Abstract
One way to restore failing heart function following myocardial infarction would be to replace lost or damaged cardiac cells by local or systemic injection. The sources of replacement cells presently discussed include embryonic stem cells, hematopoietic and non‐hematopoietic stem cells from bone marrow or cord blood and small stem cell populations thought to reside in the heart itself or in skeletal muscle. Here we review this area of stem cell research with focus particularly on recent laboratory advances towards producing cardiomyocytes from embryonic stem cells. We conclude that embryonic stem cells and cardiac progenitors in the heart itself are the only proven sources of cardiomyocytes and that reported clinical effects of bone marrow stem currently undergoing validation are likely mediated by other mechanisms.
| bFGF | = | basic fibroblast growth factor |
| BM | = | bone marrow |
| BMC | = | bone marrow cell |
| BMP | = | bone morphogenetic protein |
| BMSC | = | bone marrow stem cell |
| CM | = | cardiomoycyte |
| CPC | = | cardiac progenitor cell |
| CSC | = | cardiac stem cell |
| EB | = | embryoid body |
| ECFP | = | enhanced cyan fluorescent protein |
| ECC | = | embryonal carcinoma cell |
| ECG | = | electrocardiography |
| EGF | = | epidermal growth factor |
| EGFP | = | enhanced green fluorescent protein |
| END‐2 | = | endoderm‐like cell line‐2 |
| ESC | = | EMBRYONIC STEM CELL |
| FACS | = | fluorescence activated cell sorting |
| FCS | = | fetal calf serum |
| G‐CSF | = | granulocyte colony stimulating factor |
| GFP | = | green fluorescent protein |
| HESC | = | human embryonic stem cell |
| HLA | = | human leukocyte antigen |
| HSC | = | hematopoietic stem cell |
| IGF | = | insulin‐like growth factor |
| LIF | = | leukemia inhibiting factor |
| LVEF | = | left ventricular ejection fraction |
| MESC | = | mouse embryonic stem cell |
| MHC | = | major histocompatibility complex |
| MI | = | myocardial infarction |
| MLC | = | myosin light chain |
| MRI | = | magnetic resonance imaging |
| MSC | = | mesenchymal stem cell |
| PCR | = | polymerase chain reaction |
| RA | = | retinoic acid |
| SCID | = | severe combined immunodeficient |
| SDF | = | stromal‐cell derived factor |
| STAT | = | signal transducer and activator of transcription |
| TGFβ | = | transforming growth factor β |
| VEGF | = | vascular endothelial growth factor |
Key messages
Cardiomyocytes can be derived from (human and mouse) embryonic stem cells and as cardiac progenitors or stem cells directly from the heart. Both are promising sources of cardiac cells for transplantation.
Bone marrow‐derived cells probably do not develop to cardiomyocytes but they may contribute to vascular repair in the heart that if initiated soon after infarct will limit subsequent ischemic damage.
Clinical background
Prevention and treatment of cardiovascular diseases is an important issue, especially in the Western world where ischemic heart disease and its consequences rank first in the mortality list Citation1. With aging of the population and an improved short‐term survival after myocardial infarction (MI) over the past decades, the number of patients with heart failure is increasing. In spite of major efforts to improve their condition with life‐style alterations and medication, the natural course of the disease cannot be halted and gradual progress towards severely impaired cardiac function and death is generally inevitable. The mammalian heart is unable to regenerate the large number of cardiomyocytes (CMs) lost after infarction. Adaptational mechanisms, such as hypertrophy, are initially beneficial but in the end become detrimental. The only curative option is heart transplantation, but this is limited by donor availability and transplant rejection. If it were possible to reconstitute the myocardium by replacing lost CMs, these problems could be circumvented. Here we review new stem cell‐based strategies currently being investigated for their potential to regenerate the heart. These include CMs derived from embryonic stem cells and from adult tissues as well as reactivation of resident cells surviving the initial myocardial damage.
Embryonic stem cells
Embryonic stem cells (ESCs) are derived from the inner cell mass of blastocyst‐stage embryos Citation2, Citation3 and proliferate indefinitely in vitro in an undifferentiated state when cultured under appropriate conditions. They have the potential to differentiate into derivatives of all three primary germ layers that arise during development (ectoderm, endoderm and mesoderm Citation4) and thus all somatic cells of the adult individual. Mesoderm is the embryonic origin of cardiomyocytes. ESCs are therefore a potential cell source for tissue regeneration including that necessary in the heart following MI. Following the isolation of ESCs from humans Citation5, Citation6, many of the first approaches to controlling their growth and differentiation were based on those well developed for mouse ESCs (MESCs) generated around 17 years earlier Citation2, Citation3. Cardiomyocyte formation has been one of the most successful areas of directed lineage differentiation of MESCs as described in the following section. We use this as a basis for describing the strategies being developed for HESCs.
Mouse embryonic stem cells
When cultured as aggregates in suspension, MESCs form three‐dimensional, multi‐cellular complexes called embryoid bodies (EBs). These spherical structures resemble egg cylinders of embryos 6 to 8 days after fertilization Citation7. After about 4–8 days these EBs usually contain CMs between inner mesenchymal cells and outer epithelial cells Citation8 which start to contract spontaneously. These CMs express the major cardiac proteins and show contraction characteristics, action potentials and chronotropic responses similar to those described for embryonic CMs Citation9, Citation10.
The earliest ESC‐derived CMs to form in EBs resemble pacemaker and primary myocardial cells. Later, atrial‐, ventricular‐, nodal‐, His‐, and Purkinje‐like cells develop Citation11. The cardiac transcription factors Nkx2.5, GATA‐4 and MEF2C are expressed early during differentiation, while more mature CMs express atrial natriuretic factor (ANF), the Na+‐Ca2+ exchanger and phospholamban as well as structural proteins like myosin light chain (MLC)‐2v, α‐ and β‐myosin heavy chain, titin (Z and M band), α‐actinin, cardiac troponin T, α‐actin and M protein Citation11.
Human embryonic stem cells
The first HESC lines were derived on mouse embryonic feeder cells (MEFs) to inhibit their differentiation Citation5, Citation6, just as the first MESCs. More than 200 other lines are now thought to exist, derived under a variety of (partially) defined conditions. Like MESCs, HESCs differentiate to derivatives of all three germ layers in vitro and to teratomas in vivo, tumors which develop after transplantation of undifferentiated HESCs into immunodeficient (e.g. SCID) mice. However, unlike MESCs where leukemia inhibiting factor (LIF) can replace the requirement for MEFs for self‐renewal in culture Citation12, Citation13, HESCs do not respond to LIF. In general, they are still grown on MEFs although several studies have described successful use of human feeder cells of various origins. This is important because use of animal reagents carries the risk of pathogen transfer, termed a ‘xenorisk’ Citation14. Recently, activin A was described as being secreted by MEFs and capable of maintaining HESC pluripotency in the absence of feeder cells Citation15. Feeder‐free HESC proliferation has also been described using a combination of transforming growth factor β1 (TGFβ1), LIF, basic fibroblast growth factor (bFGF) and fibronectin matrix Citation16, or a combination of bFGF and the bone morphogenetic protein (BMP) inhibitor Noggin Citation17. Detailed mechanistic studies of these factors have not yet been described and it remains to be shown whether the factor added acts directly on the undifferentiated cells to promote self‐renewal or inhibits the growth of the differentiated cells often present in heterogeneous cultures. HESCs have also been reported to grow on an autogeneic feeder layer of fibroblast‐like cells resulting from spontaneous differentiation of the same HESC line Citation18. Moreover, a new HESC line has recently been derived and successfully maintained under entirely feeder cell‐free and serum‐free conditions Citation19. Many of these results have yet to be confirmed in independent studies.
As MESCs, HESCs can differentiate into human fetal‐like CMs when grown as aggregates or EBs in suspension Citation20–22. We described induction of CM differentiation in hES2 and hES3 HESC lines that rarely undergo spontaneous cardiogenesis Citation23 by coculturing HESCs with an endoderm‐like cell line (END‐2), that is thought to mimic the effect of extra‐embryonic visceral endoderm in the embryo. The CMs obtained (Figure ) expressed sarcomeric proteins, ANF, cardiac transcription factors and multiple cardiac ion channel genes. They have ventricular action potentials, respond as expected to positive and negative chronotropic agents, and form gap‐junctions Citation23.
Enhancing cardiomyocyte differentiation
Most protocols that support CM differentiation only result in CM formation at relatively low efficiency so that the yield of CMs is also low Citation20–22. For use in drug screens or transplantation large numbers of cells are required. This could be achieved by increasing the rate of conversion of the undifferentiated cells to cardiac precursors or mature CMs or expanding the cardiac progenitors. Some of these approaches are described in the following sections.
MESC
Various factors that could promote differentiation of ESCs to cardiac cells have been suggested. These include TGFβ1 Citation24 and bFGF Citation25 which regulate the development of cardiac tissue in explants of fetal mouse hearts. The homeobox‐containing transcription factor Nkx2.5 is expressed very early in heart development Citation26, Citation27 and is involved in its morphogenesis but does not seem to be required for CM differentiation Citation28. On the other hand, studies with P19 embryonal carcinoma cells (ECCs) suggested that another early transcription factor GATA‐4 is essential for heart development. Inhibition of GATA‐4 by anti‐sense transcripts blocked the formation of CMs in vitro and induced apoptosis Citation29, whilst overexpression enhanced cardiogenesis Citation30.
Retinoic acid (RA), the active derivative of vitamin A, has also been implicated in heart development Citation31. Treatment of differentiating MESCs with RA increased the number of CMs in a time‐ and concentration‐dependent manner Citation32. However, high concentrations of RA resulted in the formation of ectoderm and endoderm by P19 ECCs rather than mesoderm. Previously, we cloned multiple differentiated cell lines from P19 ECCs with characteristics of endoderm Citation33. When cultured on a confluent monolayer of these END‐2 cells, wild‐type P19 ECCs Citation34 as well as HESCs and MESCs aggregated and differentiated to CMs with high efficiency Citation35. Apparently, END‐2 cells produce one or more factors that promote CM differentiation.
The mouse epidermal growth factor (EGF)‐CFC family member Cripto is also involved in heart development Citation36 and appears crucial for differentiation of ESCs to CMs; MESCs lacking Cripto are unable to form beating CMs in vitroCitation37 and preferentially develop into neural cells Citation38 whilst overexpression of Cripto Citation37 or the addition of recombinant Cripto to the culture medium of Cripto‐/‐ cells inhibited neuralization and induced CM formation Citation38.
Production of nitric oxide, synthesized in vivo by nitric oxide synthases or through the conversion of nitrite, appears essential for proper heart development Citation39. Lack of nitric oxide synthases results in defective cardiogenesis Citation40, Citation41, continuous exposure of MESCs to nitric oxide synthase inhibitors similarly blocks cardiac differentiation whilst adding exogenous nitric oxide restores cardiomyogenesis Citation39 and induces apoptosis in cells not committed to the cardiac lineage Citation42.
Several growth factors play crucial roles in heart development and some promote cardiomyogenesis in vitro. Treatment of MESCs with TGFβ1 or BMP‐2 for 24 hours in the presence of LIF significantly enhanced expression of Nkx2.5 and MEF2C during EB formation. After replating, beating areas increased in size and number, and showed enhanced myofibrillogenesis compared to those that emerged from untreated cell cultures Citation43. FGF‐2 also enhanced cardiomyogenic differentiation at relatively low concentrations when administered continuously after replating, although at high concentrations it is associated with self‐renewal. Combined BMP2 and FGF were additive Citation44. Remarkably, even though Noggin is an antagonist of BMP‐2, it plays a crucial role during cardiogenesis Citation45. Noggin is transiently expressed in the heart forming area in mouse embryos and increased beating of mouse EBs was observed when Noggin was added 3 days prior to as well as during EB formation. Compared to untreated cells, Noggin‐exposed cells expressed higher levels of Brachyury‐T Citation45, a marker of mesendoderm cells that have the potential to differentiate into either endoderm or mesoderm Citation46. The narrow time window in which endogenous Noggin is expressed implies that it is involved in the conversion of undifferentiated cells into Brachyury‐T‐positive derivatives that give rise to CMs Citation45.
JAK2 is a member of the Janus Kinase family that regulates gene transcription by phosphorylating signal transducers and activators of transcription (STATs), which then translocate from the cytosol to the nucleus. Expression of JAK2 was upregulated in beating MESC‐derived CMs compared to non‐beating cells Citation47. Concomitantly, STAT3 and phosphorylated STAT3 levels were elevated approximately 3‐ and 2‐fold, respectively; the phosphorylated form of STAT3 being predominantly located in the nuclei of CMs. As expected, inhibition of either JAK2 or STAT3 suppressed CM formation whereas overexpression of JAK2 had the opposite effect Citation47.
HESC
HESCs also respond to growth as aggregates by differentiating. Beating areas appeared in 8% of HESC aggregates grown for 20 days in suspension Citation21. Xu et al. reported a significantly higher proportion of beating EBs (70%) over the same culture period in the additional presence of 5‐azacytidine Citation22 whilst He et al. found 10%–25% EBs beating after 30 days Citation20. In END‐2/HESC cocultures beating areas appeared in 35% of the wells (each containing multiple HESC aggregates) after 12 days Citation23. Although these methods suggest a relatively high efficiency in CM formation, only between 5% and 20% of the cells within each beating colony are CMs Citation48. Recently, we showed a striking inverse relationship between CM differentiation and the concentration of fetal calf serum (FCS) routinely used during coculture. By using medium supplemented only with insulin, selenium, transferrin and heat‐inactivated bovine serum albumin, a 24‐fold increase in the number of beating areas and a 39‐fold increase in the number of α‐actinin positive CMs were observed Citation48. In addition, a further 40% increase in the number of beating areas was observed after adding vitamin C to the defined media. Vitamin C has been found previously to enhance CM differentiation of MESCs. Islet‐1 positive cardiac progenitors were detected in the differentiating cultures Citation48. These represent a potential target cell for in vitro expansion and upscale. Not only are such advances essential to obtain sufficient numbers of CMs for transplantation therapy, but they also represent better in vitro models for testing factors influencing differentiation, without interfering factors from serum.
Cardiomyocyte selection
MESC
The use of ESC‐derived CMs for transplantation ultimately requires that the population be devoid of non‐cardiac cells to obtain optimal engraftment and ensure that contamination with undifferentiated stem cells does not cause teratomas in vivo. Since CM differentiation of HESCs has to date never reached 100%, development of selection methods has become imperative. Introduction of selectable markers driven by cardiac‐specific promoters would allow isolation of CMs from mixed populations after differentiation. Transfection of MESCs with the aminoglycoside phosphotransferase gene behind the α‐MHC promoter Citation49 conveyed G418 resistance to CMs formed after differentiation which resulted in >99% CM enrichment after G‐418 selection. Using a human cardiac α‐actin promoter driving lacZ Citation50 or EGFP Citation51 MESCs committed to the cardiac lineage were detected 6 days after initiation of differentiation before the appearance of spontaneous contraction Citation50. The EGFP construct was also validated in transgenic mice where EGFP was detected in the heart during early development Citation52. Fluorescence‐activated cell sorting (FACS) allows efficient selection of GFP‐expressing CMs derived from ESCs.
Although genetically modified ESCs would not be ideal for clinical use they could be used to develop novel cell surface antibodies that would be useful for cardiomyocyte selection. Analysis of gene expression and electrophysiological phenotype by patch clamp analysis has shown that MESC‐derived CMs are immature and resemble those of the embryonic heart tube Citation53 and once selected, these may convey specific advantages for cell integration after transplantation. This emphasizes the usefulness of developing proof of principle in mice, i.e. the development of cell surface antibodies to early CMs, selection from mixed differentiating MESCs and transplantation to mice with myocardial infarction.
HESC
To date, the only selection method described for HESC‐CMs is Percoll gradient centrifugation which resulted in 4‐fold enrichment, with 70% of the cell fraction containing cells staining positive for cardiac markers Citation22.
Although CM selection of HESC‐CMs by genetic methods would best be avoided in HESCs for clinical purposes, it would be very useful for in vitro studies, e.g. related to drug screening, and animal experiments to have pure HESC‐CM populations. That there are to date no publications describing HESC‐reporter lines of any lineage may reflect the difficulties in genetically modifying HESCs compared with MESCs although methods are improving Citation54. Once the problems are solved though, approaches analogous to those used in MESCs would be applicable to HESCs.
Transplantation of ESC
Assuming that problems of ESC propagation, CM differentiation and selection can be improved to the point that sufficient cells were available for patients, the best site and way of delivering them would still need to be determined. In addition, possible graft rejection is an issue still to be addressed. The fate of transplanted cells would also need to be examined. Although several authors report transplantation of MESCs and MESC‐derivatives in animal hearts Citation43, Citation49, Citation55–62, results with HESCs are still limited Citation63, Citation64 and MESC‐ and HESC‐derived CMs may not behave identically in vivo.
Animal models
Rodents have mainly been used to study the behavior of transplanted ESCs either in uninjured Citation43, Citation49, Citation57, Citation61 or infarcted Citation43, Citation55–60, Citation62, Citation65 hearts. Transgenic models, especially of mice, are available and smaller numbers of cells are required in small rodents than in large animals. However, studies in large animals with cardiac physiology similar to humans will eventually be necessary for a better understanding of risk. For example, mice may not reliably report arrhythmias, since their beat rate is ∼500 bpm and this may override any arrhythmias caused by ectopic pacemaker activity.
Methods of cell delivery
Intramyocardial injection of 10,000–500,000 MESCs or 5–150 beating areas from HESCs with a small needle (21 to 30 gauge, depending on the size of the animal) is the most commonly used technique to deliver ESCs to the heart Citation43, Citation49, Citation55–57, Citation59–64. Targeted regions can be the normal myocardium, the infarcted area or the border zone, or a combination of these. Unfortunately, a variable and often relatively small proportion of the transplanted cells is successfully delivered to, and survives in, the host myocardium Citation49 (and Van Laake et al., Hubrecht Laboratory 2005, unpublished). An alternative would be combining cells with a (degradable) matrix, as shown for MESCs in rats Citation58. Such a strategy may have the advantage of preventing cell loss and, particularly for later interventions, forming a temporary support for the thinned infarcted wall. Equivalents for HESCs have not been described.
Immune rejection
It has been postulated that HESCs, like MESCs Citation66, lack major histocompatibility complex (MHC) protein expression and, therefore, would not evoke an immune response in the host. This idea was partly rejected by evidence showing that HESCs do express MHC class I molecules Citation67, albeit at low levels. This increased upon differentiation in vitro and was stimulated by interferon‐γ. In teratomas, interferon‐α and ‐β contributed to this upregulation. Transplantation of differentiated ESCs to the heart could enhance MHC‐I protein levels in a similar way. On the other hand, the myocardium may be a relatively hospitable environment in terms of immune response Citation68 as lymph drainage is restricted. However, after injury (MI or needle stick manipulation), resulting inflammation could trigger the recruitment of immunoreactive cells.
Several authors have described using immune competent (sometimes syngeneic) wild‐type animals and nevertheless reported graft integration Citation43, Citation49, Citation55–60, Citation63. An immunodeficient mouse model (Friend leukemia virus strain B mice) has also been described Citation62. In a study with MESCs, cyclosporin was administered to rats in order to prevent immune rejection Citation61. In an investigation with HESCs, a combination of cyclosporin and methylprednisolone was administered to pigs Citation64. It is not clear whether these were purely preventive measures or previous trials with immune competent hosts had been unsuccessful. Of note is the difference in MHC protein expression between MESCs and HESCs and therefore their different immunogenic potentials. The degree of immunosuppression necessary thus remains to be determined in HESC‐transplantation studies. If graft rejection turns out to be a serious problem, other possible solutions include creating a HESC bank with a range of human leukocyte antigen (HLA) profiles, as in the UK, Sweden and Spain, or induction of tolerance in the recipient Citation68. Recently, nuclear transfer was used to create patient‐specific non‐immunogenic stem cell lines Citation69. The nucleus of a skin cell from each patient was transferred into a donated oocyte. When the resulting cloned embryos had reached the blastocyst stage, HESC lines could be derived that were genetically identical, excepting the mitochondria, to the donor of the nucleus. An even better strategy in terms of ethical acceptability, cost‐effectiveness and ‘shelfability’ for use in acute disease would be to modify ESCs to become universal non‐immunogenic cells, for example by deleting the β‐2 microglobulin gene which controls MHC‐I presentation Citation70.
Functional assessment
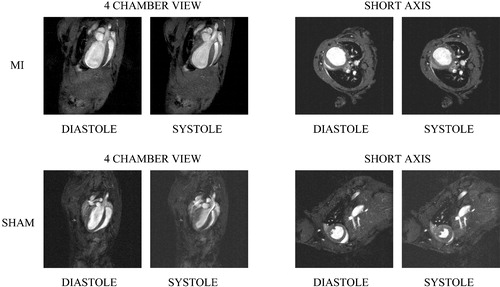
The ultimate goal of ESC‐derived CM cell transplantation is not only to improve survival of patients, but also to improve cardiac performance. Methods applied to assess cardiac function include electrocardiography (ECG) Citation56, Citation63, Citation64, measurements of cardiac pressure Citation59, Citation60, Citation62, echocardiography Citation56–60, Citation62, and electrophysiological mapping Citation63, Citation64. Magnetic resonance imaging (MRI) has been performed to locate transplanted MESCs Citation55. Each of these methods has its advantages and drawbacks. ECG is inexpensive and widely available, but has low sensitivity and specificity for quantifying heart function. Direct pressure measurements provide more information on left ventricular function, but are limited unless combined with volume measurement, which is technically more challenging. However, with the requisite specialized equipment and a skilled investigator, measuring pressure‐volume loops with a conductance‐micromanometer is an excellent way to evaluate cardiac performance after ESC transplantation Citation71. Echocardiography and MRI (Figure ) are both appealing techniques as they present direct and easily interpretable images of both cardiac kinetics and morphology. Although to date there is significantly more experience with the more often used echocardiographic visualization, MRI is expected eventually to become the method of first choice because of its higher resolution and accuracy Citation72 and the possibility of repeated non‐invasive measurements over prolonged periods.
Results
MESC‐derived cardiomyocytes
Transplantation of MESC‐derived CMs into normal and infarcted rodent hearts has been extensively studied Citation49, Citation56, Citation59–61. Immunohistochemistry and PCR confirmed the origin of engrafted MESC‐derived CMs, showed they had normal myocardial topography and contained myofibers Citation49. In another study, GFP‐positive MESC‐derived CMs were injected in the border zone and infarcted areas of rat hearts. The observation of cardiac‐like striations and the concurrent expression of cardiac Troponin I and GFP in the injected hearts confirmed the successful engraftment and differentiation of these cells. Reported functional improvements included smaller infarct areas in injected hearts, less extensive cardiac remodeling, improved left ventricle pressure indices as measured with a pressure transduction catheter, and improved fractional shortening of the left ventricle (a measure of the contractile ability of the heart) Citation59. In a similar study by the same group, injected cells were observed for a period of 32 weeks. The number of GFP positive cells was found to increase between 6 and 32 weeks after injection, the engrafted regions contained more capillaries, and the survival rate of the mice increased Citation60. Enhanced cyan fluorescent protein (ECFP)‐expressing MESC‐derived CMs that were transplanted into rats after MI were recovered as striated ECFP‐positive cells in cryosections. In addition, the animals had less adverse cardiac remodeling, increased left ventricular ejection fraction (LVEF), and positive isotropic response Citation56.
Undifferentiated MESC
The injection of undifferentiated MESCs in control and infarcted mouse and rat hearts has been reported in three studies Citation43, Citation55, Citation57. One study used MESCs pre‐loaded with superparamagnetic iron oxide to enable in vivo MRI visualization of transplanted cells. It was reported that transplanted cells could be detected 12 hours to 36 days after transplantation, suggesting long term engraftment Citation55. The graft region in rat hearts injected with GFP‐positive MESCs in a matrix gel substrate was reported to contain conglomerates of GFP‐positive cells. Although these cells did not appear to have a CM phenotype, they did express connexin‐43 and α‐actinin. Both inflammation and adverse remodeling were lower compared to MESC transplantation without the matrix gel Citation58. In yet another report, undifferentiated ECFP‐labeled MESCs were injected into normal mouse hearts. At the injection site, transplanted ECFP‐positive cells were observed within scar tissue in cryosections. MLC‐2v and connexin‐43 were also detected in these cells. Mice injected with MESCs showed improved LVEF. However, when TGFβ‐receptor mediated signaling was disrupted the undifferentiated MESCs could not differentiate to CMs but formed tumors instead Citation43.
Specific MESC sub‐populations
Specific sub‐populations of undifferentiated MESCs were injected into infarcted mouse hearts in two studies Citation57, Citation62. Early‐differentiated MESCs or early‐differentiated MESCs transfected with vascular endothelial growth factor (VEGF) were injected into post‐MI mice. In hearts injected with either MESCs or VEGF‐expressing MESCs, cells co‐staining for GFP and connexin‐43 were reported, but the VEGF‐expressing MESCs resulted in greater improvements in pressure indices and capillary number Citation62. Injection of undifferentiated GFP‐positive MESCs pre‐treated with insulin‐like growth factor‐1 (IGF‐1) into the infarct region of mouse hearts resulted in the successful engraftment of GFP‐positive cells at the injection site Citation57. These cells expressed several cardiac and vascular markers, including connexin‐43, α‐actinin, and α‐smooth muscle actin. The graft regions with IGF‐1‐treated MESCs contained more vascular structures, had more myosin heavy chain expression and showed functional improvement compared to graft regions after injection with untreated MESCs Citation57. Whether the neo‐vascularization reported in these studies was of host or transplanted cell origin was not determined.
HESC‐derived cardiomyocytes
Only two studies have described the injection of HESC‐derived CMs into animal models Citation63, Citation64. Kehat et al. observed aligned cell clusters that contained human mitochondria and expressed α‐actinin in porcine ventricles after injection with HESC‐derived CMs. Although some of these human mitochondrial antibody‐positive cells appeared to be more mature, most contained immature sarcomeric structures Citation64. Xue et al. reported functional integration of HESC‐derived CMs expressing GFP into guinea pig hearts Citation63. In both studies, HESC‐derived CMs showed the potential to electrically drive the myocardium when it is electrophysiologically silenced or slowed down, thus functioning as biological pacemakers Citation63, Citation64.
Safety
Long‐term safety analysis of HESCs transplantation is an important issue before it can be applied clinically. The longest reported follow‐up after transplantation of MESC‐derived CMs in rats is 32 weeks: this did not result in tumor formation Citation60. However, transplantation of cells into the brain has been shown to result in the formation of teratomas, irrespective of whether or not these cells were pre‐differentiated to neural cells in vitroCitation73. In contrast, in multiple studies where human embryonal carcinoma NT2 neurons were transplanted into rodent and primate brains, no tumors were found several months post‐transplantation Citation74. As described above, eliminating undifferentiated cells before transplantation should significantly reduce the risk of tumors, although it remains to be established whether this is sufficient to prevent teratoma formation Citation73.
Adult stem cells
Although postnatal growth of the mammalian heart predominantly results from hypertrophy (enlargement of individual CMs), several studies have described cells from adult tissues that have the potential to repair the heart. They can be divided broadly into: 1) circulating cells, largely of bone marrow (BM) origin but of variable identity that enter the circulation and home to the heart post‐MI, 2) cells from non‐cardiac adult tissues, either used directly or selected or expanded in culture before introduction into the circulation or heart (bone marrow stem cells (BMSCs), hematopoietic stem cells (HSCs) and mesenchymal stem cells (MSCs)), and 3) resident cardiac stem cells or cardiac progenitors (cardiac progenitor cells (CPCs), cardiac stem cells (CSCs)).
Bone marrow as a source of cells for cardiac repair
After transplantation of BM cells (BMCs) from normal male mice into female dystrophic mdx mice CMs were found that contained a Y‐chromosome whereas in hearts of normal female mice undergoing the same procedure, no cells with donor characteristics were identified, initially suggesting that BMCs could differentiate into CMs in injured hearts Citation75. Local injection of 5‐azacytidine‐stimulated BMCs into cryo‐injured hearts induced angiogenesis and improved cardiac contractile force Citation76. A recent report described the derivation of multipotent stem cells from human BM that remained multipotent for 140 population doublings and had the capacity to differentiate into cells of all three germ layers. Transplantation in infarcted nude rat hearts resulted in donor cell‐derived CMs, endothelial cells and smooth muscle cells and upregulated cardioprotective paracrine factors Citation77. However, BMCs have been described as having the ability to fuse with somatic cells, both in vitroCitation78 and in vivoCitation79, Citation80. When BM from genetically marked mice expressing β‐galactoside ubiquitously was injected into wild‐type mice, all of the blue stained cells in liver, brain and heart were shown to be the result of fusion with cells of the host.
Hematopoietic stem cells
The first suggestion that HSC can contribute to cardiac regeneration came from a study by Jackson et al. Citation81. A highly enriched preparation of CD34−/low c‐kit+ Sca‐1+ side population cells was isolated from Rosa26 transgenic mice expressing lacZ. These cells were injected into lethally irradiated mice, who were then subjected to MI. HSCs migrated into ischemic cardiac muscle and blood vessels, and differentiated into CMs and endothelial cells, contributing to the formation of functional tissue. In a different study design, Lin− c‐kit+ BMCs from EGFP‐expressing mice injected into the border zone of infarcted mouse hearts were described as regenerating 68% of the infarct area by forming new myocardium and improving cardiac function and survival Citation82. Nevertheless, Murry Citation79, Balsam Citation80 and Nygren Citation78 found that there was no ‘transdifferentiation’ of HSCs or BMCs into CMs, but that reports stating the opposite were based on the observation of cell fusion or possible misinterpretation of EGFP versus autofluorescent images. Methodological differences however could not explain this discrepancy entirely, since in one study similar methods were used Citation80.
Mesenchymal stem cells
MSCs appear to be the most potent adult stem cells in terms of CM differentiation potential Citation83. Unlike other adult stem cells, with the exception of cardiac progenitors, they do appear to have the capacity to form CMs in vitro and to engraft in the uninjured myocardium Citation84, Citation85. Purification by selection based on cardiac‐specific EGFP expression and subsequent engraftment in normal mouse hearts has also been successful Citation86.
When transduced with the anti‐apoptotic Akt1 gene and injected into rat hearts after MI, they showed extensive graft formation in the heart and completely normalized cardiac function as assessed by ex vivo pressure measurements Citation87. In another attempt to improve graft survival, autologous Lac‐Z labeled MSCs were seeded in a fibrin matrix and transplanted into pigs that had undergone ischemia‐reperfusion. The study described reduced cardiac remodeling and improved systolic performance Citation88.
Circulating stem cells in adults
Analysis of sex‐mismatched heart transplants in humans suggested that adults have circulating stem cells, since both CMs and vascular cells containing a Y‐chromosome were found in female donor hearts transplanted into male recipients Citation89–91. In rodents, enhanced mobilization of endogenous stem cells from BM by injection of granulocyte colony‐stimulating factor (G‐CSF) Citation92–95 or stromal‐cell‐derived factor‐1α (SDF‐1α) Citation96 appeared to improve cardiac function after MI. Additionally, TGF‐β enhanced differentiation Citation97.
However, results were variable and were, at least in some cases, attributable to neovascularization rather than formation of CMs Citation94. In addition, similar treatment in humans was confounded by serious side effects of G‐CSF and it appeared that, in contrast to anaesthetized rodents, G‐CSF levels in humans increased anyway as a result of stress and pain post‐MI. It is therefore unlikely that additional G‐CSF would enhance stem cell mobilization from BM.
Cell cycle reactivation
It has generally been assumed that, like the brain, the heart is a post‐mitotic organ consisting of terminally differentiated CMs. In general, attempts to induce cells to re‐enter the cell cycle have not been successful. However, recent observations of spontaneous cardiac regeneration and reactivation of the cell cycle in surviving CMs in lower vertebrates like zebrafish have revitalized interest in this area Citation98. Recently, transgenic mice expressing the cell division regulatory protein cyclin D2 under the control of the α‐myosin heavy chain promoter have been generated in which CMs undergo DNA synthesis and infarct size is significantly reduced after MI Citation99. The therapeutic potential of this approach however remains uncertain despite its theoretical promise since it is not clear how gene expression in the heart might best be mediated in the light of risks recently associated with current methods of gene therapy Citation100.
Cardiac progenitor cells and cardiac stem cells
Increasing evidence indicates that the murine myocardium harbors several different types of stem cells (i.e. CSCs) that can (re)enter the cell cycle and differentiate into CMs, endothelial cells and vascular smooth muscle cells. In addition there may be a subset of these cells (i.e CPCs) with a more restricted potential to form CMs only. The molecular identities of various subpopulations of stem cells have not yet made clear whether all of the cells have the same potential to form mature CMs in vivo and it will require careful lineage tracing and genetic marking to resolve this issue. In the following section we review the studies that have described the properties of these cells, carefully noting the differences between reports in humans and in mice or rats.
A population of stem cells from postnatal mouse hearts has been described that possesses the ability to efflux Hoechst. These so called ‘side population’ cells represent ∼1% of the total cell number in the adult heart. They were shown to enter the cell cycle in postnatal hypoplastic hearts of MEF2C dominant negative mice and appeared capable of fusion with other cells Citation101.
In a more extensive study, small stem cells with a high nucleus to cytoplasm ratio were isolated from hearts of ∼2‐year‐old Fischer rats using FACS and immunomagnetic microbeads Citation102. These cells were self‐renewing, clonogenic, multipotent, and expressed stem cell markers like c‐kit, while they were negative for markers of the blood lineage (Lin−) suggesting they were not BM derived. They did not express markers of mature myocytes, endothelial cells or fibroblasts. Notably, 7%–10% of the Lin− c‐kit+ cells expressed transcription factors Nkx2.5, GATA‐4 and MEF2C, associated with early myocytes Citation26, Citation103, Citation104, suggesting that the population is heterogeneous and contains cells already committed to the CM lineage. In vitro, the Lin− c‐kit+ cells gave rise to immature CMs, smooth muscle cells, and endothelial cells. Remarkably, they not only formed new myocardium, but also appeared to improve cardiac function, when injected into the myocardium of infarcted rats Citation102.
In a similar approach, another stem cell population but expressing stem cell antigen‐1 (Sca‐1) was isolated from adult mouse hearts Citation105. Like the rat Lin− c‐kit+ cells, these mouse Sca‐1+ cells did not express structural cardiac genes, blood cell lineage markers or hematopoetic stem cell markers. In contrast to the rat cardiac progenitors, these mouse Sca‐1+ cells were c‐kit negative, but expressed high levels of early cardiogenic transcription factors like GATA‐4, MEF2C and TEF‐1. In response to 5’‐azacytidine, the cells were reported to differentiate in vitro to CMs expressing NKx2.5, α‐myosin heavy chain, β‐myosin heavy chain and BMP receptor 1A, all involved in cardiac development. When injected intravenously, Sca‐1+ cells homed to injured myocardium after ischemia/reperfusion injury, and were found to differentiate as well as fuse with the host cells Citation105. Almost simultaneously, the presence of a Sca‐1+ stem cell population in the adult murine heart was confirmed by an independent study. Here, however, the Sca‐1+ cells expressed cardiac transcription factors and contractile proteins, and exhibited sarcomeric structures as well as spontaneous beating when treated with oxytocin Citation106.
In humans, a heterogenous population of CSCs has been isolated by mild enzymatic digestion of human atrial and ventricular biopsy Citation107. These cells formed clonal spherical clusters referred to as cardiospheres expressing endothelial as well as stem cell markers, like c‐kit, Sca‐1 and CD‐34. When cultured as single cells on collagen‐coated dishes, cardiosphere‐derived cells expressed cardiac differentiation markers. A similar population of cells grown as cardiospheres from mice started beating spontaneously.
Recently, a novel population of cells that can proliferate as well as differentiate into cardiac cells has been isolated from rat, mouse and human postnatal hearts. These cells are characterized by the expression of islet‐1, a marker of progenitor cells in the embryonic heart destined to contribute CMs primarily to the left ventricle Citation108. These cells express neither Sca‐1 nor c‐kit, but do express early cardiac differentiation markers like Nkx2.5 and GATA‐4 whilst they lack transcripts of mature myocytes. When cocultured in vitro with primary myocytes, they spontaneously acquired myocyte characteristics Citation109.
Together, these studies have shown that there are multiple but distinct populations of stem cells or progenitor cells that can be found in the rodent and human heart with the capacity to generate cells with some CM characteristics although they may also form other non‐cardiac cells. What the relationship is between these cells is still unclear, as is the question of whether they have a common precursor or intermediate. Lineage tracing using genetic markers may be a way of answering these questions.
Other adult cell types
Skeletal muscle satellite cells have also been investigated as another candidate for CM replacement therapy. Cells from adult murine skeletal muscle started to beat and show other characteristics of CMs in vitro. Upon post‐MI tail vein injection in mice they homed to the myocardium and differentiated in vivoCitation110. However, skeletal myoblasts that were transplanted into rat infarcted myocardium were not electromechanically and functionally coupled with the host CMs Citation111. Instead, they differentiated into morphologically distinct hyperexcitable myotubes, a potential source for arrhythmias.
Despite all these reports claiming to demonstrate the great potential for adult stem cells in CM replacement therapy, skepticism has arisen among investigators trying to repeat these experiments with little success. This culminated in a series of publications describing possible sources of artifacts Citation78–80. As described above, cell fusion could explain the apparent ‘transdifferentiation’ in part. In addition, autofluorescence deriving from scar tissue at the site of MI appeared to be another source of possible misinterpretation of data. Transplanting cells expressing an enzymatic marker like β‐galactoside instead of using GFP epifluorescence confirmed that transdifferentiation did not take place. HSCs and BMCs may contribute to neovascularization at the site of MI however, and this might explain reports of improved cardiac function post‐MI in mice, and to a limited extent in humans. It is not clear whether this represents long‐term improvement or is a short‐term effect.
Clinical trials
Despite ongoing discussions on the correct interpretation of the animal studies, clinical studies have been initiated in several countries, including larger numbers of patients and placebo control groups. The drive is the lack of alternatives and the lack of donor organs. Most of these studies have so far been based on transplantation with autologous BMCs Citation112. Whether controlled or not, most of these investigations report improvement of at least some parameters indicative of cardiac function, although results have not always been reproducible Citation113. From a scientific point of view, humans are the best model to study treatment of cardiac disease because of differences in physiology and pathology between species but it is essential to take every precaution that trials are safe. To date, with approximately 300 patients treated with their own BM no directly related adverse effects have been reported although stenosis associated with intracoronary delivery of cells Citation114 and arrhythmias after skeletal myoblast transplantation Citation115 in humans have been described. Studies in humans receiving autologous cell transplantations will not disclose the fate of the transplanted cells. Long‐ and short‐term cardiac function are the only aspects that can be assessed as the cells cannot be tracked in and ex vivo and the underlying histology remains unclear.
Conclusion
Although stem cells make up an appealing field of research with important clinical implications, many questions need to be addressed before ESCs can be used in cardiac cell therapy in humans. Issues include selection and upscaling of pure CM populations, assessment of their immunogenic potential, and the unambiguous determination of their fate after transplantation. Clinical trials are ongoing with various adult stem cells but their efficacy could probably be improved by understanding how they act.
Acknowledgements
DVH was supported by the Netherlands Proteomics Centre Bsik program and CLM by the Bsik Programs ‘Dutch Platform for Tissue Engineering’ and ‘Stem Cells in Development and Disease’ and ‘Embryonic Stem Cell International’. We thank Stefan Braam and Leon Tertoolen for Figure , Maurits Jansen for help with Figure , Cees Van Echteld for use of the MRI facility and Robert Passier for critical reading of the manuscript.
References
- International Cardiovascular Disease Statistics, American Heart Association (2005)
- Evans M. J., Kaufman M. H. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981; 292: 154–6
- Martin G. R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A 1981; 78: 7634–8
- Bradley A., Evans M., Kaufman M. H., Robertson E. Formation of germ‐line chimaeras from embryo‐derived teratocarcinoma cell lines. Nature 1984; 309: 255–6
- Thomson J. A., Itskovitz‐Eldor J., Shapiro S. S., Waknitz M. A., Swiergiel J. J., Marshall V. S., et al. Embryonic stem cell lines derived from human blastocysts. Science 1998; 282: 1145–7
- Reubinoff B. E., Pera M. F., Fong C. Y., Trounson A., Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol 2000; 18: 399–404
- Doetschman T. C., Eistetter H., Katz M., Schmidt W., Kemler R. The in vitro development of blastocyst‐derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol 1985; 87: 27–45
- Hescheler J., Fleischmann B. K., Lentini S., Maltsev V. A., Rohwedel J., Wobus A. M., et al. Embryonic stem cells: a model to study structural and functional properties in cardiomyogenesis. Cardiovasc Res 1997; 36: 149–62
- Maltsev V. A., Wobus A. M., Rohwedel J., Bader M., Hescheler J. Cardiomyocytes differentiated in vitro from embryonic stem cells developmentally express cardiac‐specific genes and ionic currents. Circ Res 1994; 75: 233–44
- Wobus A. M., Wallukat G., Hescheler J. Pluripotent mouse embryonic stem cells are able to differentiate into cardiomyocytes expressing chronotropic responses to adrenergic and cholinergic agents and Ca2+ channel blockers. Differentiation 1991; 48: 173–82
- Boheler K. R., Czyz J., Tweedie D., Yang H. T., Anisimov S. V., Wobus A. M. Differentiation of pluripotent embryonic stem cells into cardiomyocytes. Circ Res 2002; 91: 189–201
- Williams R. L., Hilton D. J., Pease S., Willson T. A., Stewart C. L., Gearing D. P., et al. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature 1988; 336: 684–7
- Smith A. G., Heath J. K., Donaldson D. D., Wong G. G., Moreau J., Stahl M., et al. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature 1988; 336: 688–90
- Richards M., Tan S., Fong C. Y., Biswas A., Chan W. K., Bongso A. Comparative evaluation of various human feeders for prolonged undifferentiated growth of human embryonic stem cells. Stem Cells 2003; 21: 546–556
- Beattie G. M., Lopez A. D., Bucay N., Hinton A., Firpo M. T., King C. C., et al. Activin A maintains pluripotency of human embryonic stem cells in the absence of feeder layers. Stem Cells 2005; 23: 489–95
- Amit M., Shariki C., Margulets V., Itskovitz‐Eldor J. Feeder layer‐ and serum‐free culture of human embryonic stem cells. Biol Reprod 2004; 70: 837–45
- Xu R. H., Peck R. M., Li D. S., Feng X., Ludwig T., Thomson J. A. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat Methods 2005; 2: 185–90
- Stojkovic P., Lako M., Stewart R., Przyborski S., Armstrong L., Evans J., et al. An autogeneic feeder cell system that efficiently supports growth of undifferentiated human embryonic stem cells. Stem Cells 2005; 23: 306–14
- Klimanskaya I., Chung Y., Meisner L., Johnson J., West M. D., Lanza R. Human embryonic stem cells derived without feeder cells. Lancet 2005; 365: 1636–41
- He J. Q., Ma Y., Lee Y., Thomson J. A., Kamp T. J. Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization. Circ Res 2003; 93: 32–9
- Kehat I., Kenyagin‐Karsenti D., Snir M., Segev H., Amit M., Gepstein A., et al. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest 2001; 108: 407–14
- Xu C., Police S., Rao N., Carpenter M. K. Characterization and enrichment of cardiomyocytes derived from human embryonic stem cells. Circ Res 2002; 91: 501–8
- Mummery C., Ward‐van Oostwaard D., Doevendans P., Spijker R., van den B. S., Hassink R., et al. Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm‐like cells. Circulation 2003; 107: 2733–40
- Muslin A. J., Williams L. T. Well‐defined growth factors promote cardiac development in axolotl mesodermal explants. Development 1991; 112: 1095–101
- Consigli S. A., Joseph‐Silverstein J. Immunolocalization of basic fibroblast growth factor during chicken cardiac development. J Cell Physiol 1991; 146: 379–85
- Lints T. J., Parsons L. M., Hartley L., Lyons I., Harvey R. P. Nkx‐2.5: a novel murine homeobox gene expressed in early heart progenitor cells and their myogenic descendants. Development 1993; 119: 419–31
- Tonissen K. F., Drysdale T. A., Lints T. J., Harvey R. P., Krieg P. A. XNkx‐2.5, a Xenopus gene related to Nkx‐2.5 and tinman: evidence for a conserved role in cardiac development. Dev Biol 1994; 162: 325–8
- Lyons I., Parsons L. M., Hartley L., Li R., Andrews J. E., Robb L., et al. Myogenic and morphogenetic defects in the heart tubes of murine embryos lacking the homeo box gene Nkx2‐5. Genes Dev 1995; 9: 1654–66
- Grepin C., Robitaille L., Antakly T., Nemer M. Inhibition of transcription factor GATA‐4 expression blocks in vitro cardiac muscle differentiation. Mol Cell Biol 1995; 15: 4095–102
- Grepin C., Nemer G., Nemer M. Enhanced cardiogenesis in embryonic stem cells overexpressing the GATA‐4 transcription factor. Development 1997; 124: 2387–95
- Niederreither K., Vermot J., Messaddeq N., Schuhbaur B., Chambon P., Dolle P. Embryonic retinoic acid synthesis is essential for heart morphogenesis in the mouse. Development 2001; 128: 1019–31
- Wobus A. M., Kaomei G., Shan J., Wellner M. C., Rohwedel J., Ji G., et al. Retinoic acid accelerates embryonic stem cell‐derived cardiac differentiation and enhances development of ventricular cardiomyocytes. J Mol Cell Cardiol 1997; 29: 1525–39
- Mummery C. L., Feijen A., van der Saag P. T., van den Brink C. E., de Laat S. W. Clonal variants of differentiated P19 embryonal carcinoma cells exhibit epidermal growth factor receptor kinase activity. Dev Biol 1985; 109: 402–10
- Mummery C. L., van Achterberg T. A., van den Eijnden‐van Raaij A. J., van Haaster L., Willemse A., de Laat S. W., et al. Visceral‐endoderm‐like cell lines induce differentiation of murine P19 embryonal carcinoma cells. Differentiation 1991; 46: 51–60
- Mummery C., Ward D., van den Brink C. E., Bird S. D., Doevendans P. A., Opthof T., et al. Cardiomyocyte differentiation of mouse and human embryonic stem cells. J Anat 2002; 200((Pt 3))233–42
- Dono R., Scalera L., Pacifico F., Acampora D., Persico M. G., Simeone A. The murine cripto gene: expression during mesoderm induction and early heart morphogenesis. Development 1993; 118: 1157–68
- Xu C., Liguori G., Adamson E. D., Persico M. G. Specific arrest of cardiogenesis in cultured embryonic stem cells lacking Cripto‐1. Dev Biol 1998; 196: 237–47
- Parisi S., D'Andrea D., Lago C. T., Adamson E. D., Persico M. G., Minchiotti G. Nodal‐dependent Cripto signaling promotes cardiomyogenesis and redirects the neural fate of embryonic stem cells. J Cell Biol 2003; 163: 303–14
- Bloch W., Fleischmann B. K., Lorke D. E., Andressen C., Hops B., Hescheler J., et al. Nitric oxide synthase expression and role during cardiomyogenesis. Cardiovasc Res 1999; 43: 675–84
- Lee T. C., Zhao Y. D., Courtman D. W., Stewart D. J. Abnormal aortic valve development in mice lacking endothelial nitric oxide synthase. Circulation 2000; 101: 2345–8
- Feng Q., Song W., Lu X., Hamilton J. A., Lei M., Peng T., et al. Development of heart failure and congenital septal defects in mice lacking endothelial nitric oxide synthase. Circulation 2002; 106: 873–9
- Kanno S., Kim P. K., Sallam K., Lei J., Billiar T. R., Shears L. L. Nitric oxide facilitates cardiomyogenesis in mouse embryonic stem cells. Proc Natl Acad Sci U S A 2004; 101: 12277–81
- Behfar A., Zingman L. V., Hodgson D. M., Rauzier J. M., Kane G. C., Terzic A., et al. Stem cell differentiation requires a paracrine pathway in the heart. FASEB J 2002; 16: 1558–66
- Kawai T., Takahashi T., Esaki M., Ushikoshi H., Nagano S., Fujiwara H., et al. Efficient cardiomyogenic differentiation of embryonic stem cell by fibroblast growth factor 2 and bone morphogenetic protein 2. Circ J 2004; 68: 691–702
- Yuasa S., Itabashi Y., Koshimizu U., Tanaka T., Sugimura K., Kinoshita M., et al. Transient inhibition of BMP signaling by Noggin induces cardiomyocyte differentiation of mouse embryonic stem cells. Nat Biotechnol 2005; 23: 607–11
- Kubo A., Shinozaki K., Shannon J. M., Kouskoff V., Kennedy M., Woo S., et al. Development of definitive endoderm from embryonic stem cells in culture. Development 2004; 131: 1651–62
- Foshay K., Rodriguez G., Hoel B., Narayan J., Gallicano G. I. JAK2/STAT3 directs cardiomyogenesis within murine embryonic stem cells in vitro. Stem Cells 2005; 23: 530–43
- Passier R., Oostwaard D. W., Snapper J., Kloots J., Hassink R. J., Kuijk E., et al. Increased cardiomyocyte differentiation from human embryonic stem cells in serum‐free cultures. Stem Cells 2005; 23: 772–80
- Klug M. G., Soonpaa M. H., Koh G. Y., Field L. J. Genetically selected cardiomyocytes from differentiating embronic stem cells form stable intracardiac grafts. J Clin Invest 1996; 98: 216–24
- Metzger J. M., Lin W. I., Samuelson L. C. Vital staining of cardiac myocytes during embryonic stem cell cardiogenesis in vitro. Circ Res 1996; 78: 547–52
- Kolossov E., Fleischmann B. K., Liu Q., Bloch W., Viatchenko‐Karpinski S., Manzke O., et al. Functional characteristics of ES cell‐derived cardiac precursor cells identified by tissue‐specific expression of the green fluorescent protein. J Cell Biol 1998; 143: 2045–56
- Fleischmann M., Bloch W., Kolossov E., Andressen C., Muller M., Brem G., et al. Cardiac specific expression of the green fluorescent protein during early murine embryonic development. FEBS Lett 1998; 440: 370–6
- Fijnvandraat A. C., van Ginneken A. C., de Boer P. A., Ruijter J. M., Christoffels V. M., Moorman A. F., et al. Cardiomyocytes derived from embryonic stem cells resemble cardiomyocytes of the embryonic heart tube. Cardiovasc Res 2003; 58: 399–409
- Moore J. C., van Laake L. W., Braam S. R., Xue T., Tsang S. Y., Ward D., et al. Human embryonic stem cells: Genetic manipulation on the way to cardiac cell therapies. Reprod Toxicol 2005; 20: 377–91
- Himes N., Min J. Y., Lee R., Brown C., Shea J., Huang X., et al. In vivo MRI of embryonic stem cells in a mouse model of myocardial infarction. Magn Reson Med 2004; 52: 1214–9
- Hodgson D. M., Behfar A., Zingman L. V., Kane G. C., Perez‐Terzic C., Alekseev A. E., et al. Stable benefit of embryonic stem cell therapy in myocardial infarction. Am J Physiol Heart Circ Physiol 2004; 287: H471–9
- Kofidis T., de Bruin J. L., Yamane T., Balsam L. B., Lebl D. R., Swijnenburg R. J., et al. Insulin‐like growth factor promotes engraftment, differentiation, and functional improvement after transfer of embryonic stem cells for myocardial restoration. Stem Cells 2004; 22: 1239–45
- Kofidis T., de Bruin J. L., Hoyt G., Lebl D. R., Tanaka M., Yamane T., et al. Injectable bioartificial myocardial tissue for large‐scale intramural cell transfer and functional recovery of injured heart muscle. J Thorac Cardiovasc Surg 2004; 128: 571–8
- Min J. Y., Yang Y., Converso K. L., Liu L., Huang Q., Morgan J. P., et al. Transplantation of embryonic stem cells improves cardiac function in postinfarcted rats. J Appl Physiol 2002; 92: 288–96
- Min J. Y., Yang Y., Sullivan M. F., Ke Q., Converso K. L., Chen Y., et al. Long‐term improvement of cardiac function in rats after infarction by transplantation of embryonic stem cells. J Thorac Cardiovasc Surg 2003; 125: 361–9
- Naito H., Nishizaki K., Yoshikawa M., Yamada T., Satoh H., Nagasaka S., et al. Xenogeneic embryonic stem cell‐derived cardiomyocyte transplantation. Transplant Proc 2004; 36: 2507–8
- Yang Y., Min J. Y., Rana J. S., Ke Q., Cai J., Chen Y., et al. VEGF enhances functional improvement of postinfarcted hearts by transplantation of ESC‐differentiated cells. J Appl Physiol 2002; 93: 1140–51
- Xue T., Cho H. C., Akar F. G., Tsang S. Y., Jones S. P., Marban E., et al. Functional integration of electrically active cardiac derivatives from genetically engineered human embryonic stem cells with quiescent recipient ventricular cardiomyocytes: insights into the development of cell‐based pacemakers. Circulation 2005; 111: 11–20
- Kehat I., Khimovich L., Caspi O., Gepstein A., Shofti R., Arbel G., et al. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat Biotechnol 2004; 22: 1282–9
- Min J. Y., Sullivan M. F., Yang Y., Zhang J. P., Converso K. L., Morgan J. P., et al. Significant improvement of heart function by cotransplantation of human mesenchymal stem cells and fetal cardiomyocytes in postinfarcted pigs. Ann Thorac Surg 2002; 74: 1568–75
- Tian L., Catt J. W., O'Neill C., King N. J. Expression of immunoglobulin superfamily cell adhesion molecules on murine embryonic stem cells. Biol Reprod 1997; 57: 561–8
- Drukker M., Katz G., Urbach A., Schuldiner M., Markel G., Itskovitz‐Eldor J., et al. Characterization of the expression of MHC proteins in human embryonic stem cells. Proc Natl Acad Sci U S A 2002; 99: 9864–9
- Drukker M., Benvenisty N. The immunogenicity of human embryonic stem‐derived cells. Trends Biotechnol 2004; 22: 136–41
- Hwang W. S., Roh S. I., Lee B. C., Kang S. K., Kwon D. K., Kim S., et al. Patient‐specific embryonic stem cells derived from human SCNT blastocysts. Science 2005; 308: 1777–83
- Zijlstra M., Bix M., Simister N. E., Loring J. M., Raulet D. H., Jaenisch R. Beta 2‐microglobulin deficient mice lack CD4‐8+ cytolytic T cells. Nature 1990; 344: 742–6
- Lips D. J., van der N. T., Steendijk P., Palmen M., Janssen B. J., van Dantzig J. M., et al. Left ventricular pressure‐volume measurements in mice: comparison of closed‐chest versus open‐chest approach. Basic Res Cardiol 2004; 99: 351–9
- Devlin A. M., Moore N. R., Ostman‐Smith I. A comparison of MRI and echocardiography in hypertrophic cardiomyopathy. Br J Radiol 1999; 72: 258–64
- Erdo F., Buhrle C., Blunk J., Hoehn M., Xia Y., Fleischmann B., et al. Host‐dependent tumorigenesis of embryonic stem cell transplantation in experimental stroke. J Cereb Blood Flow Metab 2003; 23: 780–5
- Newman M. B., Misiuta I., Willing A. E., Zigova T., Karl R. C., Borlongan C. V., et al. Tumorigenicity issues of embryonic carcinoma‐derived stem cells: relevance to surgical trials using NT2 and hNT neural cells. Stem Cells Dev 2005; 14: 29–43
- Bittner R. E., Schofer C., Weipoltshammer K., Ivanova S., Streubel B., Hauser E., et al. Recruitment of bone‐marrow‐derived cells by skeletal and cardiac muscle in adult dystrophic mdx mice. Anat Embryol (Berl) 1999; 199: 391–6
- Tomita S., Li R. K., Weisel R. D., Mickle D. A., Kim E. J., Sakai T., et al. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation 1999; 100((19 Suppl))II247–56
- Yoon Y. S., Wecker A., Heyd L., Park J. S., Tkebuchava T., Kusano K., et al. Clonally expanded novel multipotent stem cells from human bone marrow regenerate myocardium after myocardial infarction. J Clin Invest 2005; 115: 326–38
- Nygren J. M., Jovinge S., Breitbach M., Sawen P., Roll W., Hescheler J., et al. Bone marrow‐derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med 2004; 10: 494–501
- Murry C. E., Soonpaa M. H., Reinecke H., Nakajima H., Nakajima H. O., Rubart M., et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature 2004; 428: 664–8
- Balsam L. B., Wagers A. J., Christensen J. L., Kofidis T., Weissman I. L., Robbins R. C. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature 2004; 428: 668–73
- Jackson K. A., Majka S. M., Wang H., Pocius J., Hartley C. J., Majesky M. W., et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest 2001; 107: 1395–402
- Orlic D., Kajstura J., Chimenti S., Jakoniuk I., Anderson S. M., Li B., et al. Bone marrow cells regenerate infarcted myocardium. Nature 2001; 410: 701–5
- Smits A. M., van Vliet P., Hassink R. J., Goumans M. J., Doevendans P. A. The role of stem cells in cardiac regeneration. J Cell Mol Med 2005; 9: 25–36
- Toma C., Pittenger M. F., Cahill K. S., Byrne B. J., Kessler P. D. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation 2002; 105: 93–8
- Wang J. S., Shum‐Tim D., Galipeau J., Chedrawy E., Eliopoulos N., Chiu R. C. Marrow stromal cells for cellular cardiomyoplasty: feasibility and potential clinical advantages. J Thorac Cardiovasc Surg 2000; 120: 999–1005
- Hattan N., Kawaguchi H., Ando K., Kuwabara E., Fujita J., Murata M., et al. Purified cardiomyocytes from bone marrow mesenchymal stem cells produce stable intracardiac grafts in mice. Cardiovasc Res 2005; 65: 334–44
- Mangi A. A., Noiseux N., Kong D., He H., Rezvani M., Ingwall J. S., et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med 2003; 9: 1195–201
- Liu J., Hu Q., Wang Z., Xu C., Wang X., Gong G., et al. Autologous stem cell transplantation for myocardial repair. Am J Physiol Heart Circ Physiol 2004; 287: H501–11
- Laflamme M. A., Myerson D., Saffitz J. E., Murry C. E. Evidence for cardiomyocyte repopulation by extracardiac progenitors in transplanted human hearts. Circ Res 2002; 90: 634–40
- Muller P., Pfeiffer P., Koglin J., Schafers H. J., Seeland U., Janzen I., et al. Cardiomyocytes of noncardiac origin in myocardial biopsies of human transplanted hearts. Circulation 2002; 106: 31–5
- Quaini F., Urbanek K., Beltrami A. P., Finato N., Beltrami C. A., Nadal‐Ginard B., et al. Chimerism of the transplanted heart. N Engl J Med 2002; 346: 5–15
- Fukuhara S., Tomita S., Nakatani T., Ohtsu Y., Ishida M., Yutani C., et al. G‐CSF promotes bone marrow cells to migrate into infarcted mice heart, and differentiate into cardiomyocytes. Cell Transplant 2004; 13: 741–8
- Kawada H., Fujita J., Kinjo K., Matsuzaki Y., Tsuma M., Miyatake H., et al. Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction. Blood 2004; 104: 3581–7
- Norol F., Merlet P., Isnard R., Sebillon P., Bonnet N., Cailliot C., et al. Influence of mobilized stem cells on myocardial infarct repair in a nonhuman primate model. Blood 2003; 102: 4361–8
- Orlic D., Kajstura J., Chimenti S., Limana F., Jakoniuk I., Quaini F., et al. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci U S A 2001; 98: 10344–9
- Tang Y. L., Qian K., Zhang Y. C., Shen L., Phillips M. I. Mobilizing of haematopoietic stem cells to ischemic myocardium by plasmid mediated stromal‐cell‐derived factor‐1alpha (SDF‐1alpha) treatment. Regul Pept 2005; 125: 1–8
- Li T. S., Hayashi M., Ito H., Furutani A., Murata T., Matsuzaki M., et al. Regeneration of infarcted myocardium by intramyocardial implantation of ex vivo transforming growth factor‐beta‐preprogrammed bone marrow stem cells. Circulation 2005; 111: 2438–45
- Dowell J. D., Field L. J., Pasumarthi K. B. Cell cycle regulation to repair the infarcted myocardium. Heart Fail Rev 2003; 8: 293–303
- Pasumarthi K. B., Nakajima H., Nakajima H. O., Soonpaa M. H., Field L. J. Targeted expression of cyclin D2 results in cardiomyocyte DNA synthesis and infarct regression in transgenic mice. Circ Res 2005; 96: 110–8
- Kimmelman J. Recent developments in gene transfer: risk and ethics. BMJ 2005; 330: 79–82
- Hierlihy A. M., Seale P., Lobe C. G., Rudnicki M. A., Megeney L. A. The post‐natal heart contains a myocardial stem cell population. FEBS Lett 2002; 530: 239–43
- Beltrami A. P., Barlucchi L., Torella D., Baker M., Limana F., Chimenti S., et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 2003; 114: 763–76
- Lin T., Chao C., Saito S., Mazur S. J., Murphy M. E., Appella E., et al. p53 induces differentiation of mouse embryonic stem cells by suppressing Nanog expression. Nat Cell Biol 2005; 7: 165–71
- Watt A. J., Battle M. A., Li J., Duncan S. A. GATA4 is essential for formation of the proepicardium and regulates cardiogenesis. Proc Natl Acad Sci U S A 2004; 101: 12573–8
- Oh H., Bradfute S. B., Gallardo T. D., Nakamura T., Gaussin V., Mishina Y., et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci U S A 2003; 100: 12313–8
- Matsuura K., Nagai T., Nishigaki N., Oyama T., Nishi J., Wada H., et al. Adult cardiac Sca‐1‐positive cells differentiate into beating cardiomyocytes. J Biol Chem 2004; 279: 11384–91
- Messina E., De Angelis L., Frati G., Morrone S., Chimenti S., Fiordaliso F., et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res 2004; 95: 911–21
- Evans G. A. “MegaYAC” library. Science 1993; 260: 877
- Laugwitz K. L., Moretti A., Lam J., Gruber P., Chen Y., Woodard S., et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature 2005; 433: 647–53
- Winitsky S. O., Gopal T. V., Hassanzadeh S., Takahashi H., Gryder D., Rogawski M. A., et al. Adult murine skeletal muscle contains cells that can differentiate into beating cardiomyocytes in vitro. PLoS Biol 2005; 3: e87
- Leobon B., Garcin I., Menasche P., Vilquin J. T., Audinat E., Charpak S. Myoblasts transplanted into rat infarcted myocardium are functionally isolated from their host. Proc Natl Acad Sci U S A 2003; 100: 7808–11
- Dimmeler S., Zeiher A. M., Schneider M. D. Unchain my heart: the scientific foundations of cardiac repair. J Clin Invest 2005; 115: 572–83
- Kuethe F., Richartz B. M., Sayer H. G., Kasper C., Werner G. S., Hoffken K., et al. Lack of regeneration of myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans with large anterior myocardial infarctions. Int J Cardiol 2004; 97: 123–7
- Kang H. J., Kim H. S., Zhang S. Y., Park K. W., Cho H. J., Koo B. K., et al. Effects of intracoronary infusion of peripheral blood stem‐cells mobilised with granulocyte‐colony stimulating factor on left ventricular systolic function and restenosis after coronary stenting in myocardial infarction: the MAGIC cell randomised clinical trial. Lancet 2004; 363: 751–6
- Menasche P., Hagege A. A., Vilquin J. T., Desnos M., Abergel E., Pouzet B., et al. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J Am Coll Cardiol 2003; 41: 1078–83