Abstract
Motor dysfunctions in Parkinson's disease are considered to be primarily due to the degeneration of dopaminergic neurons in the substantia nigra pars compacta. Pharmacological therapies based on the principle of dopamine replacement are extremely valuable, but suffer from two main drawbacks: troubling side effects (e.g. dyskinesia) and loss of efficacy with disease progression. Transplantation of embryonic dopaminergic neurons has emerged as a therapeutic alternative. Enthusiasm following the success of the initial open‐label trials has been dampened by the negative outcome of double‐blind placebo controlled trials. Additionally, the emergence of graft‐related dyskinesia indicates that the experimental grafting procedure requires further refinement before it can be developed into a therapy. Shortage of embryonic donor tissue limits large‐scale clinical transplantation trials. We review three of the most attractive tissue sources of dopaminergic neurons for cell replacement therapy: human embryonic ventral mesencephalic tissue, embryonic and adult multipotent region‐specific stem cells and embryonic stem cells. Recent developments in embryonic stem cell research and on their implications for a future transplantation therapy in Parkinson's disease are described. Finally, we discuss how human embryonic stem cells can be differentiated into dopaminergic neurons, and issues such as the numbers of dopaminergic neurons required for success and the risk for teratoma formation after implantation.
| Abbreviations | ||
| bFGF | = | basic fibroblast growth factor |
| DA | = | dopamine |
| EBs | = | embryoid bodies |
| EGF | = | epidermal growth factor |
| ESCs | = | embryonic stem cells |
| FGF‐8 | = | fibroblast growth factor‐8 |
| hESCs | = | human embryonic stem cells |
| L‐DOPA | = | L‐3,4‐dihydroxyphenylalanine |
| 6‐OHDA | = | 6‐hydroxydopamine |
| PD | = | Parkinson's disease |
| SDIA | = | stromal cell‐derived inducing activity |
| SHH | = | sonic hedgehog |
| TH | = | tyrosine hydroxylase |
| VTA | = | ventral tegmental area |
Introduction
Parkinson's disease (PD) is characterized by motor symptoms, which include prominent akinesia, rigidity, tremor and postural instability. The degeneration of dopamine (DA) neurons in the substantia nigra pars compacta, with consequent reduction of DA in the striatum, plays a central role for these motor symptoms. In later stages of the disease some patients also develop dementia, depression, disturbed sleep and signs of autonomic nervous system impairment. These symptoms, known as ‘non‐dopaminergic’ symptoms are caused by the degeneration of other neuronal systems, such as noradrenergic, serotonergic and cholinergic. Thus, today the neuropathology of PD is viewed as being more complex than previously thought, involving not merely the nigrostriatal DA pathway, but also several other brain systems Citation1, Citation2.
The DA precursor L‐3,4‐dihydroxyphenylalanine (L‐DOPA) remains the key treatment for PD, providing excellent symptomatic relief during the first years after start of the therapy Citation3. Invariably, the vast majority of patients develop motor fluctuations, known as the ‘on–off’ phenomenon, after about 5 to 10 years of treatment. Thus, despite careful administration of multiple daily doses of L‐DOPA the patients oscillate between a severely akinetic state and a condition when they exhibit disabling abnormal involuntary movement, called dyskinesia Citation4. Besides this, with the progression of the disease the benefits of L‐DOPA treatment gradually diminish and the time spent in ‘on’ phase gradually declines. Recently, several DA agonists, inhibitors of DA breakdown and novel surgical approaches (e.g. deep brain stimulation) have been shown to partially ameliorate these problems Citation4. These therapies do not, however, prevent disease progression and, furthermore, existing pharmacological treatments do not really improve symptoms believed to be due to non‐dopaminergic pathology. There is little doubt that other therapeutic strategies are still needed for advanced PD Citation5.
The shortcomings of pharmacological therapies have led to the search for alternative treatments. Open‐label clinical trials applying mesencephalic DA neurons dissected from human embryos have shown that when successful cell replacement therapy is clearly beneficial Citation6–9. Other sources of DA neurons have also been examined in clinical transplantation trials. For example, autografts of sympathetic neurons Citation10, Citation11 and carotid body transplants Citation12 have been tested, but a lack of positive clinical results and evidence for graft survival have been discouraging. Embryonic pig neural cells were shown to survive transplantation into a PD patient Citation13. However, the number of surviving cells was very low suggesting that graft rejection had been taking place and there was not significant improvement of function in the patients Citation14. Most probably, intracerebral xenografts require aggressive immunosuppression and, additionally, they are associated with the potential risk of animal virus transmission to humans Citation15.
Stem cells of human origin are currently considered as the most promising future source of DA neurons for a cell‐based therapy for PD Citation16–18. There are at least two major different types of stem cells that have been studied for this purpose: multipotent region‐specific stem cells, isolated from embryonic/fetal or adult brain, and pluripotent embryonic stem cells (ESCs), which constitute the inner cell mass of blastocysts. Regardless of the source of cells, the phenotype of the DA neurons used for transplantation ought to fulfill certain criteria before they should be considered relevant to a clinical therapy. Their ability to meet these criteria can be tested by grafting in animal models of PD. For example, they must be capable of synthesizing and releasing DA in a controlled fashion; they need to extend axons that re‐innervate the striatum; and they should ameliorate motor symptoms. Recent studies indicate that the DA neurons also ought to express the G‐protein‐coupled inward rectifying K+ channel subunit (Girk2). The Girk2 protein is predominantly expressed in substantia nigra pars compacta neurons, and not in the DA neurons located in the adjacent ventral tegmental area (VTA) Citation19, Citation20. A recent study has shown that DA neurons innervating the host striatum in mouse mesencephalic grafts also express Girk2 and are located around the periphery of the grafts Citation20. In contrast, the DA neurons located in the core of the implants typically do not express Girk2. Instead, they contain the calcium‐binding protein calbindin (normally found in most DA neurons of the VTA) and do not extend axons into the surrounding host striatum. In post‐mortem analysis of the brains from two PD patients who received transplants of embryonic mesencephalic tissue Citation19 it was also observed that the Girk2 positive neurons were preferentially located around the perimeter of the graft tissue. Taken together, it appears that Girk2 is a novel and useful marker to label cells that are true substantia nigra pars compacta DA neurons and those are most likely to efficiently innervate the host striatum.
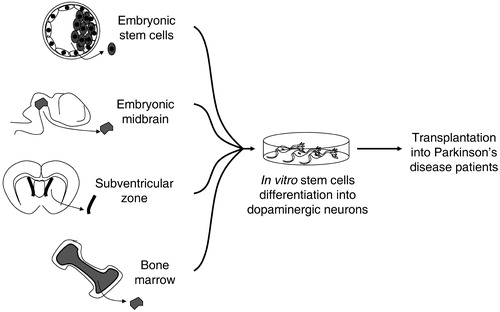
In this review we briefly overview the clinical trials using embryonic mesencephalic tissue, briefly describe different types of stem cells considered relevant to PD and provide a more detailed discussion on hESCs as a source of DA neurons in PD therapy. In Figure are represented the alternative sources of stem cells for a cell‐based therapy for Parkinson's disease discussed in this review.
Figure 1 Proposed alternative sources of stem cells for a cell‐based therapy in Parkinson's disease. These sources include pluripotent embryonic stem cells and multipotent region specific stem cells that can be isolated from embryonic tissues, such as midbrain, and from the adult, such as subventricular zone and bone marrow.
Key messages
Neural transplantation in Parkinson's disease can work.
Variable efficacy of transplants, occasional occurrence of troubling side effects and a shortage of suitable embryonic donor tissue limit a wider application of neural transplantation in Parkinson's disease.
Several types of stem cells have been suggested as sources of dopaminergic neurons for cell replacement therapy in Parkinson's disease. Human embryonic stem cells appear to be the most promising.
Why is mesencephalic tissue obtained from human embryos not the most promising source of DA neurons for a cell‐based therapy for PD?
Embryonic DA neurons after transplantation survive, extend axons, make synaptic contacts, restore reduced striatal DA levels and reverse behavioral deficits in rodent and non‐human primate models of PD Citation21, Citation22. Initial clinical trials with transplants of DA neurons obtained from aborted human embryos were also very promising Citation23, Citation24. The experience accumulated over the past 18 years indicates that transplanted DA neurons can survive, reinstate DA neurotransmission in the striatum, and ameliorate some of the symptoms of PD (see review Citation7). In the best cases, patients showed a marked symptomatic relief without L‐DOPA treatment for over 10 years Citation25. This enthusiasm was dampened by the completion of two large, double‐blind placebo controlled transplantation trials which both reported that the grafts failed to improve the PD patients on the primary endpoints Citation26, Citation27. In addition, subsets of patients develop disabling dyskinesia after neural grafting even in the absence of medication Citation27–29. The reasons why the success with symptomatic relief observed in the open‐label trials could not be matched in the controlled studies and why dyskinesia appears in some patients are not clear and have been debated in the literature. It has been suggested that differences in, for example, patient selection, immunosuppression protocols, tissue storage and preparation strongly influenced the outcome of the large controlled studies Citation7, Citation17, Citation30–32. Concerning the etiology of graft‐induced dyskinesia, it has been speculated that the possible factors are uneven graft‐derived DA innervation of the striatum Citation33, inflammation around the implants Citation17, Citation31, presence of different subpopulations of DA neurons in the graft Citation34 and inappropriate synaptic contacts Citation17 (for review see Citation35).
Regardless of the explanations for poor graft efficacy and graft‐induced dyskinesia, the future development of a neural transplantation therapy is also hampered by the lack of a good source of donor tissue. Whilst several countries consider the use of brain tissue from aborted embryos ethically justified, this is not the case everywhere Citation36. However, even when it is considered acceptable, access to embryonic tissue is unpredictable and limited. Based on animal experiments and available clinical data, it has been estimated that 100,000 grafted DA neurons need to survive in the putamen on each side of the brain in order to induce behavioral recovery Citation7. When tissue from 7 embryos was implanted bilaterally in the putamen by Kordower et al., approximately 100,000 DA neurons were detected in the grafts, leading to 25%–40% reduction of motor symptoms in the ‘off’ phase Citation29. Unfortunately, in the only study that has systematically examined the importance of cell number for graft efficacy, there were no statistically significant effects of the implants regardless of the number of cells implanted Citation26. Thus, in a large double‐blind, placebo‐controlled study neither a group of patients receiving tissue from four donors per side nor those receiving tissue from one donor per side exhibited significant recovery compared to the control group. Insufficient immunosuppression has been speculated to result in partial graft rejection and inhibited the functional effects of the implants (see e.g. Citation31). Taken together, the minimum number of surviving grafted DA neurons – and consequently the number of donor embryos– needed to achieve a considerable symptomatic relief remains unknown. Consequently, the logistics surrounding tissue retrieval and transplantation are complicated and it is difficult to standardize the procedure between patients, making large‐scale trials extremely difficult to conduct.
Neural stem cells from embryonic and adult brains as sources of dopamine neurons
Multipotent stem cells can be found in the nervous system of developing embryos/fetuses and in adults. These cells have been considered as a possible unlimited and self‐renewing source of material for transplantation. Neural stem cells isolated from the midbrain just before the formation of DA neurons can be expanded in vitro and induced to differentiate into functional DA neurons Citation37. However, after long‐term culture these midbrain‐derived stem cells lose their ability to differentiate into DA neurons. Genetic modifications and epigenetic factors (e.g. growth factors in the culture medium) have been used to help these cells to maintain their capacity to divide and differentiate Citation38. In the adult brain, neural stem cells are enriched in the subventricular zone of the ventricles and in the subgranular region of the dentate gyrus in the hippocampus Citation39. Methods for the in vitro propagation and differentiation of adult neural stem cells have been established Citation40. However, DA neurons have still not been derived from these cells and therefore they are currently not an option for transplantation into PD patients.
Another approach for a replacement therapy based on adult neural stem cells in PD is direct recruitment of endogenous neural stem cells from the proliferative regions to the site of degeneration Citation41. In this approach, the idea is that endogenous stem cells are stimulated to migrate and differentiate by signaling molecules produced by the injured brain Citation42 with no transplantation step. One recent study suggested that stem cells can differentiate into DA neurons in the adult brain and that a toxin‐induced lesion of the substantia nigra stimulates this response Citation42. However, the existence of newborn DA neurons in the adult substantia nigra has been challenged recently Citation43–45. Therefore, there is still no strong evidence that adult endogenous stem cells can differentiate into functional DA neurons in the adult brain and the validity of trying to repair the parkinsonian brain by this strategy has to be questioned Citation46.
Stem cells from tissues outside the central nervous system currently do not constitute efficient sources of DA neurons
Stem cells from non‐neural tissues have also been considered possible sources for autologous grafting Citation17, Citation47. Cells expressing neural markers have been derived under specific culture conditions from bone marrow and skin stem cells Citation48–53. Mesenchymal (stromal) stem cells from the adult bone marrow were reported to transdifferentiate into neuronal cells by several groups Citation49–51. One study even suggested that the bone marrow‐derived neurons were capable of expressing tyrosine hydroxylase (TH), the key enzyme in the biosynthesis of DA Citation49. However, there has been no further characterization of these cells showing that they are actually capable of synthesizing and releasing DA. The transdifferentiation of hematopoietic stem cells into neuronal cells has been even more controversial. A small fraction (0.3%–2.3%) of hematopoietic stem cells were reported to transdifferentiate into neurons when infused into neonatal or irradiated adult mice Citation52, Citation53. Cells expressing markers derived from the donor of hematopoietic stem cells exhibited neuronal phenotypes and seemed integrated into the host central nervous system. However, other studies reported that hematopoietic stem cells injected into the lateral ventricle of neonatal mice, only differentiated into microglial cells or astrocytes, but not into neurons Citation54. Extensive studies on cultured hematopoietic stem cells have shown that they do not readily differentiate into neurons under several tested conditions (Roybon et al., submitted) One possible explanation for the appearance of neurons expressing markers from the donor following systemic transplantation is cell fusion between hematopoietic stem cells and neurons in the host brain. Several studies have demonstrated that bone marrow cells can fuse with cerebellar Purkinje cells Citation55–60. However, so far Purkinje cells are the only type of neuron shown to exhibit cell fusion. In summary, the transdifferentiation of non‐neuronal stem cells into neurons is still controversial. It remains to be shown that multipotent stem cells isolated from embryonic or adult brains can efficiently generate DA neurons suitable for application in a cell‐based therapy in PD.
Development of hESCs for a cell‐based therapy in PD
Human ESCs (hESCs) purified from the inner cell mass of human blastocysts (5–6 days after fertilization) are truly pluripotent (i.e. are capable of generating any type of cell found in the body). Such cells could be employed as an infinite source of self‐renewing cells suitable for massive expansion and directed differentiation to DA neurons for transplantation to patients with PD. When compared with adult stem cells, hESCs are clearly easier to maintain, expand and differentiate. However, they raise controversial ethical issues due to their origin from human blastocysts Citation61, Citation62. Furthermore, unlike patient‐derived adult stem cells, grafted hESC‐derived cells are likely to be immunologically incompatible with the host and therefore cause problems of immune rejection. The current status on the development of hESCs for the treatment of PD, their future potential and the risks associated with their application are discussed below.
In vitro differentiation of hESCs into DA neurons
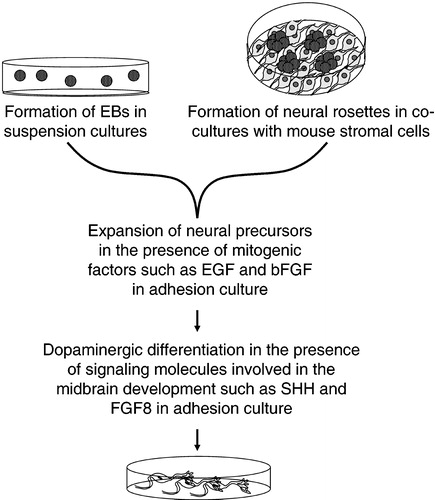
The in vitro systems that induce neuronal differentiation of ESCs rely on two major principles: the formation of embryoid bodies (EBs) Citation63–66 and the co‐culturing with a layer of feeder cells able to induce differentiation Citation67–69 (Figure ). In suspension cultures, ESCs spontaneously aggregate, differentiate and form spherical EBs containing precursor cells of all three germ layers (mesoderm, endoderm and neuroectoderm). Neural precursor cells can be expanded further by supplementation with mitogenic factors such as epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF) in the culture medium. It is then possible to achieve differentiation of cells into mature neurons by plating the EBs over the surface of specially coated culture dishes. Neuronal maturation is normally supported by the addition of signaling molecules known to be involved in the brain development. Alternatively, co‐culturing of hESCs with mouse stromal cell lines (such as the PA6 and MS5 lines) promotes their differentiation into neurons. The effects of so‐called stromal cell‐derived inducing activity (SDIA) upon ESCs were first described by Kawasaki and collaborators (2000). The SDIA involves unidentified signaling molecules present on or released by stromal feeder cells, which promote the differentiation of ESCs into neurons, and specifically generate a high proportion of DA neurons. After the initial discovery of the effects on mouse ESCs, SDIA has also been successfully applied to primate Citation70, Citation71 and human Citation68, Citation69, Citation72 ESCs. In co‐culture with mouse stromal cells, hESC colonies tend to form neuronal rosettes, which are round structures within the colonies, enriched in neuronal cells. These neuronal rosettes can then be isolated and differentiated in the presence of the specific soluble factors in adhesion or suspension cultures. The growth, differentiation and surviving‐promoting factors that have been found to be effective in this protocol are normally involved in the development and maintenance of the nervous system in vivo. The most well known factors involved in the mesencephalic DA system development are sonic hedgehog (SHH) and fibroblast growth factor (FGF)‐8. Overexpression of SHH and the addition of FGF‐8 into a co‐culture system with PA6 feeder cells leads to a substantial increase in the number of neurons expressing THCitation72. Transforming growth factor‐α (TGF‐α) is present in early embryonic structures where midbrain DA neurons develop and is believed to be essential for both induction and survival of DA neurons in vitro and in vivoCitation73. After culturing in the presence of TGF‐α for 21 days, about 15% of the hESC‐derived cells become TH‐positive and release DA Citation66. A more complex combination of factors was employed in the study performed by Perrier and co‐workers to induce the differentiation of the neuronal rosettes. The protocol included SHH, FGF‐8, brain‐derived neurotrophic factor (BDNF), glial cell line‐derived neurotrophic factor (GDNF), TGF‐β3, dibutyryl cyclic adenosine monophosphate (cAMP) and ascorbic acid. The sequential addition of combinations of these factors is believed to imitate the development of the midbrain in vivo and lead to the expression of transcription factors that are typical for midbrain neurons (such as Pax2, Aldh1, Lmx1b, En1, Nurr1 and Pitx3) in a sequence similar to that observed during normal development. After 50 days exposure to these different growth factors, more than 100 TH‐positive neurons could be generated from each undifferentiated hESC initially plated Citation68. A comparatively simpler system based on a cell suspension culture in serum‐free medium was also shown to induce the hESCs to differentiate into DA neurons Citation74. Suspension cultures in media conditioned on the human hepatocarcinoma cell line HepG2 led to the formation of cell aggregates after 2 weeks, which were then cultured on coated dishes for 5–7 days. In these adhesion cultures, 75% of the neurons were reported to be TH‐positive. These neurons exhibited action potentials, expressed markers for mesencephalic DA neurons (including Girk2) and were able to release DA Citation74.
Figure 2 General basic steps of the methods developed so far forin vitro differentiation of hESCs into dopaminergic neurons. EBs, embryoid bodies; EGF, epidermal growth factor; bFGF, basic fibroblast growth factor; FGF‐8, fibroblast growth factor‐8; SHH, sonic hedgehog.
Despite the relative success in generating DA neurons from hESCs in vitro, the existing methods have several drawbacks. All protocols published so far involve xeno‐derived feeder cells, serum and/or recombinant growth factors, which might transmit animal infectious agents to the human cells (see further discussion in ‘Ethical considerations and safety issues in hESC‐based therapies’ section below). Another limitation of these methods is the high costs associated with the culturing media and additives used.
Mouse and primate ESC‐derived DA neurons can survive and improve motor function in animal models of PD Citation70, Citation71, Citation75, Citation76. This is, so far, not the case for ESCs derived from humans. Our own and other studies have demonstrated that hESC‐derived DA neurons poorly survive implantation in the rat model of PD Citation69, Citation72, Citation77 (Bredelau and Correia et al., submitted). Improving the survival of hESC‐derived DA neurons is a major challenge that has to be overcome before clinical application of hESC in PD is possible. It has been suggested that oxidative stress, lack of trophic support and axotomy during dissection and mechanical dissociation into a cell suspension are responsible for poor survival of grafted brain tissue Citation78. Treatment with, for example, antioxidants and trophic factors, which are proven as being beneficial to grafts of primary mesencephalic tissue, could be tested in order to increase the survival of grafted hESC‐derived DA neurons.
In addition to the use of different soluble factors, genetic modification of hESCs can also be employed to induce their differentiation into DA neurons. Nuclear receptor related‐1 (Nurr1) is a transcription factor known to be involved in differentiation of midbrain precursors into DA neurons. Overexpression of Nurr1 in mouse ESCs results in a substantial increase in the proportion of neurons expressing TH, especially when the cells are treated with FGF‐8 and SHH Citation75. These neurons exhibit electrophysiological properties typical of midbrain neurons and can reverse motor deficits after being grafted into immunosuppressed rats Citation75. Another method to increase the fraction of TH‐expressing neurons would be to selectively isolate the cells expressing markers for DA neurons from the other cells in the cultures. This can be achieved by modifying the hESCs with a genetic construct containing an antibiotic resistance gene or a reporter gene (such as enhanced green fluorescence protein – eGFP) under the control of a lineage‐restricted promoter Citation79. A nearly homogeneous population of neurons was obtained from mouse ESCs by placing a neomyocin resistance gene in the Sox2 gene locus (Sox1 and 2 are expressed in the early neuroepithelium Citation80). After inducing differentiation, the antibiotic was added, resulting in the selection of neuroepithelial progenitor cells expressing Sox2. Aubert and coworkers (2003) showed that neuroepithelial cells can also be isolated by fluorescence‐activated cell sorting (FACS) from transgenic mouse embryos in which eGFP is under the control of Sox1 promoter Citation81. Genetic manipulations to label the desired cell population prior to a cell sorting step is promising from the scientific point of view. In a clinical perspective, however, it raises major safety issues. Due to the difficulty in controlling the incorporation of the transgene into the genome of a host cell, unexpected activation or inactivation of certain genes may occur. Unpredictable changes in the gene expression may therefore lead to cells with high proliferative potential, capable of forming tumors following transplantation to the patient.
Balancing the need for immature neurons against the risk of teratoma formation
Teratomas are an overgrowth of cells that differentiate into cells from all of the three germ layers (mesoderm, endoderm and ectoderm). Transplantation of undifferentiated mouse ESCs into the striatum leads to the formation of teratomas in a high proportion of animals (5 out of 25) Citation82. When mouse, primate and human ESCs differentiate to a sufficient extent in vitro before transplantation, however, there is no teratoma formation following implantation into the brain Citation70–72, Citation74–76, Citation83–85. Therefore, a certain degree of differentiation in vitro is essential prior to grafting in order to avoid teratomas. On the other hand, if stem cells are differentiated into mature neurons with long and elaborate processes already in vitro, the survival rate after implantation into the brain is likely to be low. Thus, it is important to identify a time window in the differentiation protocol that generates immature DA neurons that can survive the transplantation procedure and does not give rise to teratomas. Using a two‐step protocol for neuronal differentiation of hESCs, Ben‐Hur et al. (2004) generated cultures in which more than 90% of the cells were neuronal progenitors. When transplanted into the striatum of rats, these cells did not generate teratomas and about 0.2% of them expressed TH. In some graft recipients, up to 390 TH‐positive grafted cells were found. The authors suggest that these cells have supported partial recovery of drug‐induced motor function in the hemiparkinsonian rats Citation77.
Another approach to reduce the risk of teratoma formation is to actively eliminate pluripotent stem cells prior to the graft surgery. Bieberich et al. (2004) showed that undifferentiated ESCs could be eliminated from cultures of ESC‐derived neuronal cells by treatment with ceramide analogue N‐oleoyl serinol (S18) Citation86. Increased levels of ceramide are part of an important mechanism that promotes programmed cell death during the development of the mouse brain. Addition of ceramide analogues to mouse ESC cultures eliminates the undifferentiated cells, but leaves alive those that have differentiated into, for example, neural progenitors Citation87. Subsequent transplantation of these cultures results in graft survival without teratoma formation Citation86. In another study concerning teratoma formation, the levels of a growth factor from the EGF‐CFC family called Cripto were manipulated. Cripto is overexpressed in a wide range of epithelial cancers, it is also relevant to early development Citation88 and its repression enhances neuronal differentiation Citation89. The investigators generated ESCs from a Cripto knock‐out mouse (Cripto−/− ESCs) Citation89. When Cripto−/− ESCs were grown in the presence of SHH and FGF‐8, they generated more TH‐positive neurons than did wild‐type mouse ESCs. After grafting to immunosuppressed hemiparkinsonian rats, the Cripto−/− ESC‐derived grafts contained large numbers of TH‐positive neurons without any teratomas. In contrast, all the recipients receiving implants from wild‐type mouse ESCs (Cripto+/+) developed teratomas within 7 weeks Citation89. In summary, both the treatment with ceramide analogue and the suppression of Cripto expression appear to be interesting approaches to inhibiting of teratoma formation that could have implications for clinical trials.
Transplantation technique relevant to hESC‐derived DA neurons
As mentioned earlier, most hESC differentiation protocols involve growing the cells as monolayers or as small spheres (EBs). The disadvantage of growing hESCs as monolayers is the low cell viability after harvesting cells from the culture dish. The process of harvesting ESC‐derived neural progenitors from the dish causes trauma, which can kill them, in particular if they have long processes. Early studies on cultured rat DA primary neurons demonstrated that the survival of the neurons was reduced when they had been grown in vitro for prolonged (7 days) compared to short (2 days) periods Citation90. In the same way, the extent of development of primary DA neurons harvested from rodent and primate embryonic brains is a crucial factor determining whether the DA neurons can survive grafting. There is a donor upper age limit coinciding with the time when the immature DA neurons begin to extend the first axons. If ventral mesencephalic tissue is harvested from donors that have passed this developmental stage and is prepared into a cell suspension used for transplantation, there is little or no survival of the DA neurons. Similar rules are likely to apply concerning the survival of DA neurons derived from ESCs. That means that if they undergo full maturation in the culture dish they will die after grafting. However, because of the risk of teratoma formation from poorly differentiated hESC‐derived cells, a difficult paradox arises: hESCs need prolonged culturing to be relatively well‐differentiated before grafting, but this may dramatically reduce survival upon transplantation. One solution to the problem of cell loss during harvesting from monolayer cultures could be the development of protocols yielding hESCs‐derived DA neurons in small aggregates that can be grafted without any mechanical disruption. Aggregates of cultured DA neurons from mesencephalic tissue have already been successfully transplanted in animal studies Citation91 proving the validity of this approach. Aggregate size could be tailored so that it allows an effective transfer of nutrients and metabolites in culture and is suitably small permitting transfer directly into the transplantation instrument Citation92.
Immunological aspects of transplantation of hESC‐derived cells
Grafts of hESC‐derived cells in a clinical setting will always be allogenic, i.e. genetically dissimilar although within the same species, and consequently immunologically incompatible with the host. Problems with immune rejection of hESC‐derived grafts can be avoided if they are generated by the nuclear transfer technique using a nucleus from a cell derived from the patient – a procedure known as therapeutic nuclear transfer Citation93. Whilst therapeutic nuclear transfer could solve obstacles related to immune rejection, it comes with its own set of problems Citation94. First, it is still technically difficult to perform, and the success rate is relatively low. Second, it is not certain that the generated cells will be perfectly normal due to changes in the nucleus related to aging of the donor. Third, in cases when the patient suffers from a genetic disorder, which includes some rare forms of PD Citation2, it is not suitable to transfer the mutant DNA into the cells that are going to be transplanted. Fourth, therapeutic nuclear transfer raises specific ethical issues, especially since the technique has many features in common with methodology which could be used to clone whole human beings, an activity which is considered unethical by most cultures (see press release from the United Nations, dated August 2005, http://www.un.org/News/Press/docs/2005/ga10333.doc.htm). As used in the practice of transplantation over past three decades, an immunosuppression treatment will be needed after implantation of hESC‐derived DA neurons. Different immunosuppression protocols have been used in clinical trials employing allografted neural tissue in PD Citation7, Citation17, Citation26, Citation27, Citation95, Citation96. Immunosuppression regimen with cyclosporine A, methylprednisolone and azathioprine has been applied and some patients have been maintained on immunosuppression for several years. It has been speculated that some grafted patients who received a low dose of cyclosporine A for only six months have exhibited transient effects of the grafts that might have disappeared due to immunological rejection Citation26, Citation31. Clearly issues related to immune rejection must be carefully considered in any future stem cell based transplantation protocol for PD.
Ethical considerations and safety issues in hESC‐based therapies
Research on hESCs raises a number of ethical issues. Human blastocysts used to establish ESC lines are normally obtained as a side‐product of in vitro fertilization (IVF), which is a routine method for infertility treatment. Because the success rate of in vitro fertilization is far below 100%, a number of fertilized ‘reserved’ eggs are obtained from each couple seeking treatment and these supernumerary blastocysts, following informed consent, could be used to generate hESCs.
An important regulation restricting research on hESCs is the decision of the US President (made on 9 August 2001) to allow federal funding only for studies using already existing hESC lines that satisfy certain criteria (see eligibility criteria at http://grants.nih.gov/grants/guide/notice‐files/NOT‐OD‐02‐006.html). In Europe, there is still no consensus regarding the ethics of deriving new hESC lines, which is probably due to different ethical, philosophical and religious traditions that exist between the EU members (it is possible to find the different positions on the hESC research taken by each EU member by August 2003 at International Society for Stem Cell Research (ISSCR) website: http://www.isscr.org/scientists/legislative.htm). The hESCs karyotype is not always stable and, for example, a gain of an extra chromosome 17q has been observed in more than one hESC line Citation97. Furthermore, cell lines have been shown to vary in terms of proliferation rate and their ability to maintain undifferentiated phenotype. With this in mind, it seems unrealistic that a long‐term cell transplantation therapeutic strategy for PD could be based on a restricted number of once established hESC lines. More likely, it will be necessary to generate new cell lines at regular intervals.
Other important concerns are related to the safety of hESC‐based clinical transplantation therapies. Cells can be contaminated with microbial infections, genetic disorders from the donor, or with non‐human cell material. The transmission of undetected animal infectious agents could cause diseases (xenozoonoses) that, theoretically, could be transmitted from transplanted patients to the general public. Therefore, several recent studies describe protocols with hESC lines grown under xeno‐free conditions. These xeno‐free systems include serum replacement medium and human fibroblasts as supporting cells Citation98–100. In contrast, most culture conditions used previously include non‐human components, such as fetal calf serum, mouse cells and media supplemented by growth factors/cytokines not approved for human use Citation64–69, Citation97, Citation101, Citation102. The US regulations require that the same standards used in blood banking and organ transplantation are followed in stem cell research.
Concluding remarks
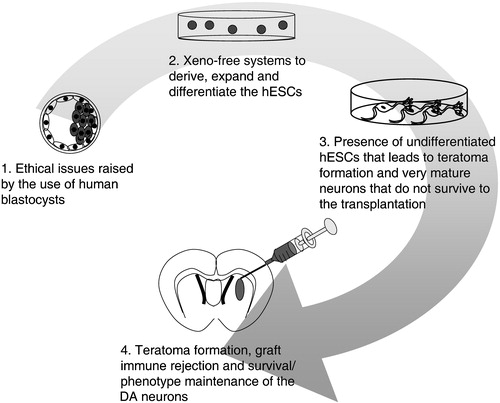
We have briefly described results obtained with clinical brain tissue transplants in PD and then considered different future sources of DA neurons for a cell‐based therapy, namely regionally specified stem cells from adults or embryos and hESCs derived from the inner cell mass of human blastocysts. We currently consider hESCs cells as the most promising option. The ability of hESCs to proliferate in vitro while maintaining their pluripotency is a great advantage. The hESCs also have a well‐documented ability to differentiate into DA neurons when cultured in vitro. However, only few hESC‐derived DA neurons survive or maintain their differentiated phenotype after implantation. As a consequence, it has not yet been possible to obtain functional grafts derived from hESCs in PD models. In addition to this fundamental problem, there are several other remaining issues that need to be solved before a hESC‐based therapy can be developed for PD (Figure ). Currently, most protocols developed to derive, maintain and differentiate the hESCs rely on non‐human components, limiting actual clinical application of the yielded cells. Moreover, the risks of teratoma formation by residual undifferentiated cells and immune rejection also question the safety of any hESC‐based therapy for PD. The problem of ‘off‐medication’‐dyskinesia seen in some of the patients implanted with DA neurons from the embryonic ventral mesencephalon needs to be understood and circumvented with hESC‐derived grafts. Future studies are also needed to address details in the phenotype of the hESC‐derived DA neurons. Probably, the neurons need to have characteristics specific to the substantia nigra pars compacta. A recent study has demonstrated that other DA neurons (e.g. those cells found in the VTA) will not provide appropriate innervation of the denervated striatum in PD Citation20. Further scientific research should aim at simplifying and improving DA neuronal differentiation and transplantation protocols so they reproducibly generate substantia nigra‐like DA neurons. Experiments are also needed to address the important safety issues mentioned above. Clearly, progress will require joint efforts from the international stem cell research community. Only then can we count on beneficial and safe treatment of PD with cell transplantation.
Figure 3 Present limitations in the development of the hESC‐based therapy for PD. Limitations are found: 1) in the derivation of new hESCs lines, 2) in the culture of these cells, 3), in the derivation of the appropriate cells for successful and safe transplantation, and 4) in the survival and integration of the grafted cells.
Acknowledgements
We acknowledge the support from the Unites States Army Medical Research Acquisition Activity (USA MRAA, Award No. W81XWH‐04‐1‐0366); National Institute of Health (Grant Number 1 R21 NS043717‐01A1) and Research Foundation of the Swedish Parkinsons Disease Association. S.V.A. is supported by Marie Curie Incoming Fellowship (MIF1‐CT‐2005‐008445). A.S.C. is supported by Fundação para a Ciência e a Tecnologia from the Portuguese government (Reference Number SFRH/BD/11804/2003).
References
- Braak H., Ghebremedhin E., Rub U., Bratzke H., Del Tredici K. Stages in the development of Parkinson's disease‐related pathology. Cell Tissue Res 2004; 318: 121–34
- Samii A., Nutt J. G., Ransom B. R. Parkinson's disease. Lancet 2004; 363: 1783–93
- Olanow C. W. The scientific basis for the current treatment of Parkinson's disease. Annu Rev Med 2004; 55: 41–60
- Brotchie J. M., Lee J., Venderova K. Levodopa‐induced dyskinesia in Parkinson's disease. J Neural Transm 2005; 112: 359–91
- Lang A. E., Obeso J. A. Challenges in Parkinson's disease: restoration of the nigrostriatal dopamine system is not enough. Lancet Neurol 2004; 3: 309–16
- Lindvall O., Hagell P. Cell therapy and transplantation in Parkinson's disease. Clin Chem Lab Med 2001; 39: 356–61
- Hagell P., Brundin P. Cell survival and clinical outcome following intrastriatal transplantation in Parkinson disease. J Neuropathol Exp Neurol 2001; 60: 741–52
- Olanow C. W., Kordower J. H., Freeman T. B. Fetal nigral transplantation as a therapy for Parkinson's disease. Trends Neurosci 1996; 19: 102–9
- Clarkson E. D., Freed C. R. Development of fetal neural transplantation as a treatment for Parkinson's disease. Life Sci 1999; 65: 2427–37
- Itakura T., Uematsu Y., Nakao N., Nakai E., Nakai K. Transplantation of autologous sympathetic ganglion into the brain with Parkinson's disease. Long‐term follow‐up of 35 cases. Stereotact Funct Neurosurg 1997; 69((Pt 2))112–5
- Nakao N., Shintani‐Mizushima A., Kakishita K., Itakura T. The ability of grafted human sympathetic neurons to synthesize and store dopamine: a potential mechanism for the clinical effect of sympathetic neuron autografts in patients with Parkinson's disease. Exp Neurol 2004; 188: 65–73
- Arjona V., Minguez‐Castellanos A., Montoro R. J., Ortega A., Escamilla F., Toledo‐Aral J. J., et al. Autotransplantation of human carotid body cell aggregates for treatment of Parkinson's disease. Neurosurgery 2003; 53: 321–8; discussion 328–30
- Deacon T., Schumacher J., Dinsmore J., Thomas C., Palmer P., Kott S., et al. Histological evidence of fetal pig neural cell survival after transplantation into a patient with Parkinson's disease. Nat Med 1997; 3: 350–3
- Schumacher J. M., Ellias S. A., Palmer E. P., Kott H. S., Dinsmore J., Dempsey P. K., et al. Transplantation of embryonic porcine mesencephalic tissue in patients with PD. Neurology 2000; 54: 1042–50
- Weiss R. A. Xenografts and retroviruses. Science 1999; 285: 1221–2
- Lindvall O., Kokaia Z., Martinez‐Serrano A. Stem cell therapy for human neurodegenerative disorders‐how to make it work. Nat Med 2004; 10(Suppl)S42–50
- Bjorklund A., Dunnett S. B., Brundin P., Stoessl A. J., Freed C. R., Breeze R. E., et al. Neural transplantation for the treatment of Parkinson's disease. Lancet Neurol 2003; 2: 437–45
- Arenas E. Stem cells in the treatment of Parkinson's disease. Brain Res Bull 2002; 57: 795–808
- Mendez I., Sanchez‐Pernaute R., Cooper O., Vinuela A., Ferrari D., Bjorklund L., et al. Cell type analysis of functional fetal dopamine cell suspension transplants in the striatum and substantia nigra of patients with Parkinson's disease. Brain 2005; 128((Pt 7))1498–510
- Thompson L., Barraud P., Andersson E., Kirik D., Bjorklund A. Identification of dopaminergic neurons of nigral and ventral tegmental area subtypes in grafts of fetal ventral mesencephalon based on cell morphology, protein expression, and efferent projections. J Neurosci 2005; 25: 6467–77
- Brundin P., Nilsson O. G., Strecker R. E., Lindvall O., Astedt B., Bjorklund A. Behavioural effects of human fetal dopamine neurons grafted in a rat model of Parkinson's disease. Exp Brain Res 1986; 65: 235–40
- Elsworth J. D., Brittan M. S., Taylor J. R., Sladek J. R Jr., al‐Tikriti M. S., Zea‐Ponce Y., et al. Restoration of dopamine transporter density in the striatum of fetal ventral mesencephalon‐grafted, but not sham‐grafted, MPTP‐treated parkinsonian monkeys. Cell Transplant 1996; 5: 315–25
- Polgar S., Morris M. E., Reilly S., Bilney B., Sanberg P. R. Reconstructive neurosurgery for Parkinson's disease: a systematic review and preliminary meta‐analysis. Brain Res Bull 2003; 60: 1–24
- Lindvall O., Hagell P. Clinical observations after neural transplantation in Parkinson's disease. Prog Brain Res 2000; 127: 299–320
- Piccini P., Brooks D. J., Bjorklund A., Gunn R. N., Grasby P. M., Rimoldi O., et al. Dopamine release from nigral transplants visualized in vivo in a Parkinson's patient. Nat Neurosci 1999; 2: 1137–40
- Olanow C. W., Goetz C. G., Kordower J. H., Stoessl A. J., Sossi V., Brin M. F., et al. A double‐blind controlled trial of bilateral fetal nigral transplantation in Parkinson's disease. Ann Neurol 2003; 54: 403–14
- Freed C. R., Greene P. E., Breeze R. E., Tsai W. Y., DuMouchel W., Kao R., et al. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N Engl J Med 2001; 344: 710–9
- Hagell P., Piccini P., Bjorklund A., Brundin P., Rehncrona S., Widner H., et al. Dyskinesias following neural transplantation in Parkinson's disease. Nat Neurosci 2002; 5: 627–8
- Kordower J. H., Freeman T. B., Snow B. J., Vingerhoets F. J., Mufson E. J., Sanberg P. R., et al. Neuropathological evifdence of graft survival and striatal reinnervation after the transplantation of fetal mesencephalic tissue in a patient with Parkinson's disease. N Engl J Med 1995; 332: 1118–24
- Lindvall O., Bjorklund A. Cell therapy in Parkinson's disease. NeuroRx 2004; 1: 382–93
- Winkler C., Kirik D., Bjorklund A. Cell transplantation in Parkinson's disease: how can we make it work?. Trends Neurosci 2005; 28: 86–92
- Olanow C. W., Fahn S. Fetal nigral transplantation as a therapy for Parkinson's disease. Restorative therapies in Parkinson's disease, C. W Olanow, P Brundin. Springer Science, Business Media B.V, New York, NYUSA 2005, In press
- Ma Y., Feigin A., Dhawan V., Fukuda M., Shi Q., Greene P., et al. Dyskinesia after fetal cell transplantation for parkinsonism: a PET study. Ann Neurol 2002; 52: 628–34
- Isacson O., Bjorklund L. M., Schumacher J. M. Toward full restoration of synaptic and terminal function of the dopaminergic system in Parkinson's disease by stem cells. Ann Neurol 2003; 53(Suppl 3)S135–46; discussion S146–8
- Cenci M. A., Hagell P. Dyskinesias and neural grafting in Parkinson's disease. Restorative therapies in Parkinson's disease, C. W Olanow, P Brundin. Springer Science, Business Media B.V, New York, NYUSA 2005, In press
- Boer G. J. Ethical issues in neurografting of human embryonic cells. Theor Med Bioeth 1999; 20: 461–75
- Studer L., Tabar V., McKay R. D. Transplantation of expanded mesencephalic precursors leads to recovery in bparkinsonian rats. Nat Neurosci 1998; 1: 290–5
- Martinez‐Serrano A., Rubio F. J., Navarro B., Bueno C., Villa A. Human neural stem and progenitor cells: in vitro and in vivo properties, and potential for gene therapy and cell replacement in the CNS. Curr Gene Ther 2001; 1: 279–99
- Frisen J., Johansson C. B., Lothian C., Lendahl U. Central nervous system stem cells in the embryo and adult. Cell Mol Life Sci 1998; 54: 935–45
- Westerlund U., Moe M. C., Varghese M., Berg‐Johnsen J., Ohlsson M., Langmoen I. A., et al. Stem cells from the adult human brain develop into functional neurons in culture. Exp Cell Res 2003; 289: 378–83
- Lie D. C., Song H., Colamarino S. A., Ming G. L., Gage F. H. Neurogenesis in the adult brain: new strategies for central nervous system diseases. Annu Rev Pharmacol Toxicol 2004; 44: 399–421
- Zhao M., Momma S., Delfani K., Carlen M., Cassidy R. M., Johansson V. B., et al. Evidence for neurogenesis in the adultmammalian substantia nigra. Proc Natl Acad Sci U S A 2003; 100: 7925–30
- Lie D. C., Dziewczapolski G., Willhoite A. R., Kaspar B. K., Shults C. W., Gage F. H. The adult substantia nigra contains progenitor cells with neurogenic potential. J Neurosci 2002; 22: 6639–49
- Cooper, Isacson O. Intrastriatal transforming growth factor alpha delivery to a model of Parkinson's disease induces proliferation and migration of endogenous adult neural progenitor cells without differentiation into dopaminergic neurons. J Neurosci 2004; 24: 8924–31
- Frielingsdorf H., Schwarz K., Brundin P., Mohapel P. No evidence for new dopaminergic neurons in the adult mammalian substantia nigra. Proc Natl Acad Sci U S A 2004; 101: 10177–82
- Mohapel P., Brundin P. Harnessing endogenous stem cells to treat neurodegenerative disorders of the basal ganglia. Parkinsonism Relat Disord 2004; 10: 259–64
- Roybon L., Christophersen N. S., Brundin P., Li J. Y. Stem cell therapy for Parkinson's disease: where do we stand?. Cell Tissue Res 2004; 318: 261–73
- Toma J. G., Akhavan M., Fernandes K. J., Barnabe‐Heider F., Sadikot A., Kaplan D. R., et al. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol 2001; 3: 778–84
- Jiang Y., Jahagirdar B. N., Reinhardt R. L., Schwartz R. E., Keene C. D., Ortiz‐Gonzalez X. R., et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 2002; 418: 41–9
- Woodbury D., Schwarz E. J., Prockop D. J., Black I. B. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res 2000; 61: 364–70
- Deng W., Obrocka M., Fischer I., Prockop D. J. In vitro differentiation of human marrow stromal cells into early progenitors of neural cells by conditions that increase intracellular cyclic AMP. Biochem Biophys Res Commun 2001; 282: 148–52
- Brazelton T. R., Rossi F. M., Keshet G. I., Blau H. M. From marrow to brain: expression of neuronal phenotypes in adult mice. Science 2000; 290: 1775–9
- Mezey E., Chandross K. J., Harta G., Maki R. A., McKercher S. R. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science 2000; 290: 1779–82
- Vitry S., Bertrand J. Y., Cumano A., Dubois‐Dalcq M. Primordial hematopoietic stem cells generate microglia but not myelin‐forming cells in a neural environment. J Neurosci 2003; 23: 10724–31
- Ying Q. L., Nichols J., Evans E. P., Smith A. G. Changing potency by spontaneous fusion. Nature 2002; 416: 545–8
- Weimann J. M., Charlton C. A., Brazelton T. R., Hackman R. C., Blau H. M. Contribution of transplanted bone marrow cells to Purkinje neurons in human adult brains. Proc Natl Acad Sci U S A 2003; 100: 2088–93
- Wang X., Willenbring H., Akkari Y., Torimaru Y., Foster M., Al‐Dhalimy M., et al. Cell fusion is the principal source of bone‐marrow‐derived hepatocytes. Nature 2003; 422: 897–901
- Terada N., Hamazaki T., Oka M., Hoki M., Mastalerz D. M., Nakano Y., et al. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature 2002; 416: 542–5
- Priller J., Persons D. A., Klett F. F., Kempermann G., Kreutzberg G. W., Dirnagl U. Neogenesis of cerebellar Purkinje neurons from gene‐marked bone marrow cells in vivo. J Cell Biol 2001; 155: 733–8
- Alvarez‐Dolado M., Pardal R., Garcia‐Verdugo J. M., Fike J. R., Lee H. O., Pfeffer K., et al. Fusion of bone‐marrow‐derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature 2003; 425: 968–73
- Henningson C. T Jr., Stanislaus M. A., Gewirtz A. M. 28. Embryonic and adult stem cell therapy. J Allergy Clin Immunol 2003; 111((2 Suppl))S745–53
- Henon P. R. Human embryonic or adult stem cells: an overview on ethics and perspectives for tissue engineering. Adv Exp Med Biol 2003; 534: 27–45
- Zhang S. C., Wernig M., Duncan I. D., Brustle O., Thomson J. A. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol 2001; 19: 1129–33
- Carpenter M. K., Inokuma M. S., Denham J., Mujtaba T., Chiu C. P., Rao M. S. Enrichment of neurons and neural precursors from human embryonic stem cells. Exp Neurol 2001; 172: 383–97
- Schulz T. C., Palmarini G. M., Noggle S. A., Weiler D. A., Mitalipova M. M., Condie B. G. Directed neuronal differentiation of human embryonic stem cells. BMC Neurosci 2003; 4: 27
- Park S., Lee K. S., Lee Y. J., Shin H. A., Cho H. Y., Wang K. C., et al. Generation of dopaminergic neurons in vitro from human embryonic stem cells treated with neurotrophic factors. Neurosci Lett 2004; 359: 99–103
- Reubinoff B. E., Itsykson P., Turetsky T., Pera M. F., Reinhartz E., Itzik A., et al. Neural progenitors from human embryonic stem cells. Nat Biotechnol 2001; 19: 1134–40
- Perrier A. L., Tabar V., Barberi T., Rubio M. E., Bruses J., Topf N., et al. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci U S A 2004; 101: 12543–8
- Zeng X., Cai J., Chen J., Luo Y., You Z. B., Fotter E., et al. Dopaminergic differentiation of human embryonic stem cells. Stem Cells 2004; 22: 925–40
- Kawasaki H., Suemori H., Mizuseki K., Watanabe K., Urano F., Ichinose H., et al. Generation of dopaminergic neurons and pigmented epithelia from primate ES cells by stromal cell‐derived inducing activity. Proc Natl Acad Sci U S A 2002; 99: 1580–5
- Takagi Y., Takahashi J., Saiki H., Morizane A., Hayashi T., Kishi Y., et al. Dopaminergic neurons generated from monkey embryonic stem cells function in a Parkinson primate model. J Clin Invest 2005; 115: 102–9
- Park C. H., Minn Y. K., Lee J. Y., Choi D. H., Chang M. Y., Shim J. W., et al. In vitro and in vivo analyses of human embryonic stem cell‐derived dopamine neurons. J Neurochem 2005; 92: 1265–76
- Farkas L. M., Dunker N., Roussa E., Unsicker K., Krieglstein K. Transforming growth factor‐beta(s) are essential for the development of midbrain dopaminergic neurons in vitro and in vivo. J Neurosci 2003; 23: 5178–86
- Schulz T. C., Noggle S. A., Palmarini G. M., Weiler D. A., Lyons I. G., Pensa K. A., et al. Differentiation of human embryonic stem cells to dopaminergic neurons in serum‐free suspension culture. Stem Cells 2004; 22: 1218–38
- Kim J. H., Auerbach J. M., Rodriguez‐Gomez J. A., Velasco I., Gavin D., Lumelsky N., et al. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson's disease. Nature 2002; 418: 50–6
- Barberi T., Klivenyi P., Calingasan N. Y., Lee H., Kawamata H., Loonam K., et al. Neural subtype specification of fertilization and nuclear transfer embryonic stem cells and application in parkinsonian mice. Nat Biotechnol 2003; 21: 1200–7
- Ben‐Hur T., Idelson M., Khaner H., Pera M., Reinhartz E., Itzik A., et al. Transplantation of human embryonic stem cell‐derived neural progenitors improves behavioral deficit in Parkinsonian rats. Stem Cells 2004; 22: 1246–55
- Brundin P., Karlsson J., Emgard M., Schierle G. S., Hansson O., Petersen A., et al. Improving the survival of grafted dopaminergic neurons: a review over current approaches. Cell Transplant 2000; 9: 179–95
- O'Shea K. S. Directed differentiation of embryonic stem cells: genetic and epigenetic methods. Wound Repair Regen 2001; 9: 443–59
- Li M., Pevny L., Lovell‐Badge R., Smith A. Generation of purified neural precursors from embryonic stem cells by lineage selection. Curr Biol 1998; 8: 971–4
- Aubert J., Stavridis M. P., Tweedie S., O'Reilly M., Vierlinger K., Li M., et al. Screening for mammalian neural genes via fluorescence‐activated cell sorter purification of neural precursors from Sox1‐gfp knock‐in mice. Proc Natl Acad Sci U S A 2003; 100(Suppl 1)11836–41
- Bjorklund L. M., Sanchez‐Pernaute R., Chung S., Andersson T., Chen I. Y., McNaught K. S., et al. Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson rat model. Proc Natl Acad Sci U S A 2002; 99: 2344–9
- Morizane A., Takahashi J., Takagi Y., Sasai Y., Hashimoto N. Optimal conditions for in vivo induction of dopaminergic neurons from embryonic stem cells through stromal cell‐derived inducing activity. J Neurosci Res 2002; 69: 934–9
- Kawasaki H., Mizuseki K., Nishikawa S., Kaneko S., Kuwana Y., Nakanishi S., et al. Induction of midbrain dopaminergic neurons from ES cells by stromal cell‐derived inducing activity. Neuron 2000; 28: 31–40
- Tabar V., Panagiotakos G., Greenberg E. D., Chan B. K., Sadelain M., Gutin P. H., et al. Migration and differentiation of neural precursors derived from human embryonic stem cells in the rat brain. Nat Biotechnol 2005; 23: 601–6
- Bieberich E., Silva J., Wang G., Krishnamurthy K., Condie B. G. Selective apoptosis of pluripotent mouse and human stem cells by novel ceramide analogues prevents teratoma formation and enriches for neural precursors in ES cell‐derived neural transplants. J Cell Biol 2004; 167: 723–34
- Bieberich E., MacKinnon S., Silva J., Noggle S., Condie B. G. Regulation of cell death in mitotic neural progenitor cells by asymmetric distribution of prostate apoptosis response 4 (PAR‐4) and simultaneous elevation of endogenous ceramide. J Cell Biol 2003; 162: 469–79
- Anisimov S. V., Tarasov K. V., Riordon D., Wobus A. M., Boheler K. R. SAGE identification of differentiation responsive genes in P19 embryonic cells induced to form cardiomyocytes in vitro. Mech Dev 2002; 117: 25–74
- Parish C. L., Parisi S., Persico M. G., Arenas E., Minchiotti G. Cripto as a target for improving embryonic stem cell‐based therapy in Parkinson's disease. Stem Cells 2005; 23: 471–6
- Brundin P., Strecker R. E., Widner H. E., Clarke D. J., Nilsson O. G., Astedt B., et al. Human fetal dopamine neurons grafted in a rat model of Parkinson's disease: immunological aspects, spontaneous and drug‐induced behaviour, and dopamine release. Exp Brain Res 1988; 70: 192–208
- Spector D. H., Boss B. D., Strecker R. E. A model three‐dimensional culture system for mammalian dopaminergic precursor cells: application for functional intracerebral transplantation. Exp Neurol 1993; 124: 253–64
- Freeman T. B., Brundin P. Important aspects of surgical methodology for transplantation in Parkinson's disease. Restorative therapies in Parkinson's disease, C. W Olanow, P Brundin. Springer Science, Business Media B.V, New York, NYUSA 2005, In press
- Hwang W. S., Ryu Y. J., Park J. H., Park E. S., Lee E. G., Koo J. M., et al. Evidence of a Pluripotent Human Embryonic Stem Cell Line Derived from a Cloned Blastocyst. Science 2004; 303: 1669–74
- Hochedlinger K., Rideout W. M., Kyba M., Daley G. Q., Blelloch R., Jaenisch R. Nuclear transplantation, embryonic stem cells and the potential for cell therapy. Hematol J 2004; 5(Suppl 3)S114–7
- Hauser R. A., Freeman T. B., Snow B. J., Nauert M., Gauger L., Kordower J. H., et al. Long‐term evaluation of bilateral fetal nigral transplantation in Parkinson disease. Arch Neurol 1999; 56: 179–87
- Lindvall O., Rehncrona S., Brundin P., Gustavii B., Astedt B., Widner H., et al. Neural transplantation in Parkinson's disease: the Swedish experience. Prog Brain Res 1990; 82: 729–34
- Draper J. S., Smith K., Gokhale P., Moore H. D., Maltby E., Johnson J., et al. Recurrent gain of chromosomes 17q and 12 in cultured human embryonic stem cells. Nat Biotechnol 2004; 22: 53–4
- Inzunza J., Gertow K., Stromberg M. A., Matilainen E., Blennow E., Skottman H., et al. Derivation of human embryonic stem cell lines in serum replacement medium using postnatal human fibroblasts as feeder cells. Stem Cells 2005; 23: 544–9
- Amit M., Shariki C., Margulets V., Itskovitz‐Eldor J. Feeder layer‐ and serum‐free culture of human embryonic stem cells. Biol Reprod 2004; 70: 837–45
- Amit M., Margulets V., Segev H., Shariki K., Laevsky I., Coleman R., et al. Human feeder layers for human embryonic stem cells. Biol Reprod 2003; 68: 2150–6
- Reubinoff B. E., Pera M. F., Fong C. Y., Trounson A., Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol 2000; 18: 399–404
- Thomson J. A., Itskovitz‐Eldor J., Shapiro S. S., Waknitz M. A., Swiergiel J. J., Marshall V. S., et al. Embryonic stem cell lines derived from human blastocysts. Science 1998; 282: 1145–7