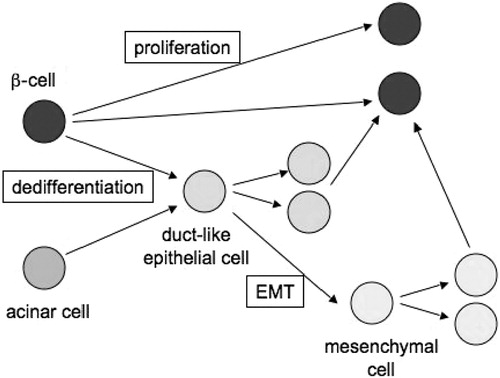
Based on the recent observations described above, the idea of pancreatic islet plasticity emerges as an important concept. This implies that in addition to proliferation of the beta cells, which is known to be minimal in the human islets, the islet cell mass could expand through a transient dedifferentiation and proliferation phase. This notion would not necessarily imply the existence of a specific tissue stem cell type and would not conflict with the observations made in mice showing that only self‐duplication of beta cells is responsible for the expansion of this cell type Citation8 (Figure ). However, many conflicting results have been published, and the existence of a specific rare pancreatic stem cell type cannot be ruled out Citation30. Whether it will ever be possible to translate these experimental studies into clinical therapies, remains unclear. In theory, specific stimulation of beta cell neogenesis could be the optimal way to restore the beta‐cell mass in type 1 diabetes, assuming that autoimmunity could be controlled.
Figure 1 Potential pathways of beta‐cell expansion. Direct proliferation is relatively active in many rodents, but human beta cells have an extremely low capacity to replicate. Instead, it appears that human beta cells can expand through a phase of transient dedifferentiation into a more primitive duct‐like epithelial cell, or even through epithelial‐mesenchymal transition (EMT) into a mesenchyme‐like islet precursor.
Other types of somatic stem cells
Bone marrow (BM) has been shown to contain multipotent stem cells that can differentiate even towards ectodermal or endodermal directions Citation31. Although the true multipotency of BM‐derived stem cells is quite controversial, it is of particular interest in this context that several studies have suggested that BM stem cells can contribute to the pancreatic islet cell population in various models of islet regeneration Citation32, Citation33. It has also been shown that BM‐derived stem cells can differentiate in vitro into insulin‐producing cells with many characteristics of true beta cells Citation34. The idea of using a diabetic patient's own bone marrow as a source of autologous insulin‐producing cells for beta cell replacement would be very attractive. However, the evidence for this is until now rather fragmentary and based only on rodent models.
Liver stem cells would be another attractive source for new beta cells because pancreas and liver arise from common endodermal progenitors. Convincing evidence has been presented demonstrating the transdifferentiation of immortalized rat hepatocytes into functional insulin‐producing cells after transduction with a superactive form of the Pdx‐1 gene Citation35. After a period of maturation induced by hyperglycemia, the phenotype of these cells closely resembled that of true beta cells, and transplantation of the cells in diabetic NOD‐Scid mice could reverse their hyperglycemia. Pdx‐1 overexpression in cell lines derived from human fetal hepatocytes has yielded similarly promising results Citation36. Liver stem cells could thus provide another clinically relevant source of precursors to be used for the generation of transplantable insulin‐producing cells.
Embryonic stem cells
Whether or not specific beta‐cell precursors exist in the adult pancreas, it is clear that true pancreatic stem cells exist during embryonic and fetal development. Since the establishment of human embryonic stem cell (hESC) lines in 1998 Citation37, major expectations have been set on these cells as a renewable source of cells for cell replacement therapies, due to their remarkable potential to differentiate into all cell types of an organism. The first mouse embryonic stem cell (mESC) lines were established already in 1981 Citation38, Citation39. So far, most studies aiming to form insulin‐producing beta‐cells from embryonic stem (ES) cells have been made on mouse cells.
The first report of successful generation of insulin‐producing cells from mES cells was published in 2000 Citation40. A cell‐trapping system utilizing the human insulin promoter was used for selection of insulin‐producing cells from spontaneously differentiated ES cells, followed by a maturation stage. When transplanted into diabetic mice, these cells were able to restore normoglycemia. However, only 1% of the cells were insulin‐positive and the reproducibility of the protocol was poor. This strategy has later been enhanced by using the Nkx6.1 promoter in the gene trap Citation41. Two other groups showed that cells of all islet lineages, including insulin‐producing cells, do develop during the spontaneous differentiation of mES cells as embryoid bodies Citation42, Citation43. However, the number of insulin‐positive cells in these cultures remained very low – less than 0.1%.
These publications indicated that although mouse ES cells indeed have the potential to differentiate into insulin‐producing cells, the proportion of this specific population in the heterogeneous cell mass is very small, and more efficient ways of directing the differentiation have to be developed. This can in principle be done in two ways, either by modifying culture conditions, for example by addition of specific growth factors, or by genetic manipulation of the ES cells aiming at the over‐expression of master regulators of the desired differentiation pathway.
In 2001, much interest was raised by a report describing the generation of insulin‐producing islet‐like structures from mouse ES cells through a five‐step protocol Citation44. It was modified from protocols originally generated for neuronal differentiation Citation45, based on the idea that as neural and pancreatic beta‐cells share several features and expression markers during development and both were thought to be derived from nestin‐expressing precursors, both cell types could be generated from the same precursor population. Several modifications of the protocol were published, including the addition of a phosphoinositide 3‐kinase (PI3K) inhibitor to the differentiation step Citation46, or genetic manipulation of the ES cells to further induce islet‐like differentiation Citation47–49. These modifications resulted in insulin‐positive cell clusters with more beta‐cell specific characteristics, including the ability to rescue diabetic mice upon transplantation Citation46, Citation47. It was, however, later shown that many of the insulin‐positive cells generated using this protocol were not synthesizing insulin de novo, but that the insulin was concentrated from the culture medium and released by apoptotic cells Citation50, Citation51. Furthermore, it was shown that the generated insulin‐expressing cells had neuronal features. Several of the genes generally used as markers for beta‐cell development have been shown to be expressed also in developing neurons, and therefore cells taken for immature beta‐cells might as well be developing neurons Citation52. In fact, it has been proposed that the potential of ES cells to generate cells of the endoderm lineage and consequently pancreatic precursors is lost when the cells are predetermined to an ectodermal fate by selectively enriching the cultures with cells expressing nestin Citation52, Citation53, and that induction of pancreatic differentiation of cells committed to a neural fate may activate apoptotic pathways Citation54.
Several protocols have been developed during recent years in order to obtain more viable and functional insulin‐producing cells from mouse ES cells. Blyzczyk et al. showed that by omitting the nestin selection step and instead allowing the cells to spontaneously differentiate into ‘multi‐lineage progenitor cells’, islet‐like clusters with embryonic beta‐cell characteristics could be generated. These clusters released insulin in a glucose‐dependent manner, but were not able to restore normoglycemia in diabetic mice. The performance of the cells was further enhanced by overexpression of Pax4, a transcription factor of importance for later stages of beta‐cell differentiation, and this time even the cell transplantation experiments were successful Citation53. Islet‐like cell clusters containing cells capable of insulin synthesis and secretion have also been generated by various growth factor combinationsCitation55–57.
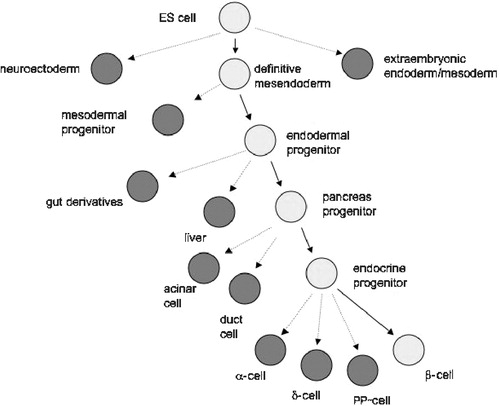
In spite of some promising results, it appears that many of the results published so far on mESC differentiation towards a ß‐cell like phenotype represent an aberrant differentiation pathway of neuroectodermally committed cells, and do not provide a platform for the generation of long‐term viable and physiologically functioning ß‐cells. A more natural approach to generate pancreatic precursors and eventually beta‐cells is based on initial commitment towards definitive endoderm, thereby mimicking in vivo differentiation. This has proven to be a difficult challenge because the spontaneous differentiation of ES cells in vitro is directed towards neuroectoderm rather than mesoderm or endoderm. Overexpression of the transforming growth factor (TGF)‐ß family growth factor nodal in mESC led to a marked increase in extra‐embryonic endoderm differentiation but there was no evidence of the induction of definitive endoderm Citation58. It has, however, recently been shown that definitive endoderm commitment also occurs during differentiation within the embryoid bodies. This is characterized by the appearance of a cell population mimicking the early ‘mesendodermal’ cells found in the anterior part of the primitive streak, co‐expressing the transciption factors FoxA2 and Brachyuru Citation59. These observations provide a clear basis for future attempts to isolate and further differentiate these cells into definitive endoderm derivatives, including pancreatic islets. Some progress along these lines has already been reported Citation60 (Figure ).
Figure 2 The challenge of generating physiologically functioning insulin‐producing cells from embryonic stem (ES) cells requires that the differentiating cell follows a pathway resembling that occurring in the developing embryo.
Human ES cell research is still at an early stage. However, it has become apparent that many principles established in mESC cannot be directly applied in hESC. Insulin has been detected in differentiating human embryoid bodies, but it is not clear if this reflects true pancreatic differentiation Citation61. Insulin‐producing clusters were also generated from hESCby the nestin‐selection protocol Citation44 with minor modifications, increasing the insulin content of the clusters by 30‐fold compared to monolayer cells Citation62. Whether these are true immature beta‐cells or neuronal cells remains to be shown.
Much controversy thus remains regarding whether the insulin‐producing cells generated in vitro from ES cells are true (immature) beta‐cells. It was also recently suggested that the ES cell‐derived insulin‐expressing cells might be of extra‐embryonic endoderm origin, displaying some endodermal and beta‐cell characteristics Citation63. The same conclusion could be drawn from a comparison of insulin‐producing cells generated from wild type versus hepatocyte nuclear factor (HNF)‐6 knock‐out mouse ES cells, suggesting that the insulin‐producing cells seen in spontaneously differentiating embryoid bodies are of visceral endodermal lineage, not beta‐cells Citation64.
These findings further stress the importance of thorough phenotypic analysis of the cells obtained after the differentiation process. This should include not only the demonstration of insulin protein and mRNA expression, but also evidence of C‐peptide cleavage and the expression of key proteins needed for glucose sensing and regulated exocytosis, as well as functional analyses of insulin release induced by glucose and other secretagogues. Furthermore, cell transplantation experiments are very important, not only to demonstrate the maturation and long‐term viability of transplanted cells, but also to detect the possible formation of teratomas Citation53, Citation57. At the moment, human ES cells clearly have the potential to serve as a source of physiological insulin‐producing cells for future cell replacement therapies. However, much basic research is still required to establish the foundations needed for the planning of clinical trials.
Acknowledgements
The authors are grateful to Dr. Timo Tuuri for critical comments on the manuscript. Our studies on human beta‐cell differentiation have been supported by grants from the Juvenile Diabetes Foundation International (JDRFI), The Academy of Finland, The Sigrid Jusélius Foundation and the research funds of The Helsinki University Hospital.
References
- Shapiro A. M., Lakey J. R., Ryan E. A., Korbutt G. S., Toth E., Warnock G. L., et al. Islet Transplantation in Seven Patients With Type 1 Diabetes Mellitus Using a Glucocorticoid‐Free Immunosuppressive Regimen. N Engl J Med 2000; 343: 230–8
- Ricordi C., Strom T. B. Clinical islet transplantation: advances and immunological challenges. Nat Rev Immunol 2004; 4: 259–68
- Jensen J., Heller R. S., Funder‐Nielsen T., Pedersen E. E., Lindsell C., Weinmaster G., et al. Independent Development of Pancreatic Alpha‐ and Beta‐Cells From Neurogenin 3‐Expressing Precursors: a Role for the Notch Pathway in Repression of Premature Differentiation. Diabetes 2000; 49: 163–76
- Wilson M. E., Scheel D., German M. S. Gene expression cascades in pancreatic development. Mech Dev 2003; 120: 65–80
- Bonner‐Weir S., Baxter L. A., Schuppin G. T., Smith F. E. A second pathway for regeneration of adult exocrine and endocrine pancreas: A possible recapitulation of embryonic development. Diabetes 1993; 42: 1715–20
- Vinik A., Pittenger G., Rafaeloff R., Rosenberg L., Duguid W. P. Determinants of pancreatic islet cell mass: a balance between neogenesis and senescence/apoptosis. Diab Rev 1996; 4: 235–63
- Zulewski H., Abraham E. J., Gerlach M. J., Daniel P. B., Moritz W., Muller B., et al. Multipotential nestin‐positive stem cells isolated from adult pancreatic islets differentiate ex vivo into pancreatic endocrine, exocrine, and hepatic phenotypes. Diabetes 2001; 50: 521–33
- Dor Y., Brown J., Martinez O. I., Melton D. A. Adult pancreatic beta‐cells are formed by self‐duplication rather than stem‐cell differentiation. Nature 2004; 429: 41–6
- Ramiya V. K., Maraist M., Arfors K. E., Schatz D. A., Peck A. B., Cornelius J. G. Reversal of Insulin‐Dependent Diabetes Using Islets Generated in Vitro From Pancreatic Stem Cells. Nat Med 2000; 6: 278–82
- Bonner‐Weir S., Taneja M., Weir G. C., Tatarkiewicz K., Song K. H., Sharma A., et al. In Vitro Cultivation of Human Islets From Expanded Ductal Tissue. Proc Natl Acad Sci USA 2000; 97: 7999–8004
- Gao R., Ustinov J., Pulkkinen M. A., Lundin K., Korsgren O., Otonkoski T. Characterization of endocrine progenitor cells and critical factors for their differentiation in human adult pancreatic cell culture. Diabetes 2003; 52: 2007–15
- Wang T. C., Bonner‐Weir S., Oates P. S., Chulak M., Simon B., Merlino G. T., et al. Pancreatic gastrin stimulates islet differentiation of transforming growth factor †‐induced ductular precursor cells. J Clin Invest 1993; 92: 1349–56
- Rooman I., Lardon J., Bouwens L. Gastrin stimulates beta‐cell neogenesis and increases islet mass from transdifferentiated but not from normal exocrine pancreas tissue. Diabetes 2002; 51: 686–90
- Suarez‐Pinzon W. L., Lakey J. R., Brand S. J., Rabinovitch A. Combination Therapy with Epidermal Growth Factor and Gastrin Induces Neogenesis of Human Islet {beta}‐Cells from Pancreatic Duct Cells and an Increase in Functional {beta}‐Cell Mass. J Clin Endocrinol Metab 2005; 90: 3401–9
- Heremans Y., Van De Casteele M., In't Veld P., Gradwohl G., Serup P., Madsen O., et al. Recapitulation of embryonic neuroendocrine differentiation in adult human pancreatic duct cells expressing neurogenin 3. J Cell Biol 2002; 159: 303–12
- Klein T., Ling Z., Heimberg H., Madsen O. D., Heller R. S., Serup P. Nestin is expressed in vascular endothelial cells in the adult human pancreas. J Histochem Cytochem 2003; 51: 697–706
- Lardon J., Rooman I., Bouwens L. Nestin expression in pancreatic stellate cells and angiogenic endothelial cells. Histochem Cell Biol 2002; 117: 535–40
- Seaberg R. M., Smukler S. R., Kieffer T. J., Enikolopov G., Asghar Z., Wheeler M. B., et al. Clonal identification of multipotent precursors from adult mouse pancreas that generate neural and pancreatic lineages. Nat Biotechnol 2004; 22: 1115–24
- Piper K., Ball S. G., Turnpenny L. W., Brickwood S., Wilson D. I., Hanley N. A. Beta‐cell differentiation during human development does not rely on nestin‐positive precursors: implications for stem cell‐derived replacement therapy. Diabetologia 2002; 45: 1045–7
- Treutelaar M. K., Skidmore J. M., Dias‐Leme C. L., Hara M., Zhang L., Simeone D., et al. Nestin‐lineage cells contribute to the microvasculature but not endocrine cells of the islet. Diabetes 2003; 52: 2503–12
- Delacour A., Nepote V., Trumpp A., Herrera P. L. Nestin expression in pancreatic exocrine cell lineages. Mech Dev 2004; 121: 3–14
- Rooman I., Heremans Y., Heimberg H., Bouwens L. Modulation of rat pancreatic acinoductal transdifferentiation and expression of PDX‐1 in vitro. Diabetologia 2000; 43: 907–14
- Lardon J., De Breuck S., Rooman I., Van Lommel L., Kruhoffer M., Orntoft T., et al. Plasticity in the adult rat pancreas: transdifferentiation of exocrine to hepatocyte‐like cells in primary culture. Hepatology 2004; 39: 1499–507
- Baeyens L., De Breuck S., Lardon J., Mfopou J. K., Rooman I., Bouwens L. In vitro generation of insulin‐producing beta cells from adult exocrine pancreatic cells. Diabetologia 2005; 48: 49–57
- Beattie G. M., Itkin‐Ansari P., Cirulli V., Leibowitz G., Lopez A. D., Bossie S., et al. Sustained Proliferation of Pdx‐1(+) Cells Derived From Human Islets. Diabetes 1999; 48: 1013–9
- Gao R., Ustinov J., Korsgren O., Otonkoski T. In vitro neogenesis of human islets reflects the plasticity of differentiated human pancreatic cells. 2005. In press
- Gershengorn M. C., Hardikar A. A., Wei C., Geras‐Raaka E., Marcus‐Samuels B., Raaka B. M. Epithelial‐to‐mesenchymal transition generates proliferative human islet precursor cells. Science 2004; 306: 2261–4
- Lechner A., Nolan A. L., Blacken R. A., Habener J. F. Redifferentiation of insulin‐secreting cells after in vitro expansion of adult human pancreatic islet tissue. Biochem Biophys Res Commun 2005; 327: 581–8
- Choi Y., Ta M., Atouf F., Lumelsky N. Adult pancreas generates multipotent stem cells and pancreatic and nonpancreatic progeny. Stem Cells 2004; 22: 1070–84
- Suzuki A., Nakauchi H., Taniguchi H. Prospective isolation of multipotent pancreatic progenitors using flow‐cytometric cell sorting. Diabetes 2004; 53: 2143–52
- Jiang Y., Jahagirdar B. N., Reinhardt R. L., Schwartz R. E., Keene C. D., Ortiz‐Gonzalez X. R., et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 2002; 418: 41–9
- Ianus A., Holz G. G., Theise N. D., Hussain M. A. In vivo derivation of glucose‐competent pancreatic endocrine cells from bone marrow without evidence of cell fusion. J Clin Invest 2003; 111: 843–50
- Hess D., Li L., Martin M., Sakano S., Hill D., Strutt B., et al. Bone marrow‐derived stem cells initiate pancreatic regeneration. Nat Biotechnol 2003; 21: 763–70
- Tang D. Q., Cao L. Z., Burkhardt B. R., Xia C. Q., Litherland S. A., Atkinson M. A., et al. In vivo and in vitro characterization of insulin‐producing cells obtained from murine bone marrow. Diabetes 2004; 53: 1721–32
- Cao L. Z., Tang D. Q., Horb M. E., Li S. W., Yang L. J. High glucose is necessary for complete maturation of Pdx1‐VP16‐expressing hepatic cells into functional insulin‐producing cells. Diabetes 2004; 53: 3168–78
- Zalzman M., Gupta S., Giri R. K., Berkovich I., Sappal B. S., Karnieli O., et al. Reversal of hyperglycemia in mice by using human expandable insulin‐producing cells differentiated from fetal liver progenitor cells. Proc Natl Acad Sci U S A 2003; 100: 7253–8
- Thomson J. A., Itskovitz‐Eldor J., Shapiro S. S., Waknitz M. A., Swiergiel J. J., Marshall V. S., et al. Embryonic stem cell lines derived from human blastocysts. Science 1998; 282: 1145–7
- Evans M. J., Kaufman M. H. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981; 292: 154–6
- Martin G. R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA 1981; 78: 7634–8
- Soria B., Roche E., Berna G., Leon‐Quinto T., Reig J. A., Martin F. Insulin‐Secreting Cells Derived From Embryonic Stem Cells Normalize Glycemia in Streptozotocin‐Induced Diabetic Mice. Diabetes 2000; 49: 157–62
- Leon‐Quinto T., Jones J., Skoudy A., Burcin M., Soria B. In vitro directed differentiation of mouse embryonic stem cells into insulin‐producing cells. Diabetologia 2004; 47: 1442–51
- Shiroi A., Yoshikawa M., Yokota H., Fukui H., Ishizaka S., Tatsumi K., et al. Identification of insulin‐producing cells derived from embryonic stem cells by zinc‐chelating dithizone. Stem Cells 2002; 20: 284–92
- Kahan B. W., Jacobson L. M., Hullett D. A., Ochoada J. M., Oberley T. D., Lang K. M., et al. Pancreatic precursors and differentiated islet cell types from murine embryonic stem cells: an in vitro model to study islet differentiation. Diabetes 2003; 52: 2016–24
- Lumelsky N., Blondel O., Laeng P., Velasco I., Ravin R., McKay R. Differentiation of embryonic stem cells to insulin‐secreting structures similar to pancreatic islets. Science 2001; 292: 1389–94
- Lee S. H., Lumelsky N., Studer L., Auerbach J. M., McKay R. D. Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nat Biotechnol 2000; 18: 675–9
- Hori Y., Rulifson I. C., Tsai B. C., Heit J. J., Cahoy J. D., Kim S. K. Growth inhibitors promote differentiation of insulin‐producing tissue from embryonic stem cells. Proc Natl Acad Sci USA 2002; 99: 16105–10
- Blyszczuk P., Czyz J., Kania G., Wagner M., Roll U., St‐Onge L., et al. Expression of Pax4 in embryonic stem cells promotes differentiation of nestin‐positive progenitor and insulin‐producing cells. Proc Natl Acad Sci USA 2003; 100: 998–1003
- Miyazaki S., Yamato E., Miyazaki J. Regulated expression of pdx‐1 promotes in vitro differentiation of insulin‐producing cells from embryonic stem cells. Diabetes 2004; 53: 1030–7
- Moritoh Y., Yamato E., Yasui Y., Miyazaki S., Miyazaki J. Analysis of insulin‐producing cells during in vitro differentiation from feeder‐free embryonic stem cells. Diabetes 2003; 52: 1163–8
- Rajagopal J., Anderson W. J., Kume S., Martinez O. I., Melton D. A. Insulin staining of ES cell progeny from insulin uptake. Science 2003; 299: 363
- Hansson M., Tonning A., Frandsen U., Petri A., Rajagopal J., Englund M. C., et al. Artifactual insulin release from differentiated embryonic stem cells. Diabetes 2004; 53: 2603–9
- Sipione S., Eshpeter A., Lyon J. G., Korbutt G. S., Bleackley R. C. Insulin expressing cells from differentiated embryonic stem cells are not beta cells. Diabetologia 2004; 47: 499–508
- Blyszczuk P., Asbrand C., Rozzo A., Kania G., St‐Onge L., Rupnik M., et al. Embryonic stem cells differentiate into insulin‐producing cells without selection of nestin‐expressing cells. Int J Dev Biol 2004; 48: 1095–104
- Kania G., Blyszczuk P., Wobus A. M. The generation of insulin‐producing cells from embryonic stem cells ‐ a discussion of controversial findings. Int J Dev Biol 2004; 48: 1061–4
- Kim D., Gu Y. J., Ishii M., Fujimiya M., Qi M. G., Nakamura N., et al. In vivo functioning and transplantable mature pancreatic islet‐like cell clusters differentiated from embryonic stem cell. Pancreas 2003; 27: E34–41
- Micallef S. J., Janes M. E., Knezevic K., Davis R. P., Elefanty A. G., Stanley E. G. Retinoic acid induces Pdx1‐positive endoderm in differentiating mouse embryonic stem cells. Diabetes 2005; 54: 301–5
- Shi Y., Hou L., Tang F., Jiang W., Wang P., Ding M., et al. Inducing embryonic stem cells to differentiate into pancreatic beta cells by a novel three‐step approach with activin A and all‐trans retinoic acid. Stem Cells 2005; 23: 656–62
- Vallier L., Reynolds D., Pedersen R. A. Nodal inhibits differentiation of human embryonic stem cells along the neuroectodermal default pathway. Dev Biol 2004; 275: 403–21
- Kubo A., Shinozaki K., Shannon J. M., Kouskoff V., Kennedy M., Woo S., et al. Development of definitive endoderm from embryonic stem cells in culture. Development 2004; 131: 1651–62
- Ku H. T., Zhang N., Kubo A., O'Connor R., Mao M., Keller G., et al. Committing embryonic stem cells to early endocrine pancreas in vitro. Stem Cells 2004; 22: 1205–17
- Assady S., Maor G., Amit M., Itskovitz‐Eldor J., Skorecki K. L., Tzukerman M. Insulin production by human embryonic stem cells. Diabetes 2001; 50: 1691–7
- Segev H., Fishman B., Ziskind A., Shulman M., Itskovitz‐Eldor J. Differentiation of human embryonic stem cells into insulin‐producing clusters. Stem Cells 2004; 22: 265–74
- Milne H. M., Burns C. J., Kitsou‐Mylona I., Luther M. J., Minger S. L., Persaud S. J., et al. Generation of insulin‐expressing cells from mouse embryonic stem cells. Biochem Biophys Res Commun 2005; 328: 399–403
- Houard N., Rousseau G. G., Lemaigre F. P. HNF‐6‐independent differentiation of mouse embryonic stem cells into insulin‐producing cells. Diabetologia 2003; 46: 378–85