Abstract
The pathogenesis of bipolar disorder may involve, at least in part, aberrations in serotonergic neurotransmission. Hence, serotonergic genes are attractive targets for association studies of bipolar disorder. We have reviewed the literature in this field. It is difficult to synthesize results as only one polymorphism per gene was typically investigated in relatively small samples. Nevertheless, suggestive associations are available for the 5HT2A receptor and the serotonin transporter genes. With the availability of extensive polymorphism data and high throughput genotyping techniques, comprehensive evaluation of these genes using adequately powered samples is warranted. We also report on our investigations of the serotonin transporter, SLC6A4 (17q11.1‐q12). An insertion/deletion polymorphism (5HTTLPR) in the promoter region of this gene has been investigated intensively. However, the results have been inconsistent. We reasoned that other polymorphism/s may contribute to the associations and the inconsistencies may be due to variations in linkage disequilibrium (LD) patterns between samples. Therefore, we conducted LD analyses, as well as association and linkage using 12 polymorphisms, including 5HTTLPR. We evaluated two samples. The first sample consisted of 135 US Caucasian nuclear families having a proband with bipolar I disorder (BDI, DSM IV criteria) and available parents. For case‐control analyses, the patients from these families were compared with cord blood samples from local Caucasian live births (n = 182). Our second, independent sample was recruited through the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP‐BD, 545 cases, 548 controls). No significant associations were detected at the individual polymorphism or haplotype level using the case‐control or family‐based analyses. Our analyses do not support association between SLC6A4 and BDI families. Further studies using sub‐groups of BDI are worthwhile.
Introduction
Twin, adoption and family studies have suggested substantial genetic contributions to the etiology of bipolar I disorder (BDI) Citation1. It has proven difficult to identify individual genetic risk factors, possibly because the contribution of such factors is relatively small, and complex interactions may be involved Citation2. Complementing conventional linkage analyses, association studies have become increasingly popular for gene mapping studies of BDI as they may detect susceptibility loci with small effects Citation3.
There is suggestive evidence implicating dysregulation of serotonin (5‐hydroxytryptamine, 5‐HT) in the pathogenesis of a range of psychiatric disorders, including mood disorders Citation4–6. Serotonergic neurons are also targets for antidepressants Citation7–9. Hence genes regulating the synthesis, transport, and metabolism of 5‐HT, as well as genes encoding multiple 5‐HT receptors have been extensively analyzed. In the following section, we review peer‐reviewed publications in English describing genetic association studies of serotonergic polymorphisms and BDI. Our association and linkage analyses of the serotonin transporter (SLC6A4) follow. Serotonin related genes are also of interest for pharmacogenetic analyses, but the majority of these studies have focused on depression and therefore are not reviewed here Citation10–12.
Tryptophan hydoxylase (TPH)
TPH encodes the rate‐limiting enzyme that catalyzes the oxidation of tryptophan to 5‐hydroxytryptophan and is thus critical for the synthesis of 5‐HT. The gene for human TPH has been cloned Citation13 and mapped to chromosome 11p14‐p15.3 Citation14. Recently, a second isoform of TPH has been discovered and has been named TPH2 Citation15. TPH2 is localized to chromosome 12q21.1. TPH2 is preferentially expressed in the brain, whileTPH1 in mainly expressed in peripheral tissues Citation15,16.
Published association studies for the TPH1 gene and bipolar disorder have been inconsistent. A significant association with an intronic A218C polymorphism was first reported in a French sample. In this case‐control study, 152 patients (103 patients with BDI and 49 patients with BDII) were compared with 94 healthy control individuals (odds ratio, OR = 3.96 for probands homozygous for allele 218A) Citation17. However, significant associations were not detected in subsequent studies using case‐control or family‐based samples Citation18–22. Most of the studies lacked power to replicate the initial association. Recently, an association with a functional polymorphism of TPH2 and major depressive disorder was reported Citation23. A subsequent study did not detect association with bipolar disorder or suicidal behavior Citation24. Hence, it is difficult to make a definitive pronouncement about this association. This is a recurring theme for the other candidate genes reviewed below.
Serotonin 2A receptor (HTR2A)
HTR2A is thought to play a key role in the action of antidepressants such as selective serotonin reuptake inhibitors (SSRIs) Citation4. Several polymorphisms of HTR2A, localized to chromosome 13q14‐q21 have been extensively analyzed in genetic association studies of BDI. A number of significant associations have been published. In one of the earliest studies, the frequency of allele C102 at the 102T/C (rs6313) single nucleotide polymorphism (SNP) was elevated among Caucasian cases compared with unrelated controls (OR 1.79) Citation18. The 102T/C SNP is localized to exon 1 and represents a synonymous substitution. Another association has been observed at ‐1438A/G (rs6311, a SNP localized to the putative promoter region) in separate samples of Caucasian and Korean ethnicity, albeit with different alleles Citation25,26. However, the majority of studies did not detect associations at HTR2ACitation27–33 (see 34).
Most studies investigated cases and unrelated controls, so the significant associations could be attributed to population sub‐structure secondary to population admixture Citation35. In other words, differing ethnic backgrounds, rather than the disorder of interest may account for the case‐control difference. On the other hand, the majority of the studies with non‐significant associations investigated only one polymorphism and did not investigate all the documented variants. Thus, it is difficult to conclude that the negative reports represent absence of association. Indeed, when we investigated nine SNPs, a significant association at 1354C/T was detected using case‐control as well as family‐based analyses Citation34. In addition, an association with 516C/T was detected using case‐control, but not family‐based analyses. Though 1354C/T represents a synonymous substitution, it is in significant LD with 102T/C. Comprehensive analyses of all polymorphisms at this locus are warranted, using samples with adequate power.
Serotonin 2C receptor (HTR2C)
HTR2C has been mapped to chromosome Xq24 Citation36 and is a plausible candidate as it is involved in affective/perceptual states and endocrine function Citation37. An exonic polymorphism (Cys23Ser) was investigated among 88 bipolar patients and 113 controls from Spain, but significant case‐control differences were not detected. However, a suggestive association with the Ser23 allele was observed among female cases (P = 0.04, not corrected for multiple comparisons) Citation38. These results were supported by a later study of 42 bipolar patients and 40 healthy controls from Croatia (P = 0.051) Citation39. However, association could not be detected in two studies analyzing two polymorphic di‐nucleotide repeats in the promoter region, namely (GT)12‐18 and (CT)4‐5 Citation40,41. Based on these results, the Cys23Ser SNP was analyzed in a large multi‐center study comprising 649 bipolar patients and 901 normal controls from nine European countries. A significant excess of Ser23 allele carriers in patients compared to controls was observed (chi square = 5.45, df 1, P = 0.02) Citation42. However a significant variation in the allele frequency for Ser23 was noted across population groups included in the study, raising the possibility of a ‘spurious’ association attributable to ethnic admixture. Associations at HTR2C have not been detected in other, smaller samples Citation18,Citation43. Thus, the putative associations are reminiscent of the HTR2A association studies and merit comprehensive evaluation.
Other serotonergic receptors
The HTR1A, HTR1D alpha, HTR1D beta, HTR3A, HTR4 and HTR7 receptors have all been investigated in one or two studies Citation44,Citation18,Citation45. For example, in a case‐control study from Japan (53 patients, 187 controls), significant association of four polymorphisms at HTR4 gene with bipolar disorder was reported (OR 1.5–2) Citation45. These associations were supported by transmission disequilibrium test (TDT) analysis in 69 multiplex pedigrees from the National Institute of Mental Health (NIMH) Genetics Initiatives Bipolar Pedigrees. In view of the suggestive associations, further analyses at HTR4 are warranted.
Key messages
Dysfunction of the serotonergic neurotransmission has long been suggested to play a role in the pathogenesis of bipolar disorders.
Extensive research was conducted analyzing polymorphisms of several serotonin genes. However, consistent associations did not emerge. The inconsistencies may be due to inadequately powered samples and/or insufficient analysis of polymorphisms at these loci.
Associations between bipolar I disorder and polymorphisms of the serotonin transporter gene were not detected.
Monoamine oxidase (MAO)
Monoamine oxidase type A (MAOA) and type B (MAOB) are enzymes that catalyze the degradation of biogenic amines including serotonin Citation46. Hence, they are of great interest in psychiatric research and may even mediate the pathogenesis of affective disorders Citation47. Genes encoding these enzymes are tightly linked and arranged in a ‘tail‐to‐tail’ pattern on chromosome Xp11.23‐11.4 Citation48–50.
Several polymorphisms of MAOA have been investigated in genetic association in bipolar disorders. They include a di‐nucleotide repeat polymorphism in the second intron (MAOA‐CA) Citation51, an Fnu4HI restriction fragment length polymorphism (RFLP) in exon 8, an RFLP resulting from single base substitution in the third base of a triplet codon (G → T at position 941, also called ‘MAOA‐RFLP) Citation52 and variable number of tandem repeats (VNTR) polymorphism in intron 1 (‘MAOA‐VNTR’) Citation53. A significant association was detected at three polymorphisms in a sample of 57 British bipolar patients and 59 unaffected controls. A significant association with alleles of a MAOB polymorphism was not detected (di‐nucleotide sequence in the 2nd intron; MAOB‐GT) Citation54. These promising results were supported by three other studies. The first reported significant associations with the MAOA‐CA marker in a sample of 58 Japanese bipolar patients and 68 controls (P = 0.029) Citation55. An association with the same polymorphism was also detected in another sample of UK patients, though an association with the Fnu4HI RFLP was not detected Citation56. In France and Switzerland, 272 patients with bipolar disorder and 122 healthy subjects were investigated for the MAOA‐CA repeat, the MAOA‐RFLP, and the VNTR. A significant difference in the distribution of the alleles for the MAOA‐CA repeat was observed between the female bipolar patients and the comparison group Citation57. In contrast, no evidence for association was found with MAOA‐CA, Fnu4H1 RFLP or MAOA‐VNTR in another British sample composed of 84 bipolar patients (70 of them had BDI) and 84 controls Citation58. The same MAOA polymorphisms, as well as an additional MAOB marker were investigated in a Japanese sample consisting of 60 patients and 100 controls. Significant associations were not detected Citation59. Another case‐control study from Japan (132 patients, 169 controls) did not detect associations with two polymorphisms (T/941/G and A/1609/G)Citation60. No association could be found after analysis of the MAOA‐LPR polymorphism in a larger sample from Germany (174 patients with affective disorder, 229 controls) Citation61. A number of other investigators have analyzed MAO polymorphisms using family‐based controls and these have been published, although none of their findings were significant Citation62–64, Citation21. Thus, there are no credible associations at MAOA or MAOB, though it should be noted that many of the replication samples were underpowered and polymorphisms at these genes have not been investigated comprehensively. A similar conclusion was reached in a recent review Citation65.
Serotonin transporter (5‐HTT, hSERT, SLC6A4)
The serotonin transporter has received considerable attention, as it mediates serotonin reuptake into pre‐synaptic neurons. It is also the likely site of action of tricyclic antidepressants and serotonin re‐uptake inhibitors. Hence, the gene for the serotonin transporter (SLC6A4), which maps to chromosome 17q11.1‐q12, is a plausible candidate in the pathogenesis of BDI.
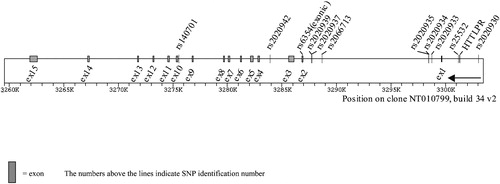
SLC6A4 polymorphisms have been investigated widely among cases and population‐based controls (). A study in the UK involving Caucasian BDI patients and unrelated controls first reported an association with allele 12 of a VNTR in the second intron Citation66. These results received support from another study conducted independently in the UK Citation67. This group also reported association with bipolar affective puerperal psychosis Citation68. A study from France did not detect the association with bipolar disorder, but reported association with increased homozygosity for the short variant of a 44 bp insertion/deletion polymorphism in the 5' untranslated region (5' UTR) denoted as 5‐HTTLPR Citation69. This polymorphism is considered to be important as it affects the transcription, and hence possibly the function of the serotonin transporter Citation70. The results from the French study are also plausible because the VNTR and 5‐HTTLPR polymorphisms are in strong linkage disequilibrium (LD) Citation71. In other words, allelic status at the VNTR and 5‐HTTLPR are significantly correlated at the population level. A third UK study examined both polymorphisms. Though significant associations were not detected in this sample, meta‐analysis of published studies suggested a significant association with allele 2 (the shorter allele) at the (5‐HTTLPR) marker Citation72 (). A recent meta‐analysis study applied to case‐control studies of 5‐HTTLPR revealed that the short allele(s) of the 44‐bp ins/deletion polymorphism showed a modest but significant association for bipolar disorder (OR = 1.13, P = 0.001) Citation73. Another recent Japanese study not included in the meta‐analysis, however, did not reveal a significant association Citation74.
Table I. Published BD1 case‐control association studies using SLC6A4 polymorphisms.
A number of family‐based association studies involving SLC6A4 polymorphisms have also been reported. A study involving 122 case‐parent trio families did not report significant association with either polymorphism using the transmission disequilibrium test (TDT). Since this sample included cases previously reported Citation66, analysis was repeated among 98 independently ascertained families. Significant excess transmission of the 12‐repeat VNTR allele was observed in this sub‐group. The TDT results for the VNTR polymorphism were supported by haplotype‐based analyses involving the VNTR and 5‐HTTLPR polymorphisms Citation71. Another study reported a significant TDT result for the 5HTTLPR polymorphism in BDI Citation75. Significant transmission distortion suggests association in the presence of linkage, providing further credence to the results. Non‐significant TDT results have also been reported at SLC6A4Citation76,77,Citation21.
Thus, a number of reports suggest associations with a functional polymorphism. However, the non‐significant results need to be considered also. There are several explanations for the inconsistent associations. First, the sample sizes of the published studies are relatively small, so each sample had limited power to detect small effects on liability. Second, most of the studies have examined one or two polymorphisms, which limits the power to detect association if the measured polymorphisms are only in LD with polymorphisms having a direct effect on liability. Indeed, it is now known that 5‐HTTLPR is not a binary insertion/deletion polymorphism, but involves a more complex repeat structure Citation78. Since the majority of studies employed unrelated controls, varying levels of population sub‐structure could also explain the inconsistencies Citation79.
To evaluate these possibilities, we investigated twelve polymorphisms at SLC6A4. To increase the informativeness of the available polymorphisms, we conducted additional haplotype‐based analyses. We employed case‐control, as well as family‐based analyses in order to understand artifacts due to ethnic substructure.
Materials and methods: Clinical
Pittsburgh sample
Probands with bipolar I disorder (DSM IV Criteria), and available parents were included. Details of ascertainment and diagnosis are described elsewhere Citation34,Citation80. Briefly, consenting patients were interviewed using the Diagnostic Interview for Genetic Studies (DIGS), a structured diagnostic interview schedule Citation81. Additional clinical information was obtained from available clinical records and from relatives. This information was synthesized and a consensus diagnosis assigned. The parents provided blood samples, but detailed diagnostic interviews were not conducted.
Unscreened controls
Cord blood samples were obtained from live births at Magee‐Women's Hospital, Pittsburgh and served as unscreened, population‐based controls. No information apart from ethnicity was available for these samples.
STEP‐BD sample
Patients
We obtained genomic DNA samples from patients with a baseline diagnosis of bipolar I disorder (DSM IV criteria) recruited through the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP‐BD). This is a longitudinal study aimed at improving treatment for Bipolar Disorder Citation82. STEP‐BD used a network of eighteen U.S. treatment centers for standardized evaluation and treatment of patients including interview schedules based on the Structured Clinical Interview for DSM‐IV (SCID), as well as the Mini‐International Neuropsychiatric Interview (MINI) Citation83,84.
Unscreened controls
Control samples were not collected as part of the STEP‐BD cohort. For comparison with the STEP‐BD cases, additional cord blood samples were obtained from Pittsburgh, independent of the unscreened ‘Pittsburgh’ samples described above.
All participants reported Caucasian ethnicity (maternal report for neonatal samples). We obtained written informed consent from participants, according to the guidelines of the University of Pittsburgh Institutional Review Board (IRB), as well as IRBs at relevant STEP‐BD recruitment sites.
Materials and methods: Laboratory
Genomic DNA was extracted from venous blood samples using the phenol chloroform method.
SNP selection
Fifteen polymorphisms were selected over a 38 kb genomic region spanning SLC6A4. These polymorphisms were identified by other investigators following bi‐directional sequencing of 10 unrelated autism probands Citation85. Among the polymorphisms that were analyzed initially; three were discarded due to inconsistent genotyping assays (rs6352 A/C, rs2020941 T/C and rs2020936 T/C). Thus, 12 polymorphisms were used in the analyses. They included the insertion/deletion polymorphism (5‐HTTLPR), as well as 11 additional SNPs, which are listed in public databases (http://www.ncbi.nlm.nih.gov/genome/guide/human/). Genomic DNA from a panel of eight individuals was re‐sequenced for each of the 11 SNPs. For 5HTTLPR, we re‐sequenced 48 individuals. These samples served as positive controls for subsequent genotype assays.
Genotype assays
Variation in 5HTTLPR was analyzed by re‐sequencing, as well as a polymerase chain reaction (PCR) based assay Citation86. Genotype assays for the SNPs were based on multiplex PCR followed by single base extension analysis (SnaPShot assays, ABI Inc; details available online at www.pitt.edu/∼nimga, supplemental ).
All allele calls were re‐checked independently, blind to diagnostic status. Genotypes were then evaluated for Mendelian consistency using PedCheck in the family‐based sample Citation87. In case of any discrepancy, samples were re‐typed. All clinical and genetic data were double‐checked to guard against data entry errors.
Statistical analysis
We evaluated linkage disequilibrium (LD), defined as the non‐random association of variants at linked loci Citation88. Several measures of LD are available, the classic measure being ‘D’ Citation89. As D is influenced by allele frequencies, we used two scaled measures, namely D' and r2 Citation90–92. The latter was estimated using a method based on a hierarchical clustering procedure called ‘H‐clust’. This method also enables selection of ‘tag SNPs’, i.e. SNPs that can be used to successfully predict unmeasured SNPs over a selected region and thus can be said to represent polymorphism in that region Citation93. The software program GENEHUNTER (GH) was used for TDT analysis Citation94. For family‐based association analyses, we used the ‘uncertain haplotypes’ option in the TDTPHASE program to analyze SNP based and haplotype based data (software package UNPHASED) Citation95. For case‐control comparisons, we utilized the Armitage trends test to evaluate genotype frequency differences between our cases and unrelated controls Citation96.
Results
The Pittsburgh family‐based sample included 135 cases; both parents were available for 61 families, while a single parent was available for the remaining 74 families. All the patients from these families were compared with 182 controls. The STEP‐BD sample included 545 BDI cases and 548 controls. No Mendelian inconsistencies were noted for the family based samples. All genotype distributions were in Hardy‐Weinberg equilibrium (HWE) for cases, parents and neonatal controls, with the exception of 5‐HTTLPR. A heterozygote deficit was noted for this polymorphism among the STEP‐BD cases (P = 0.005). Since departure from HWE can arise due to genotyping or book keeping errors, all genotypes from the STEP‐BD samples were re‐read. In case of ambiguity, samples were genotyped again. Results from the final assays are presented below.
Genomic structure of 5‐HTTLPR:
Consistent with published results, we detected a complex polymorphism after re‐sequencing 48 individual samples Citation86. The polymorphism consists of a 44 bp repeat in the promoter region. Further, we identified another SNP flanking 5HTTLPR, localized to the repeat motif (rs25532). In our sample, rs25532 is homozygous among all individuals homozygous for the 5HTTLPR insertion.
LD structure:
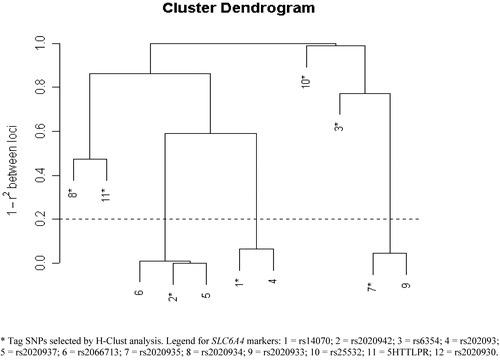
The relative positions of the other polymorphisms are displayed in . Pair‐wise LD patterns were evaluated between the polymorphisms among Pittsburgh patients, their parents, the controls, and the STEP‐BD controls, and tag SNPs selected by H‐clust with r2 = 80% Citation93 ( and ; ; haploview analysis available online at www.pitt.edu/∼nimga supplemental and )
Table II. Association and linkage analysis of ploymorphisms at SLC6A4 using Pittsburgh BD1 sample.
Using Haploview, two distinct ‘blocks’ were identified; the first composed of six SNPs, (rs140701, rs2020942, rs6354, rs2020939, rs2020937 and rs2066713) and the second composed of four other SNPs (rs2020935, rs2020934, rs2020933 and rs2020930). The remaining two SNPs, including 5HTTLPR, did not form LD blocks, although 5HTTLPR is in significant LD with rs2020934 Citation91.
Analyses using ‘H‐clust’ were similar overall to the ‘blocks’ outlined using Haploview, although there were some distinctions. Close clustering was observed for five of the six SNPs that composed ‘block 1’, but the remaining SNP (rs6354) did not cluster with the other SNPs, unlike the Haploview analysis (see in this text, and online www.pitt.edu/∼nimga). There was more disparity between these two methods with regard to ‘block 2’. Unlike the D' analyses, rs2020934 was not closely related to rs2020935 or rs2020933. Instead, it clustered with 5HTTLPR.
Association and linkage analysis:
Pittsburgh sample
No significant associations were detected after comparing genotype and allele distributions for each polymorphism between patients with BDI and neonatal controls. Consistent with these results, no significant transmission distortion was observed using the TDT for 5HTTLPR, individual SNPs or ‘sliding window’ haplotypes based on 2, 3 or 4 contiguous polymorphisms (). Significant associations were also not detected using the UNPHASED program at the SNP or at the haplotype level. An excess of the short allele of 5HTTLPR was previously observed in cases with psychotic symptoms Citation97. However, additional analyses comparing our Pittsburgh BDI cases with psychotic features against neonatal controls did not detect this effect (data not shown).
STEP‐BD sample
Allele frequencies for individual polymorphisms were consistent with those observed in the Pittsburgh samples. The case‐control comparisons conducted in the Pittsburgh sample were repeated in the STEP‐BD sample. No significant associations were detected using the larger STEP‐BD sample at the individual polymorphism or at the haplotype levels ().
Table III. Association analysis of polymorphisms at SLC6A4 using the STEP‐BD sample.
Table IV. Linkage disequilibrium across SLC6A4 using D' and r2 values among Pittsburgh BD1 cases.
Discussion
Our review of published studies did not reveal a clear pattern of association with any serotonergic (polymorphism, though a number of intriguing and plausible associations have been reported. Most of these studies were published prior to initiation of the Hapmap project (http://www.hapmap.org/). Hence only one or two polymorphisms were typically analyzed at each gene. As a large number of polymorphisms are now documented for most of these genes, it may be appropriate to conduct analyses using such polymorphisms. It will be economical to identify ‘tag SNPs’ that can represent a significant proportion of the variations across the entire gene, as was demonstrated here for SLC6A4Citation93. Here, we have suggested a method for selection of tag SNPs. In addition, much larger samples will soon become available to the scientific community Citation82 (http://www.nimh.nih.gov/researchfunding/geneticsinitiative.cfm).
In view of these concerns, we conducted a more comprehensive evaluation of the serotonin transporter (SLC6A4). In addition to the well‐known 5‐HTTLPR marker, we included 11 additional SNPs spaced across the gene. Our initial analyses revealed a lack of significant LD between 5‐HTTLPR and most of the other SNPs analyzed here, emphasizing the need for evaluation of multiple polymorphisms. To understand variations due to differences in control selection, we conducted association analyses using case‐control, as well as family‐based designs. We did not detect a significant association with any of the SLC6A4 polymorphisms in two independent samples composed of cases and community based controls. Our case‐control results are consistent with other previous negative studies Citation98–101,Citation18. In addition, we did not find significant associations using the TDT, in agreement with two prior reports Citation76,77.
Some limitations of our analyses should be noted. Our analyses were restricted to individuals reporting Caucasian ancestry. Our ‘Pittsburgh sample’ was relatively small, particularly for the family‐based analyses. The requirement for heterozygous parents restricts the number of family units that can be used for TDT analyses. The TDT also has limited usefulness and has reduced power when only one parent is available in a family unit Citation102,103. Since a proportion of our sample included single‐parent family units, we also compared our cases with unrelated controls.
Departure from Hardy Weinberg equilibrium was noted only among the STEP‐BD patients with regard to 5‐HTTLPR genotypes. We note that the P value (0.005) does not remain significant following correction for multiple comparisons (12 polymorphisms; two sets of patients, two set of controls and one set of parents). Nevertheless, concerns about genotype errors lead us to re‐evaluate our results. As such errors do not explain the departure from HWE, we attribute it to chance variation.
Since we did not re‐sequence SLC6A4 comprehensively in our samples, it is possible that other unidentified polymorphisms may yet be associated with BDI at SLC6A4. Some investigators have reported an association with a VNTR in the second intron. This polymorphism was not analyzed here as it is reported to be in strong LD with 5HTTLPR Citation71. At present, twelve SNPs have been analyzed by the HapMap project. Haploview analysis revealed that the SNPs form two blocks (data not shown). Four SNPs present in these blocks were also analyzed in our set. Thus, our set appears to have a reasonable representation of polymorphisms at SLC6A4, at least with respect to ‘common’ SNPs. In addition, our set also includes ‘tag’ SNPs from H‐Clust analysis (). The disparity between the cluster analyses and the Haploview analyses is due to quite different approaches and assumptions about the pattern of LD in the genome. For example, two point LD analysis, on which Haploview is based, is highly dependent on sample size, unlike H‐Clust analysis. Analyses of LD suggest that the genome does not show perfect block like structure, but rather can show a very patchy pattern of LD Citation93.
Conclusion
There is suggestive evidence for dysfunction in serotonergic neurotransmission among patients with BDI. Hence, it is appropriate to investigate genetic associations with serotonergic genes. Polymorphisms of several such genes have been analyzed, but consistent associations have not emerged. The inconsistencies could be due to differences in sample size, number of markers analyzed, artifacts introduced by ethnic admixture, heterogeneity or may indicate the absence of a ‘true’ association. The time may be ripe for comprehensive analysis of these genes systematically and comprehensively, using adequately powered samples.
Our investigation of Caucasian cases, controls, and parents did not reveal significant association between serotonin transporter (SLC6A4) gene polymorphisms and BDI. Our results are concordant with a majority of prior reports. Though our analyses did not reveal associations with SLC6A4, associations at this locus among sub‐groups of BDI are possible, such as those based on mixed or rapid cycling, clinical course, vulnerability to adverse life events, or response to relevant therapies.
Acknowledgements
We gratefully acknowledge statistical advice from Dr Bernie Devlin and Mr Vibhor Sonpal. We thank Ms Amber Johnson for technical assistance. Supported in part by grants from the National Institutes of Health (MH63420, MH56242 and MH66263 to VLN) and the Mental Health Intervention Research Center (# MH 30915 to DJK).
References
- Gershon E. S., Martinez M., Goldin L. R., Gejman P. V. Genetic mapping of common diseases: the challenges of manic‐depressive illness and schizophrenia. Trends Genet 1990; 6: 282–7
- Craddock N., Jones I. Genetics of bipolar disorder. J Med Genet 1999; 36: 585–94
- Risch N., Merikangas K. The future of genetic studies of complex human diseases. Science 1996; 273: 1516–7
- Veenstra‐VanderWeele J., Anderson G. M., Cook E. H., Jr. Pharmacogenetics and the serotonin system: initial studies and future directions. Eur J Pharmacol 2000; 410: 165–81
- Lesch K. P., Mossner R. Genetically driven variation in serotonin uptake: is there a link to affective spectrum, neurodevelopmental, and neurodegenerative disorders?. Biol Psychiatry 1998; 44: 179–92
- Owens M. J., Nemeroff C. B. Role of serotonin in the pathophysiology of depression: focus on the serotonin transporter. Clin Chem 1994; 40: 288–95
- Sibille E., Pavlides C., Benke D., Toth M. Genetic inactivation of the Serotonin(1A) receptor in mice results in downregulation of major GABA(A) receptor alpha subunits, reduction of GABA(A) receptor binding, and benzodiazepine‐resistant anxiety. J Neurosci 2000; 20: 2758–65
- Gingrich J. A., Hen R. Dissecting the role of the serotonin system in neuropsychiatric disorders using knockout mice. Psychopharmacology (Berl) 2001; 155: 1–10
- Murphy D. L., Uhl G. R., Holmes A., Ren‐Patterson R., Hall F. S., Sora I., et al. Experimental gene interaction studies with SERT mutant mice as models for human polygenic and epistatic traits and disorders. Genes Brain Behav 2003; 2: 350–64
- Joyce P. R., Mulder R. T., Luty S. E., McKenzie J. M., Miller A. L., Rogers G. R., et al. Age‐dependent antidepressant pharmacogenomics: polymorphisms of the serotonin transporter and G protein beta3 subunit as predictors of response to fluoxetine and nortriptyline. Int J Neuropsychopharmacology 2003; 6: 339–46
- Peters E. J., Slager S. L., McGrath P. J., Knowles J. A., Hamilton S. P. Investigation of serotonin‐related genes in antidepressant response. Mol Psychiatry 2004; 9: 879–89
- Neumeister A., Young T., Stastny J. Implications of genetic research on the role of the serotonin in depression: emphasis on the serotonin type 1A receptor and the serotonin transporter. Psychopharmacology (Berl) 2004; 174: 512–24, Epub. 2004 Jul 13
- Boularand S., Darmon M. C., Ganem Y., Launay J. M., Mallet J. Complete coding sequence of human tryptophan hydroxylase. Nucleic Acids Res 1990; 18: 4257
- Craig S. P., Boularand S., Darmon M. C., Mallet J., Craig I. W. Localization of human tryptophan hydroxylase (TPH) to chromosome 11p15.3‐p14 by in situ hybridization. Cytogenet Cell Genet 1991; 56: 157–9
- Walther D. J., Peter J. U., Bashammakh S., Hortnagl H., Voits M., Fink H., et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science 2003; 299: 76
- Zhang X., Beaulieu J. M., Sotnikova T. D., Gainetdinov R. R., Caron M. G. Tryptophan hydroxylase‐2 controls brain serotonin synthesis. Science 2004; 305: 217
- Bellivier F., Leboyer M., Courtet P., Buresi C., Beaufils B., Samolyk D., et al. Association Between the Tryptophan Hydroxylase Gene and Manic‐depressive Illness. Arch Gen Psychiatry 1998; 55: 33–7
- Vincent J. B., Masellis M., Lawrence J., Choi V., Gurling H. M., Parikh S. V., et al. Genetic association analysis of serotonin system genes in bipolar affective disorder. Am J Psychiatry 1999; 156: 136–8
- Rietschel M., Schorr A., Albus M., Franzek E., Kreiner R., Held T., et al. Association study of the tryptophan hydroxylase gene and bipolar affective disorder using family‐based internal controls. Am J Med Genet 2000; 96: 310–1
- Furlong R. A., Ho L., Rubinsztein J. S., Walsh C., Paykel E. S., Rubinsztein D. C. No association of the tryptophan hydroxylase gene with bipolar affective disorder, unipolar affective disorder, or suicidal behaviour in major affective disorder. Am J Med Genet 1998; 81: 245–7
- Serretti A., Cristina S., Lilli R., Cusin C., Lattuada E., Lorenzi C., et al. Family‐based association study of 5‐HTTLPR, TPH, MAO‐A, and DRD4 polymorphisms in mood disorders. Am J Med Genet 2002; 114: 361–9
- Kirov G., Owen M. J., Jones I., McCandless F., Craddock N. Tryptophan hydroxylase gene and manic‐depressive illness [letter; comment]. Arch Gen Psychiatry 1999; 56: 98–9
- Zhang X., Gainetdinov R. R., Beaulieu J. M., Sotnikova T. D., Burch L. H., Williams R. B., et al. Loss‐of‐function mutation in tryptophan hydroxylase‐2 identified in unipolar major depression. Analysis of the novel TPH2 gene in bipolar disorder and suicidality. Neuron 2005; 45: 11–6
- De Luca V., Mueller D. J., Tharmalingam S., King N., Kennedy J. L. Analysis of the novel TPH2 gene in bipolar disorder and suicidality. Mol Psychiatry 2004; 9: 896–7
- Bonnier B., Gorwood P., Hamon M., Sarfati Y., Boni C., Hardy‐Bayle M. C. Association of 5‐HT(2A) receptor gene polymorphism with major affective disorders: the case of a subgroup of bipolar disorder with low suicide risk. Biol Psychiatry 2002; 51: 762–5
- Chee I. S., Lee S. W., Kim J. L., Wang S. K., Shin Y. O., Shin S. C., et al. 5‐HT2A receptor gene promoter polymorphism‐1438A/G and bipolar disorder. Psychiatr Genet 2001; 11: 111–4
- Mahieu B., Souery D., Lipp O., Mendelbaum K., Verheyen G., De Maertelaer V., et al. No association between bipolar affective disorder and a serotonin receptor (5‐HT2A) polymorphism. Psychiatry Res 1997; 70: 65–9
- Gutierrez B., Bertranpetit J., Collier D., Arranz M. J., Valles V., Guillamat R., et al. Genetic variation of the 5‐HT2A receptor gene and bipolar affective disorder. Hum Genet 1997; 100: 582–4
- Massat I., Souery D., Lipp O., Blairy S., Papadimitriou G., Dikeos D., et al. A European multicenter association study of HTR2A receptor polymorphism in bipolar affective disorder. Am J Med Genet 2000; 96: 136–40
- Tut T. G., Wang J. L., Lim C. C. Negative association between T102C polymorphism at the 5‐HT2A receptor gene and bipolar affective disorders in Singaporean Chinese. J Affect Disord 2000; 58: 211–4
- Serretti A., Lattuada E., Catalano M., Smeraldi E. Serotonin transporter gene not associated with psychotic symptomatology of mood disorders. Psychiatry Res 1999; 86: 59–65
- Blairy S., Massat I., Staner L., Le Bon O., Van Gestel S., Van Broeckhoven C., et al. 5‐HT2a receptor polymorphism gene in bipolar disorder and harm avoidance personality trait. Am J Med Genet 2000; 96: 360–4
- Murphy V. E., Mynett‐Johnson L. A., Claffey E., Shields D. C., McKeon P. No association between 5HT‐2A and bipolar disorder irrespective of genomic imprinting. Am J Med Genet 2001; 105: 422–5
- Ranade S. S., Mansour H., Wood J., Chowdari K. V., Brar L. K., Kupfer D. J., et al. Linkage and association between serotonin 2A receptor gene polymorphisms and bipolar I disorder. Am J Med Genet 2003; 121B: 28–34
- Ewens W. J., Spielman R. S. The transmission/disequilibrium test: history, subdivision, and admixture. Am J Hum Genet 1995; 57: 455–64
- Milatovich A., Hsieh C. L., Bonaminio G., Tecott L., Julius D., Francke U. Serotonin receptor 1c gene assigned to X chromosome in human (band q24) and mouse (bands D‐F4). Hum Mol Genet 1992; 1: 681–4
- Naughton M., Mulrooney J. B., Leonard B. E. A review of the role of serotonin receptors in psychiatric disorders. Hum Psychopharmacol 2000; 15: 397–415
- Gutierrez B., Fananas L., Arranz M. J., Valles V., Guillamat R., van Os J., et al. Allelic association analysis of the 5‐HT2C receptor gene in bipolar affective disorder. Neurosci Lett 1996; 212: 65–7
- Oruc L., Verheyen G. R., Furac I., Jakovljevic M., Ivezic S., Raeymaekers P., et al. Association analysis of the 5‐HT2C receptor and 5‐HT transporter genes in bipolar disorder. Am J Med Genet 1997; 74((5))504–6
- Gutierrez B., Arias B., Papiol S., Rosa A., Fananas L. Association study between novel promoter variants at the 5‐HT2C receptor gene and human patients with bipolar affective disorder. Neurosci Lett 2001; 309: 135–7
- Meyer J., Saam W., Mossner R., Cangir O., Ortega G. R., Tatschner T., et al. Evolutionary conserved microsatellites in the promoter region of the 5‐hydroxytryptamine receptor 2C gene (HTR2C) are not associated with bipolar disorder in females. J Neural Transm 2002; 109: 939–46
- Lerer B., Macciardi F., Segman R. H., Adolfsson R., Blackwood D., Blairy S., et al. Variability of 5‐HT2C receptor cys23ser polymorphism among European populations and vulnerability to affective disorder. Mol Psychiatry 2001; 6: 579–85
- Serretti A., Lilli R., Lorenzi C., Lattuada E., Smeraldi E. Serotonin‐2C and serotonin‐1A receptor genes are not associated with psychotic symptomatology of mood disorders. Am J Med Genet 2000; 96: 161–6
- Niesler B., Flohr T., Nothen M. M., Fischer C., Rietschel M., Franzek E., et al. Association between the 5' UTR variant C178T of the serotonin receptor gene HTR3A and bipolar affective disorder. Pharmacogenetics 2001; 11: 471–5
- Ohtsuki T., Ishiguro H., Detera‐Wadleigh S. D., Toyota T., Shimizu H., Yamada K., et al. Association between serotonin 4 receptor gene polymorphisms and bipolar disorder in Japanese case‐control samples and the NIMH Genetics Initiative Bipolar Pedigrees. Mol Psychiatry 2002; 7: 954–61
- Weyler W., Hsu Y. P., Breakefield X. O. Biochemistry and genetics of monoamine oxidase. Pharmacol Ther 1990; 47: 391–417
- Goodwin F. K., Jamison K. R. Manic‐Depressive Illness. Oxford University Press, New York, Oxford 1990
- Lan N. C., Heinzmann C., Gal A., Klisak I., Orth U., Lai E., et al. Human monoamine oxidase A and B genes map to Xp 11.23 and are deleted in a patient with Norrie disease. Genomics 1989; 4: 552–9
- Chen Z. Y., Powell J. F., Hsu Y. P., Breakefield X. O., Craig I. W. Organization of the human monoamine oxidase genes and long‐range physical mapping around them. Genomics 1992; 14: 75–82
- Sabol S. Z., Hu S., Hamer D. A functional polymorphism in the monoamine oxidase A gene promoter. Hum Genet 1998; 103: 273–9
- Black G. C., Chen Z. Y., Craig I. W., Powell J. F. Dinucleotide repeat polymorphism at the MAOA locus. Nucleic Acids Res 1991; 19: 689
- Brunner H. G., Nelen M., Breakefield X. O., Ropers H. H., van Oost B. A. Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science 1993; 262: 578–80
- Hinds H. L., Hendriks R. W., Craig I. W., Chen Z. Y. Characterization of a highly polymorphic region near the first exon of the human MAOA gene containing a GT dinucleotide and a novel VNTR motif. Genomics 1992; 13: 896–7
- Lim L. C., Powell J., Sham P., Castle D., Hunt N., Murray R., et al. Evidence for a genetic association between alleles of monoamine oxidase A gene and bipolar affective disorder. Am J Med Genet 1995; 60: 325–31
- Kawada Y., Hattori M., Dai X. Y., Nanko S. Possible association between monoamine oxidase A gene and bipolar affective disorder. Am J Hum Genet 1995; 56: 335–6
- Rubinsztein D. C., Leggo J., Goodburn S., Walsh C., Jain S., Paykel E. S. Genetic association between monoamine oxidase A microsatellite and RFLP alleles and bipolar affective disorder: analysis and meta‐analysis. Hum Mol Genet 1996; 5: 779–82
- Preisig M., Bellivier F., Fenton B. T., Baud P., Berney A., Courtet P., et al. Association between bipolar disorder and monoamine oxidase A gene polymorphisms: results of a multicenter study. Am J Psychiatry 2000; 157: 948–55
- Craddock N., Daniels J., Roberts E., Rees M., McGuffin P., Owen M. J. No evidence for allelic association between bipolar disorder and monoamine oxidase A gene polymorphisms. Am J Med Genet 1995; 60: 322–4
- Muramatsu T., Matsushita S., Kanba S., Higuchi S., Manki H., Suzuki E., et al. Monoamine oxidase genes polymorphisms and mood disorder. Am J Med Genet 1997; 74: 494–6
- Sasaki T., Hattori M., Sakai T., Kato T., Kunugi H., Hirose T., et al. The monoamine oxidase‐A gene and major psychosis in Japanese subjects. Biol Psychiatry 1998; 44: 922–4
- Syagailo Y. V., Stober G., GraBle M., Reimer E., Knapp M., Jungkunz G., et al. Association analysis of the functional monoamine oxidase a gene promoter polymorphism in psychiatric disorders. Am J Med Genet 2001; 105: 168–171
- Nothen M. M., Eggermann K., Albus M., Borrmann M., Rietschel M., Korner J., et al. Association analysis of the monoamine oxidase A gene in bipolar affective disorder by using family‐based internal controls. Am J Hum Genet 1995; 57: 975–8
- Parsian A., Todd R. D. Genetic association between monoamine oxidase and manic‐depressive illness: comparison of relative risk and haplotype relative risk data. Am J Med Genet 1997; 74: 475–9
- Kirov G., Norton N., Jones I., McCandless F., Craddock N., Owen M. J. A functional polymorphism in the promoter of monoamine oxidase A gene and bipolar affective disorder. Int J Neuropsychopharmcol 1999; 2: 293–8
- Preisig M., Ferrero F., Malafosse A. Monoamine oxidase a and tryptophan hydroxylase gene polymorphisms: are they associated with bipolar disorder?. Am J Pharmacogenomics 2005; 5: 45–52
- Collier D. A., Arranz M. J., Sham P., Battersby S., Vallada H., Gill P., et al. The serotonin transporter is a potential susceptibility factor for bipolar affective disorder. Neuroreport 1996; 7: 1675–9
- Craddock N., Rees M., Norton N., Feldman E., McGuffin P., Owen M. J. Association between bipolar disorder and the VNTR polymorphism in intron 2 of the human serotonin transporter gene (HSERT). Psychiatr Genet 1996; 6: 147
- Coyle N., Jones I., Robertson E., Lendon C., Craddock N. Variation at the serotonin transporter gene influences susceptibility to bipolar affective puerperal psychosis. Lancet 2000; 356: 1490–1
- Bellivier F., Henry C., Szoke A., Schurhoff F., Nosten‐Bertrand M., Feingold J., et al. Serotonin transporter gene polymorphisms in patients with unipolar or bipolar depression. Neurosci Lett 1998; 255: 143–6
- Lesch K. P., Balling U., Gross J., Strauss K., Wolozin B. L., Murphy D. L., et al. Organization of the human serotonin transporter gene. J Neural Transm (General Sect) 1994; 95: 157–62
- Kirov G., Rees M., Jones I., MacCandless F., Owen M. J., Craddock N. Bipolar disorder and the serotonin transporter gene: a family‐based association study. Psychol Med 1999; 29: 1249–54
- Furlong R. A., Ho L., Walsh C., Rubinsztein J. S., Jain S., Paykel E. S., et al. Analysis and meta‐analysis of two serotonin transporter gene polymorphisms in bipolar and unipolar affective disorders. Am J Med Genet 1998; 81: 58–63
- Lasky‐Su J. A., Faraone S. V., Glatt S. J., Tsuang M. T. Meta‐analysis of the association between two polymorphisms in the serotonin transporter gene and affective disorders. Am J Med Genet B (Neuropsychiatr Genet) 2005; 133: 110–5
- Ikeda M., Iwata N., Suzuki T., Kitajima T., Yamanouchi Y., Kinoshita Y., et al. No association of serotonin transporter gene (SLC6A4) with schizophrenia and bipolar disorder in Japanese patients: association analysis based on linkage disequilibrium. J Neural Transm 2005; 5: 5
- Mynett‐Johnson L., Kealey C., Claffey E., Curtis D., Bouchier‐Hayes L., Powell C., et al. Multimarker haplotypes within the serotonin transporter gene suggest evidence of an association with bipolar disorder. Am J Med Genet 2000; 96: 845–9
- Esterling L. E., Yoshikawa T., Turner G., Badner J. A., Bengel D., Gershon E. S., et al. Serotonin transporter (5‐HTT) gene and bipolar affective disorder. Am J Med Genet 1998; 81: 37–40
- Mundo E., Walker M., Tims H., Macciardi F., Kennedy J. L. Lack of linkage disequilibrium between serotonin transporter protein gene (SLC6A4) and bipolar disorder. Am J Med Genet 2000; 96: 379–83
- Nakamura M., Ueno S., Sano A., Tanabe H. The human serotonin transporter gene linked polymorphism (5‐HTTLPR) shows ten novel allelic variants. Mol Psychiatry 2000; 5: 32–8
- Jorde L. B. Linkage disequilibrium as a gene‐mapping tool. American Journal of Human Genetics 1995; 56: 11–4
- Chowdari K. V., Mirnics K., Semwal P., Wood J., Lawrence E., Bhatia T., et al. Association and linkage analyses of RGS4 polymorphisms in schizophrenia. Hum Mol Genet 2002; 11: 1373–80
- Nurnberger J. I., Jr., Blehar M. C., Kaufmann C. A., York‐Cooler C., Simpson S. G., Harkavy‐Friedman J., et al. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry 1994; 51: 849–59;discussion 863–4
- Sachs G. S., Thase M. E., Otto M. W., Bauer M., Miklowitz D., Wisniewski S. R., et al. Rationale, design, and methods of the systematic treatment enhancement program for bipolar disorder (STEP‐BD). Biol Psychiatry 2003; 53: 1028–42
- Sheehan D. V., Lecrubier Y., Sheehan K. H., Amorim P., Janavs J., Weiller E., et al. The Mini‐International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM‐IV and ICD‐10. J Clin Psychiatry 1998; 59((Suppl. 20))22–33, quiz 34–57
- Spitzer R., Williams J., Gibbon M. Structured Clinical Interview for DSM‐IV, Outpatient Version (SCID‐OP). Biometrics Research Department, New York State Psychiatric Institute, New York 1996
- Kim S. J., Cox N., Courchesne R., Lord C., Corsello C., Akshoomoff N., et al. Transmission disequilibrium mapping at the serotonin transporter gene (SLC6A4) region in autistic disorder. Mol Psychiatry 2002; 7: 278–88
- Heils A., Teufel A., Petri S., Stober G., Riederer P., Bengel D., et al. Allelic variation of human serotonin transporter gene expression. J Neurochem 1996; 66: 2621–4
- O'Connell J. R., Weeks D. E. PedCheck: a program for identification of genotype incompatibilties in linakge analysis. American Journal of Human Genetics 1998; 63: 259–266
- Jorde L. B. Linkage disequilibrium and the search for complex disease genes. Genome Res 2000; 10: 1435–44
- Lewontin R. C. On measures of gametic disequilibrium. Genetics 1988; 120: 849–52
- Weiss K. M., Clark A. G. Linkage disequilibrium and the mapping of complex human traits. Trends Genet 2002; 18: 19–24
- Barrett J. C., Fry B., Maller J., Daly M. J. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 2005; 21: 263–5, Epub. 2004 Aug 05
- Devlin B., Risch N. A comparison of linkage disequilibrium measures for fine‐scale mapping. Genomics 1995; 29: 311–22
- Rinaldo A., Bacanu S. A., Devlin B., Sonpar V., Wasserman L., Roeder K. Characterization of multilocus linkage disequilibrium. Genet Epidemiol 2005; 6: 6
- Lander E., Kruglyak L. Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nat Genet 1995; 11: 241–247
- Dudbridge F. Pedigree disequilibrium tests for multilocus haplotypes. Genet Epidemiol 2003; 25: 115–21
- Devlin B., Roeder K. Genomic control for association studies. Biometrics 1999; 55: 997–1004
- Ospina‐Duque J., Duque C., Carvajal‐Carmona L., Ortiz‐Barrientos D., Soto I., Pineda N., et al. An association study of bipolar mood disorder (type I) with the 5‐HTTLPR serotonin transporter polymorphism in a human population isolate from Colombia. Neurosci Lett 2000; 292: 199–202
- Mendlewicz J., Massat I., Souery D., Del‐Favero J., Oruc L., Nothen M. M., et al. Serotonin transporter 5HTTLPR polymorphism and affective disorders: no evidence of association in a large European multicenter study. Eur J Hum Genet 2004; 12: 377–82, Write to the Help Desk NCBI | NLM | NIH Department of Health & Human Services Privacy Statement | Freedom of Information Act | Disclaimer
- Hoehe M. R., Wendel B., Grunewald I., Chiaroni P., Levy N., Morris‐Rosendahl D., et al. Serotonin transporter (5‐HTT) gene polymorphisms are not associated with susceptibility to mood disorders. Am J Med Genet 1998; 81: 1–3
- Ohara K., Nagai M., Tsukamoto T., Tani K., Suzuki Y. Functional polymorphism in the serotonin transporter promoter at the SLC6A4 locus and mood disorders. Biol Psychiatry 1998; 44: 550–4
- Gutierrez B., Arranz M. J., Collier D. A., Valles V., Guillamat R., Bertranpetit J., et al. Serotonin transporter gene and risk for bipolar affective disorder: an association study in Spanish population. Biol Psychiatry 1998; 43: 843–7
- Sham P. C., Curtis D. An extended transmission/disequilibrium test (TDT) for multi‐allele marker loci. Ann Hum Genet 1995; 59((Pt 3))323–36
- Sun F., Flanders W. D., Yang Q., Khoury M. J. Transmission disequilibrium test (TDT) when only one parent is available: the 1‐TDT. Am J Epidemiology 1999; 150: 97–104
- Kunugi H., Tatsumi M., Sakai T., Hattori M., Nanko S. Serotonin transporter gene polymorphism and affective disorder [letter; comment]. Lancet 1996; 347: 1340
- Bellivier F., Laplanche J. L., Leboyer M., Feingold J., Bottos C., Allilaire J. F., et al. Serotonin transporter gene and manic depressive illness: an association study. Biol Psychiatry 1997; 41: 750–2
- Rees M., Norton N., Jones I., McCandless F., Scourfield J., Holmans P., et al. Association studies of bipolar disorder at the human serotonin transporter gene (hSERT; 5HTT). Mol Psychiatry 1997; 2: 398–402
- Oliveira J. R., Carvalho D. R., Pontual D., Gallindo R. M., Sougey E. B., Gentil V., et al. Analysis of the serotonin transporter polymorphism (5‐HTTLPR) in Brazilian patients affected by dysthymia, major depression and bipolar disorder. Mol Psychiatry 2000; 5: 348–9
- Saleem Q., Ganesh S., Vijaykumar M., Reddy Y. C., Brahmachari S. K., Jain S. Association analysis of 5HT transporter gene in bipolar disorder in the Indian population. Am J Med Genet 2000; 96: 170–2