Abstract
BACKGROUND. Infections are associated with atherogenic changes in serum.
AIM. To elucidate effects of recent infections on risk factors for coronary heart disease in children.
SUBJECTS AND METHODS. In 1983 and again 3 years later, 2458 individuals aged 9, 12, 15, 18 and 21 years were investigated. In 1986, 106 subjects had symptoms of infection during the past 2 weeks before their follow‐up visit. Their serum albumin and lipid concentrations were compared to those in 1983 when these individuals probably were healthy. An age‐ and sex‐matched healthy control group from the cohort 1986 was chosen for comparison. For cholesterol age, sex and body mass index specific Z‐scores in addition to actual values were used in statistical comparisons.
RESULTS. Serum albumin was 42 g/L in subjects with positive history of infection and 46 g/L in healthy controls (P<0.0001). HDL‐cholesterol and the ratio of HDL‐ to total cholesterol were lower with increasing evidence of infection. Elevated serum C‐reactive protein (CRP) or orosomucoid grouped the subjects with high and low serum HDL‐cholesterol concentrations better than history of infection alone.
CONCLUSION. A mild infection lowers serum HDL‐cholesterol and serum albumin concentrations, which both favour atherogenesis.
| Abbreviations | ||
| APPs | = | Acute phase proteins |
| BMI | = | Body mass index |
| CHD | = | Coronary heart disease |
| CRP | = | C‐reactive protein |
| HDL | = | High density lipoprotein |
| LDL | = | Low density lipoprotein |
Introduction
Coronary heart disease (CHD) is the leading cause of death in western countries and increasing in the developing countries. Its roots lie in childhood Citation1. The classical risk factors for CHD explain only a part of its incidence. Both acute and chronic infections have been suggested to be risk factors for CHD Citation2. Various kinds of infections might even have an additive effect by acting simultaneously and increasing the risk for CHD Citation3. HDL‐cholesterol has antioxidant properties and functions as a reverse cholesterol transporter, binding to peripheral tissues by HDL receptors and transferring cholesterol from the tissues to the liver Citation4. Lowering of HDL‐cholesterol and serum lipoprotein lipase have been reported to be associated with infections Citation5.
Decreased concentration of serum albumin has been suggested to be a ‘new’ risk factor for CHD Citation6. Low serum albumin due to inflammation is common among hospitalized patients and albumin is classified among acute phase proteins (APPs) whose plasma concentration decreases due to an inflammatory stimulus Citation7. Low serum albumin concentration may increase the risk for CHD by several mechanisms. Albumin has antioxidant properties; it inhibits the production of free hydroxyl radicals and may inhibit endothelial apoptosis Citation8. It modulates arachidonic acid release and membrane fluidity, and protects against myocardial injury from ischaemia and reperfusion Citation9. Low serum albumin level may be a marker of persistent injury to arteries and progression of atherosclerosis and thrombosis Citation10. This kind of phenomenon is seen in young (<1year of age) Kawasaki disease patients. Those with low serum albumin seem to run a very high risk for coronary artery lesions Citation11. Infections as modulators of serum cholesterol levels and serum albumin were the topic of this study.
Key messages
Mild infection lowers serum HDL‐cholesterol and serum albumin concentrations.
Acute phase reaction induces atherogenic changes in the serum.
Increasing evidence of infection is associated with increased atherogenity of the serum.
Subjects and methods
This study is a part of the Cardiovascular Risk in Young Finns Study where 2458 individuals aged 9, 12, 15, 18 and 21 years, were investigated in a visit to the out‐patient department of paediatrics in the university hospital or local health centres in 1983 and again 3 years later. The study was approved by local ethics committees.
Subjects were asked in 1986 about symptoms of infection such as rhinitis, cough, sore throat, earache, or diarrhoea in association with fever over 38°C during the 2 weeks before their follow‐up visit. Symptoms of infection appeared in 106 subjects out of 2458 (4.3%) according to questionnaire. Even if no enquiry was made in 1983 the subjects were considered to be healthy because it is unlikely that the same 106 patients who had been infected in 1986 would have been also infected in 1983. For this study cohort the same number (106) of healthy age‐and sex‐matched controls was chosen in 1986.
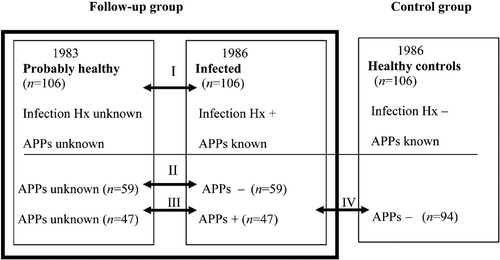
Details of the study design are presented in . The lipid parameters of the subjects in 1986 and 1983 were compared (step I). Body mass index (BMI) and feeding habits were similar for both groups. Acute phase proteins (APPs) concentrations (serum CRP, orosomucoid and albumin) were measured in infected subjects and healthy controls in 1986 which helped to find objective evidence of infection. Cholesterol parameters in 59 subjects with a positive history of infection and non‐elevated APPs were compared to their values in 1983 when infection history and APPs were not known (step II). CRP or orosomucoid were elevated (⩾5mg/L and ⩾1.1 g/L correspondingly) in 47 subjects with positive history of infection. Lipid concentrations in these subjects were further compared to their values in 1983 when history of infection and APPs were not enquired into (step III). Cholesterol values of healthy age‐ and sex‐ matched controls with negative APPs (n = 94) were compared to those with elevated APPs from infection group (n = 47) (step IV).
Figure 1. Study design: the cholesterol concentrations of subjects with history of infection in 1986 were compared to the concentrations during probable infection‐free period in 1983, and to those of healthy controls in 1986. APPs = acute phase proteins; Hx = history; + = positive/elevated; − = negative/normal. Numbers between the squares refer to , , and , which represent analysis steps I, II, III and IV correspondingly.
Age, sex and study year dependent Z‐scores for lipid parameters and BMI were calculated from the whole cohort of 2458 subjects separately for 1983 and 1986. The Z‐score for an item indicates how far and in what direction, that item deviates from its distribution's mean, expressed in units of its distribution's standard deviation. The mathematics of the Z‐score transformation is such that if every item in a distribution is converted to its Z‐score, the transformed scores will necessarily have a mean of zero and a standard deviation of one. Albumin concentrations in study groups in 1986 were also compared.
Blood samples were taken after 12 hours fasting during October‐November in both study years in order to rule out the effect of seasonal variation. The samples were analysed with the same method immediately after each study year in the same laboratory, which was under the control of the WHO laboratory in Prague Citation12. Serum cholesterol concentrations were measured using fully enzymatic Boehringer CHOD‐PAP kits Citation13 with an OLLI 3000 analyser. The average within‐run and between‐run coefficient of variation was 1.6% and 2.2%, respectively Citation11.
The amount of serum HDL‐cholesterol was measured using the dextran sulphate 500 000 method Citation14. LDL‐cholesterol concentrations were calculated using the Friedewald formula Citation15. Samples for determination of APPs were done by immunoturbidimetry using a Roche Modular P 800 analysis system and a calibrator based upon the international calibrator CRM 470 Citation16.
Statistical analysis
Z‐scores in addition to actual cholesterol values and BMI values were used in statistical analyses. Paired student's t‐test and StatView (SAS Inst.5.0) as a statistical software package Citation17 was used. P‐value of <0.05 was taken as significant.
Results
One hundred and six subjects out of 2458 (4.3%) reported to have had symptoms of infection during the preceding 2 weeks (). All had had fever, but 63 of them reported temperatures over 38°C. The mean temperature was 38.3°C and the mean duration of fever was 2.6 days. The mean time from the end of fever to blood sampling was 6.7 days. The mean number of additional symptoms was 1.9. CRP was elevated in 22 subjects and orosomucoid alone in 25 subjects.
Table I. Symptoms of infection during the last 2 weeks before blood sampling in the infected group (n = 106).
Step 1 of analysis
Symptoms of mild infections were associated with lower levels of HDL‐, LDL‐, total cholesterol and the ratio of HDL/total cholesterol (). These values were lower in 1986 (positive history of infection) than in 1983 (non‐infection group) as reported earlier using multifactorial analysis Citation18. When using Z‐scores HDL‐cholesterol proved to be significantly decreased (P = 0.01). BMI values were normal in both groups and the Z‐scores were similar ().
Table II. Serum cholesterol concentrations and BMI values in unknown history of infection (1983) and in history of infection (1986) groups, the actual values and the Z–score values adjusted for age and sex, n = 106.
Step 2 of analysis
In 59 subjects investigated both in 1983 and 1986 and having non‐elevated levels of APPs (CRP and orosomucoid) but positive history of infection in 1986, the HDL‐cholesterol Z‐scores were significantly lower during infection in 1986 (). There were no differences in BMI Z‐scores.
Table III. Cholesterol parameters in 59 subjects with positive history of infection and normal levels of APPs compared to their values during probable infection‐free period (1986 values compared to 1983 values). APPs not known.
Step 3 of analysis
In 47 subjects with elevated CRP ⩾ 5mg/L or orosomucoid ⩾ 1.1g/L in 1986 and probably normal (not measured) values in 1983. The difference in HDL Z‐score was even higher than that based on the grouping based on the history alone (0.49 as compared to 0.19). BMI actual values were normal and the Z‐scores did not differ ().
Table IV. Cholesterol parameters in 47 subjects with positive history of infection and elevated APPs compared to values during probable infection‐free period (1986 values compared to 1983 values) and APPs not known. CRP⩾5 mg/l or orosomucoid⩾1.1 g/l is APPs positive.
Step 4 of analysis
In 1986 serum CRP or orosomucoid concentrations were elevated in 47/106 subjects with positive history of infection and were normal in 94/106 of age‐and sex‐matched healthy controls. The Z‐score difference of 0.79 (P = 0.0001) in serum HDL‐cholesterol was especially high. Even total cholesterol concentration was significantly decreased (Z‐score difference 0.61, P = 0.002). Actual values of BMI were normal and Z‐scores similar ().
Table V. Cholesterol and albumin concentrations in 1986 in subjects: A. With positive history of infection and elevated APPs (n = 47). B. With positive history of infection (n = 106). C. With no history of infection and non‐elevated APPs (n = 94).
Mean serum albumin concentration was 46 g/L in controls and 42 g/L in infection group in 1986 (P<0.0001). There was no significant correlation between serum albumin concentration values and HDL‐cholesterol Z‐score values.
Discussion
Comparing the concentrations of serum lipids in different study years may lead to misleading interpretation. By using the Z‐scores it was possible to take into account the effects of age, sex, weight and cohort. The effect of seasonal variation was ruled out by doing the studies during the same time of the year. The Z‐scores of HDL‐cholesterol were lower in subjects when history of infection was positive as compared to their earlier ‘non‐infection‐associated values’. The difference between the values increased with increasing evidence of inflammation. The underlying mechanism seems to be inflammation as evidenced by the high association of lowered HDL‐cholesterol concentration to inflammation markers CRP and orosomucoid. In addition, serum albumin concentration was lower during the post‐infection period. Theoretically, repeated bouts of infection with temporarily increased risk factor levels could increase the risk for later CHD as suggested by Ross and Glomset Citation19. APPs are elevated but not very high suggesting viral infection or past bacterial infection Citation20.
Infections are usually documented by the appearance of symptoms; however, history alone is quite a poor indicator. Patients may exaggerate or neglect their symptoms, do not remember them or infections have been totally subclinical. Therefore, the real influence of infections is somewhat larger than analyses based on the history alone. This is supported by the present findings. When elevated APPs were used as an objective evidence of a past infection, the change of the HDL‐cholesterol was most significant. Especially HDL‐cholesterol decreased during an infection. BMI was normal in all groups and there were no differences in BMI Z‐scores. Obesity increases CRP but there were no obese children in any group. In an earlier study in patients with severe infections, the decrease in LDL‐cholesterol concentration was maximal during an acute phase of infection and the decrease of the HDL‐cholesterol concentration during the convalescence phase 4 weeks after the infection Citation5.
The pro‐atherogenic changes of lipoproteins may establish a link between infection/inflammation and atherosclerosis. Lipid alterations may be associated with the host's response to infection mediated by various cytokines. A large number of cytokines including tumour necrosis factor‐α (TNF‐α), interleukins and interferon decrease serum HDL‐cholesterol level Citation21. Alterations in concentrations, composition and metabolism of plasma proteins associated with HDL metabolism (apolipoprotein A‐I, serum amyloid A, transferrin etc.) could decrease the ability of HDL to protect against atherogenesis through its antioxidation and reverse cholesterol transport mechanisms Citation22. The susceptibility of LDL to oxidation increases during the acute phase response after viral infection, which may increase the risk for atherosclerotic clinical events Citation23. Acute infections in children have been shown to be accompanied by oxidative modification of LDL‐cholesterol Citation24. Oxidized LDL is a risk factor for CHD Citation25. Unfortunately, oxidized LDL was not measured in our samples.
Albumin is a protein molecule with half‐life of 26 days. Albumin has numerous physiological functions, which include maintenance of blood osmotic pressure and capillary permeability. Physiological concentrations of albumin selectively inhibit TNF‐α induced vascular cell adhesion molecule‐1 (VCAM‐1) expression, monocyte adhesion and nuclear factor‐KB (NF‐KB) activation in human aortic endothelial cells Citation26. Disrupted micro‐vascular barrier during infection may lead to albumin leakage.
One can speculate that episodes of infections will lead to endothelial cell damage, increased insudation of albumin through the arterial wall and repeated bouts of subendothelial oedema. In animals treated repeatedly with intravenous cholesterol or adrenalin, the oedematous changes may progress markedly and lead to irreversible damage (27).
Low serum albumin is reported to be associated with an increased mortality rate among healthy subjects without evidence of inflammation (28). Djousse and colleagues found that low levels of serum albumin and serum bilirubin are associated with a greater than expected risk of myocardial infarction (29). Increasing of serum albumin levels has been suggested as an effective strategy to lower cardiovascular risk Citation26.
The limitations of our study are that both false‐negative and false‐positive infection cases are included in the groupings, but this kind of error cannot be totally avoided, even if antibody titres had been measured. It was not possible to conclude whether the long‐term changes in serum lipids were persistent or transient as we have done blood sampling only once. It was impossible to distinguish whether the infections were viral or bacterial, even though the quite low APPs sugest viral etiology. The lack of baseline values for APPs, of definite information about infections in 1983, and the small size of groups with both positive history and elevated APPs are limitations.
Conclusion
Mild infections especially with elevated APPs are associated with lowered serum albumin and serum HDL‐cholesterol concentrations. Decreased concentrations of protective HDL‐cholesterol in combination with decreased serum albumin levels could promote the risk of atherosclerosis and CHD.
Acknowledgements
Grants were received from Lund University Hospital, Sweden and The Academy of Finland (grant number 53392), the Juho Vainio Foundation, and the research grant from Turku University Central Hospital, Finland.
References
- Pesonen E., Liuba P. Footprints of atherosclerotic coronary heart disease in children. Rev Port Cardiol 2004; 23: 127–31
- Libby P., Egan D., Skarlatos S. Role of infectious agents in atherosclerosis and restenosis: an assessment of the evidence and need for future research. Circulation 1997; 96: 4095–103
- Zhu J., Quyyumi A. A., Norman J. E., Csako G., Waclawiw M. A., Shearer G. M. Effects of total pathogen burden on coronary artery disease risk and C‐reactive protein levels. Am J Cardiol 2000; 85: 140–6
- Kovanen P. T. Atheroma formation: defective control in the intimal round‐trip of cholesterol. Eur Heart J 1990; 11((Suppl E))238–46
- Sammalkorpi K., Valtonen V., Kerttula Y., Nikkilä E., Taskinen M‐R. Changes in serum lipoprotein pattern induced by acute infections. Metabolism 1988; 119: 557–61
- Djousse L., Rothman K. J., Cupples L. A., Levy D., Ellison R C. Serum albumin and risk of myocardial infarction and all cause mortality in the Framingham offspring study. Circulation 2002; 106: 2919–24
- Gabay C., Kushner I. Acute–phase proteins and other systemic responses to inflammation. N Engl J Med 1999; 340: 448–54
- Zoellner H., Hofler M., Beckman R., Hufnagl P., Vanyek E., Wojta J., et al. Serum albumin is a specific inhibitor of apoptosis in human endothelial cells. J Cell Sci 1996; 109: 2571–80
- Belayev L., Zhao W., Pattany P. M., Weaver R. G., Huh P. W., Lin B., et al. Diffusion weighted magnetic resonance imaging confirms marked neuroprotective efficacy of albumin therapy in focal cerebral ischemia. Stroke 1998; 29: 2587–99
- Kuller L. H., Eicner J. E., Orcard T. J., Grandits G. A., McCallum, Tracy R. P. The relation between serum albumin levels and risk of coronary heart disease in the multiple risk factor intervention trial. Am J Epidemiol 1991; 134: 1266–77
- Honkanen V. E., McCrindle B. W., Laxer R. M., Feldman B. M., Schneider R., Silverman E. D. Clinical relevance of the risk factors for coronary artery inflammation in Kawasaki disease. Pediatr Cardiol 2003; 24: 122–6
- Viikari J., Rönnemaa T., Seppänen A., Marniemi J., Porkka K., Räsänen L., et al. Serum lipids and lipoproteins in children, adolescents and young adults in. 1980–1986. Ann Med 1991; 23: 53–9
- Siegel J., Schlumberger H., Klose S., Ziegenhorn J., Wahlefeld A. W. Improved reagent for the enzymatic determination of serum cholesterol. J Clin Chem Clin Biochem 1981; 19: 838–9
- Kostner G. M. Enzymatic determination of cholesterol in high density lipoprotein fractions prepared by polyanion precipitation. Clin Chem 1976; 22: 695
- Friedewald W. T., Levy R., Fredrickson D. S. Estimation of the concentration of low density lipoprotein cholesterol in plasma without use of preparative ultracentrifuge. Clin Chem 1972; 18: 499–502
- Dati F., Schumann G., Thomas L., Aguzzi F., Baudner S., Bienvenu J., et al. Consensus of a group of professional societies and diagnostic companies on guidelines for interim reference ranges for 14 proteins in serum based on the standardization against the IFCC/BCR/CAP Reference material (CRM 470). International federation of Clinical Chemistry. Community Bureau of Reference of the Commission of the European Communities. College of American Pathologists. Eur J Clin Chem Clin Biochem 1996; 34: 517–20
- SAS/STAT User's Guide. SAS Institute Inc, Cary NC 1998
- Pesonen E., Rapola J., Viikari J., Turtinen J., Åkerblom H. K. Altered serum lipid profile after systemic infection in children: risk factor for CHD?. Eur Heart J 1993; 14((Suppl K))7–11
- Ross R., Glomset J. A. The pathogenesis of atherosclerosis. N Engl J Med 1976; 295: 369–77 and 420–5
- Korppi M. Non‐specific host response markers in the differentiation between pneumococcal and viral pneumonia: What is the most accurate combination?. Pediatr Int 2004; 46: 545–50
- Feingold K. R., Grunfeld C. Role of cytokines in inducing hyperlipidemia. Diabetes 1992; 41((Suppl 2))97–101
- Khovidhunkit W., Memon R. A., Feingold K. R., Grunfeld C. Infection and inflammation‐induced proatherogenic changes of lipoproteins. J Infect Dis 2000; 181((Suppl 3))462–72
- Van Lenten B. J., Wagner A. C., Nayak D. P., Hama S., Navab M., Fogelman A. M. High‐density lipoprotein loses its anti‐inflammatory properties during acute influenza A infection. Circulation 2001; 103: 2283–8
- Liuba P., Persson J., Luoma J., Ylä‐Herttuala S., Pesonen E. Acute infections in children are accompanied by oxidative modification of LDL and decrease of HDL cholesterol, and are followed by thickening of carotid intima‐media. Eur Heart J 2003; 24: 515–21
- Laaksonen R., Janatuinen T., Vesalainen R., Lehtimäki T., Elovaara I., Jaakkola O., et al. High oxidized LDL and elevated plasma homocysteine contribute to the early reduction of myocardial flow reserve in healthy adults. Eur J Clin Invest 2002; 32: 795–802
- Zhang W. J., Frei B. Albumin selectively inhibits TNFalpha–induced expression of vascular cell adhesion molecule–1 in human aortic endothelial cells. Cardiovasc Res 2002; 55: 820–9
- Shimamoto T. The relationship of edematous reaction in arteries to atherosclerosis and thrombosis. J Atheroscler Res 1963; 3: 87–102
- Reuben D. B., Ferrucci L., Wallace R., Tracy R. B., Corti M. C., Heimovitz H., et al. The prognostic value of serum albumin in healthy older persons with low and high serum interleukin–6 (IL–6) levels. J Am Geriatr Soc 2000; 48: 1404–7
- Djousse L., Rothman K. J., Cupples L. A., Levy D., Ellison C. Effects of serum Albumin and Bilirubin on the Risk of Myocardial Infarction (the Framingham Offspring Study). Am J Cardiol 2003; 91: 485–8
Erratum
Magliano et al: How to best define the metabolic syndrome
Annals of Medicine. 2006;38:34–41
Page 36, Line 18. The word “medicated” should be “mediated”.
Page 37, Table 1. In column one, to the left: “waist‐hip ratio” should read “waist:hip ratio”. In the note below the table “the American college of Endocrinology Task Force on the Insulin Resistance Syndrome” should be deleted. “International Diabetes Association” should be changed to “International Diabetes Federation”.