Abstract
Airway mucus hypersecretion is now recognized as a key pathophysiological feature in many patients with asthma, chronic obstructive pulmonary disease (COPD) and cystic fibrosis. Consequently, it is important to develop drugs that inhibit mucus hypersecretion in these susceptible patients. Conventional therapies, including anticholinergics, β2‐adrenoceptor agonists, corticosteroids, mucolytics and macrolide antibiotics, have variable efficacy in inhibiting airway mucus hypersecretion, and are less effective in COPD than in asthma. Novel pharmacotherapeutic targets are being investigated, including inhibitors of nerve activity (e.g. large conductance calcium‐activated potassium, BKCa, channel activators), tachykinin receptor antagonists, epoxygenase inducers (e.g. benzafibrate), inhibitors of mucin exocytosis (e.g. anti‐myristoylated alanine‐rich C kinase substrate (MARCKS), peptide and Munc‐18B blockers), inhibitors of mucin synthesis and goblet cell hyperplasia (e.g. epidermal growth factor (EGF), receptor tyrosine kinase inhibitors, p38 mitogen‐activated protein (MAP), kinase inhibitors, MAP kinase kinase/extracellular signal‐regulated kinase (MEK/ERK), inhibitors, human calcium‐activated chloride (hCACL2), channel blockers and retinoic acid receptor‐α antagonists), inducers of goblet cell apoptosis (e.g. Bax inducers or Bcl‐2 inhibitors), and purinoceptor P2Y2 antagonists to inhibit mucin secretion or P2Y2 agonists to hydrate secretions. However, real and theoretical differences delineate the mucus hypersecretory phenotype in asthma from that in COPD. More information is required on these differences to identify specific therapeutic targets which, in turn, should lead to rational design of anti‐hypersecretory drugs for treatment of airway mucus hypersecretion in asthma and COPD.
Introduction
Mucus secretion in the airways normally represents first‐line defence of the respiratory tract and is an important part of innate immunity. However, if secretion becomes abnormal, mucus accumulates and may obstruct the airway lumen (). Long‐term and excessive mucus production, termed chronic mucus hypersecretion, can lead to significant airflow limitation in a number of severe respiratory conditions including asthma Citation1, chronic obstructive pulmonary disease (COPD) Citation2,3 and cystic fibrosis (CF) Citation4. It should be noted, however, that the impact of airway mucus hypersecretion on morbidity and mortality is not unequivocal or pertinent to all patients. Nevertheless, there are certain groups of patients, for example those with COPD who have mucus hypersecretion and are prone to chest infections Citation5, where a causal association has been demonstrated. Consequently, there is the perception that inhibiting mucus hypersecretion should have clinical benefit in hypersecretory conditions of the airways. Numerous pharmaceutical and other treatments are available or in development that are aimed, either directly or indirectly, at inhibiting mucus hypersecretion. However, although asthma and COPD share mucus obstruction as a clinical feature, the pathophysiological mechanisms underlying the impairment in mucus clearance may be different, to a greater or lesser extent, for each condition. For example, there are real and theoretical differences in the pathophysiology of airway mucus hypersecretion between asthma and COPD (). In addition, the features of airway inflammation and remodelling that uniquely characterize different respiratory diseases are also factors that influence mucus hypersecretion. Consequently, no single therapy is likely to be effective across the spectrum of mucus obstructive conditions of the respiratory tract. It is possible that disease specific therapy will be more successful.
Figure 1. Mucus obstruction of the airways in asthma and COPD: gross pathology. A: Lung from an asthmatic patient cut through to show gelatinous plugs (P) in the large airways (arrows). Courtesy Dr Catherine Corbishley (thumb, T, and finger, F, holding specimen). B: Luminal mucus (M) partially blocking an extrapulmonary bronchus (arrow) in a long‐term elderly male cigarette smoker with chronic sputum production.
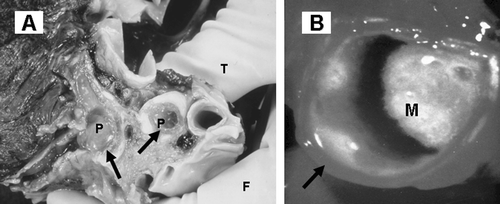
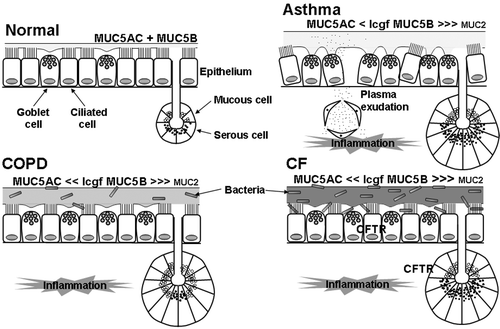
Figure 2. Putative differences in pathophysiology of the airway mucus hypersecretory phenotype in asthma, chronic obstructive pulmonary disease(COPD) and cystic fibrosis (CF). Compared with normal, in asthma there is airway inflammation, increased luminal mucus, with an increased ratio of MUC5B (low charge glycoform (lcgf)) to MUC5AC, possibly small amounts of MUC2 present in the mucus, epithelial ‘fragility’ with loss of ciliated cells, marked goblet cell hyperplasia, submucosal gland hypertrophy (although without a marked increase in mucous to serous ratio), ‘tethering’ of mucus to goblet cells, and plasma exudation. In COPD, there is airway inflammation, increased luminal mucus, goblet cell hyperplasia, submucosal gland hypertrophy (with an increased proportion of mucous to serous acini), an increased ratio of lgcf MUC5B to MUC5AC above that in asthma, possibly small amounts of MUC2 in the mucus, and a susceptibility to infection. In CF, there is airway inflammation, increased luminal mucus, goblet cell hyperplasia, submucosal gland hypertrophy, an increased ratio of lgcf MUC5B to MUC5AC, small amounts of MUC2 in the mucus, and a marked susceptibility to infection. Many of these differences require confirmation (or otherwise) by data from greater numbers of subjects.
The present review discusses airway mucus hypersecretion, using asthma and COPD as examples of respiratory conditions that share excess airway mucus as a clinical feature but with specific differences in mucus pathophysiology. Cystic fibrosis will be briefly mentioned where appropriate for comparative purposes as a third respiratory condition with its own pathophysiology and mucus problems. However, the mucus problems in CF pertain predominantly to the genetic defect in the cystic fibrosis transmembrane conductance regulator (CFTR) and the associated abnormality in sodium channels, and are beyond the scope of the present article. The interested reader is referred to two recent reviews Citation4,Citation6. Factors involved in the rational design of pharmacotherapeutic compounds to inhibit airway mucus hypersecretion in asthma and COPD will be considered. A brief description of airway mucus is given first.
Airway mucus, mucins and MUC genes
Airway luminal mucus is a complex dilute aqueous solution of lipids, glycoconjugates and proteins. It comprises salts, enzymes and anti‐enzymes, oxidants and antioxidants, exogenous bacterial products, endogenous antibacterial agents, cell‐derived mediators and proteins, plasma‐derived mediators and proteins, and cell debris such as DNA. Airway mucus is considered to form a liquid bi‐layer whereby an upper gel layer floats above a lower, more watery sol, or periciliary liquid, layer Citation6. The gel layer traps particles and is moved on the tips of the cilia. Respiratory tract mucus requires the correct combination of viscosity and elasticity for optimal efficiency of ciliary interaction. Viscoelasticity is conferred on the mucus primarily by high molecular weight mucous glycoproteins, termed mucins, which comprise up to 2% by weight of the mucus Citation7. In the airways, mucins are produced by goblet cells in the epithelium Citation8 and sero‐mucous glands in the submucosa Citation9. Mucins are thread‐like molecules comprising a linear peptide sequence (termed apomucin), often with tandemly repeated regions, that is highly glycosylated, predominantly via O‐linkages (). Apomucins are encoded by specific mucin (MUC) genes, with 19 human MUC genes currently recognized, namely MUC1, 2, 3A, 3B, 4, 5AC, 5B, 6–9, 11–13 and 16–20 Citation10–15. Of these, however, it is only the MUC5AC and MUC5B gene products that comprise the major gel‐forming mucins in respiratory secretions from normal subjects. The mucin content of secretions from patients with hypersecretory respiratory diseases may differ from normal (see below).
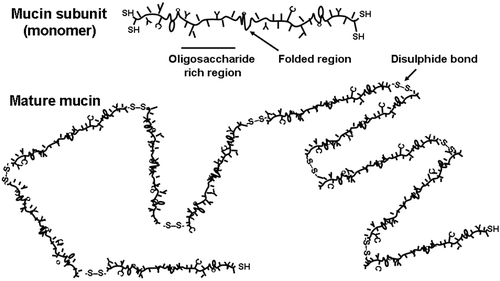
Figure 3. Schematic representation of a gel‐forming mucin molecule. The mucin subunit (∼500 nm in length) comprises an amino acid backbone with highly glycosylated areas and folded regions, stabilized via disulphide bonds, with little or no glycosylation. Glycosylation is via O‐linkages and is highly diverse. In secretions, the subunits are joined end‐to‐end by disulphide bonds (S‐S) into long thread‐like mature mucin molecules.
Key messages
Airway mucus hypersecretion is now recognized as an important contributor to morbidity and mortality in many patients with severe chronic lung diseases.
The airway mucus hypersecretory phenotypes of asthma, COPD and cystic fibrosis have many important differences.
Effective novel pharmacotherapy of airway mucus hypersecretion may have to be disease specific.
Mucus obstruction of the airways in asthma and COPD
Asthma and COPD are chronic, severe inflammatory conditions of the airways in which mucus hypersecretion is a pathophysiological feature. The increase in airway luminal mucus is associated with increases in amount of mucus‐secreting tissue.
Asthma
Asthma is a chronic inflammatory condition of the airways that has specific clinical and pathophysiological features Citation16, including mucus obstruction of the airways Citation17 (). The latter is particularly evident in a proportion of patients who die in status asthmaticus, where many airways are occluded by tenacious mucus plugs Citation18–20. There is also more mucus in the central and peripheral airways of both chronic and severe asthmatics compared with control subjects Citation21. The increased amount of luminal mucus reflects an increase in amount of airway secretory tissue, due to both goblet cell hyperplasia Citation21,22, and submucosal gland hypertrophy Citation19, although the latter is not characteristic of all patients with asthma Citation21.
COPD
COPD comprises three overlapping conditions, namely chronic obstructive bronchitis (commonly associated with increased airway mucus hypersecretion), chronic bronchiolitis (small airways disease) and emphysema (airspace enlargement due to alveolar destruction) Citation23. The following discussion considers the ‘bronchitic’ component of COPD. The airways of patients with COPD contain excessive amounts of mucus Citation24, which is markedly increased above that in control subjects Citation25,26 (). The increased mucus is linked specifically with an increase in MUC5B in the bronchiolar lumen Citation27. The excessive luminal mucus is associated with increased amounts of mucus‐secreting tissue, namely goblet cell hyperplasia Citation24,Citation28,29 and submucosal gland hypertrophy Citation24,25,Citation30,31. The amount of gland correlates with the amount of luminal mucus Citation25.
Differences in pathophysiology relating to mucus obstruction in asthma, COPD and CF
In order to develop appropriate models of airway mucus hypersecretion and develop drugs to inhibit hypersecretion, it is necessary to understand the similarities and differences in the features of mucus obstruction for different hypersecretory conditions. There are a number of differences in the pathophysiology of mucus hypersecretion between asthma, COPD and CF (). Firstly, although the underlying pulmonary inflammation of asthma and COPD share many common features, there are specific characteristics unique to each condition Citation32–35. Asthma is invariably an allergic disease that affects the airways, rather than the lung parenchyma, and is characterized by Th2‐lymphocytic orchestration of pulmonary eosinophilia. In contrast, COPD is currently perceived as predominantly a neutrophilic disorder governed largely by macrophages and epithelial cells. It is associated primarily with cigarette smoking. The differences between asthma and COPD in their profile of airway inflammation and remodelling may in turn exert different influences on the development of airway mucus obstruction in the two conditions.
Airway mucus in asthma is more viscous than in COPD or CF, with the airways of asthmatic patients tending to develop, and subsequently become blocked by, gelatinous ‘mucus’ plugs Citation17. Whether or not mucus in asthma has an intrinsic biochemical abnormality is unclear. In general terms, sputum from patients with asthma is more viscous than that from patients with chronic bronchitis or bronchiectasis Citation36–38. Mucus plugs in asthma differ from airway mucus gels in chronic bronchitis and CF in that they are stabilized by non‐covalent interactions between extremely large mucins assembled from conventionally sized subunits Citation39. This suggests an intrinsic defect in assembly of the mucin molecules, and could account for the increased viscosity of the mucus plugs in asthma. Plug formation may also be due, at least in part, to increased airway plasma exudation in asthma compared with COPD Citation40. In addition, and in direct contrast to COPD, exocytosed mucins in asthma are not released fully from the goblet cells, leading to ‘tethering’ of luminal mucins to the airway epithelium Citation41. This tethering may also contribute to plug formation. One explanation of mucus tethering is that, in COPD, neutrophil proteases cleave goblet cell‐attached mucins, whereas in asthma the inflammatory cell profile, predominantly an eosinophilia, does not generate the appropriate proteases to facilitate mucin release.
Different MUC gene products may be present in respiratory secretions in asthma and COPD. MUC5AC and a low‐charge glycoform of MUC5B are the major mucin species in airway secretions from patients with asthma, COPD and CF Citation42–46. There is significantly more of the low‐charge glycoform of MUC5B in the respiratory diseases than in normal control secretions Citation46. Interestingly, there is a proportional increase in the MUC5B mucin over the MUC5AC mucin in airway secretions from patients with COPD or CF, compared with secretions from patients with asthma Citation42. The significance of the change in MUC5B glycoforms to bacterial colonization between the different diseases is unclear. However, it is interesting that it is observed in COPD and CF, where patients are prone to bacterial chest infections Citation4,Citation23, rather than in asthma where patients are not notably prone to infection.
In contrast to normal airways, there is upregulation of MUC5AC in the airway epithelium of patients with COPD compared with smoking (and non‐smoking) controls Citation27. Goblet cells in the airways from patients with COPD contain not only MUC5AC but also MUC5B Citation44,Citation47 and MUC2 Citation7,Citation48. The latter distribution is different to that in the airways of patients with asthma or CF, where MUC5AC and MUC5B show a similar localization to that in normal subjects Citation49,50. It is noteworthy that although MUC2 is located in goblet cells in irritated airways, and MUC2 mRNA is found in the airways of smokers Citation26, MUC2 mucin is either not found in airway secretions from normal subjects or patients with chronic bronchitis Citation43, or is found only in very small amounts in asthma, COPD and CF Citation46,Citation51. The significance of the above combined observations is unclear, but suggests that there are differences in goblet cell phenotype between asthma and COPD.
Another notable difference between asthma and COPD is in the bronchial submucosal glands Citation52. In asthma, although hypertrophied, the glands are morphologically normal with an even distribution of mucous and serous cells. In contrast, in chronic bronchitis, gland hypertrophy is characterized by an increased number of mucous cells relative to serous cells, particularly in severe bronchitis Citation52.
From the above, it may be seen that there are theoretical and actual differences in the nature of airway mucus between asthma and COPD. How these differences relate to pathophysiology and clinical symptoms in the two conditions is, for the most part, unclear. However, these dissimilarities suggest that different treatments may be required for effective treatment of airway mucus obstruction in asthma and COPD.
Pharmacotherapy of airway mucus hypersecretion
The prevalence of patient presentation with cough and expectoration of sputum and the perceived importance of mucus in the pathophysiology of severe lung conditions, such as asthma and COPD, have led to development of drugs intended to treat airway mucus hypersecretion Citation53. There are two objectives for treatment of airway mucus obstruction: short‐term relief of symptoms and long‐term benefit (). Pharmacotherapy of airway mucus hypersecretion in asthma and COPD has been discussed in detail recently Citation17,Citation54 and is summarized below and in . Essentially, pharmacotherapy divides into two sections. The first is anti‐inflammatory treatment of airway inflammation, probably the most beneficial therapy overall. The second is therapies directed specifically at different aspects of the pathophysiology of mucus hypersecretion, from inhibition of the secretagogue effects of specific endogenous inflammatory secretagogues to aiding expectoration of sputum by use of mucolytics.
Table I. Objectives for effective pharmacotherapy of mucus pathophysiology in chronic airway hypersecretory diseases.
Table II. Potential therapeutic targets and inhibitors of airway mucus hypersecretion.
Conventional pharmacotherapy
The medications used currently in clinical management of COPD and asthma, namely bronchodilators (anticholinergics, β2‐adrenoceptor agonists and methylxanthines) and anti‐inflammatories (primarily glucocorticosteroids), are not administered necessarily to target airway hypersecretion, but may nevertheless exert some of their beneficial effects via actions on mucus Citation55.
Decreasing the viscosity of airway mucus with mucolytic drugs should improve mucus clearance, both by mucociliary transport and by cough. However, although numerous mucolytic drugs are available worldwide, their effectiveness in treatment of asthma and COPD has not been clearly established Citation56. Similarly, although antibiotics are recommended in clinical management of exacerbations of COPD, there is no evidence for prophylactic antibiotic treatment of stable COPD Citation23,Citation57.
Novel pharmacotherapy
There is a range of novel pharmacotherapeutics in development for inhibition of airway mucus hypersecretion, including inhibitors of mucin exocytosis and inhibitors of goblet cell hyperplasia ().
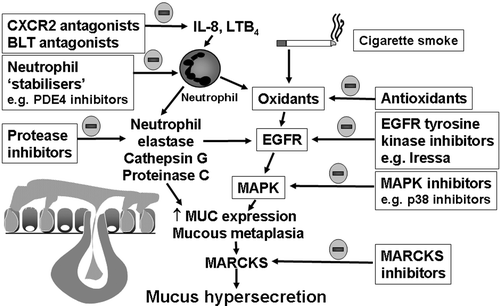
Figure 4. Possibilities for novel inhibition of airway mucus hypersecretion in chronic obstructive pulmonary disease(COPD). Theoretically, recruitment and activation of neutrophils and inhalation of cigarette smoke increases the lung burden of proteases and oxidants, which in turn activate the epidermal growth factor (EGF) intracellular signalling pathway leading to increased mucin (MUC) gene expression and mucin synthesis, mucous metaplasia (goblet cell hyperplasia and submucosal gland hypertrophy) and increased mucin secretion (via myristoylated alanine‐rich C kinase substrate (MARCKS)). The neutrophil chemotactic activity of interleukin (IL)‐8 and leukotriene (LT) B4 can be inhibited by selective antagonists at the receptors for CXC chemokines (CXCR2 antagonists) and for LTB4 (BLT antagonists), respectively, and neutrophil activity can be inhibited by inhibitors of phosphodiesterase (PDE) 4. The activity of proteases and oxidants can be inhibited by protease inhibitors and antioxidants. The EGF receptor and mitogen activated protein kinase cascade, as well as MARCKS activity, can be inhibited by appropriate inhibitors.
Exocytosis inhibitors
Inhibition of mucin exocytosis is a therapeutic option for mucus hypersecretion in asthma and COPD. Myristoylated alanine‐rich C kinase substrate (MARCKS) protein is a key intracellular molecule involved in intracellular movement and exocytosis of mucin granules Citation58. Blockade of MARCKS by a synthetic peptide to its N‐terminal region (MANS peptide) inhibited mucin secretion by normal human bronchial epithelial cells in vitroCitation58 and by mouse airway epithelium in vivoCitation59. The Sec1/Munc18 family are critical to exocytosis in airway goblet cells, and experimental induction of Munc18B (mammalian homologue of the nematode unc‐18 gene product, also known as syntaxin‐binding protein 1) induces a marked airway hypersecretory phenotype Citation60. Clostridium botulinum neurotoxins (BoNT) inhibit neurotransmitter (e.g. acetylcholine) release by cleaving soluble N‐ethyl‐maleimide‐sensitive factor attachment protein receptor (SNARE) proteins in the nerve terminal Citation61. Conversely, BoNTs could be re‐targeted to airway secretory cells via a fusion ligand to selectively inhibit mucin exocytosis, and thereby reduce mucus output. For example, the LHN fragment (representing the light chain and the N‐terminal domain of the heavy chain coupled via a single disulphide bond) of BoNT‐C fused to EGF (termed EGF‐LHN‐C) as targeting ligand for the EGF‐R on airway epithelial cells markedly inhibits stimulated mucin output from a human respiratory epithelial cell line Citation62.
Inhibition of mucin synthesis and goblet cell hyperplasia
Increased MUC gene expression, mucin synthesis and goblet cell hyperplasia appear to be linked processes that are regulated by a number of inflammatory mechanisms. For example, airway epidermal growth factor receptor (EGF‐R) upregulation and signalling via EGF‐R tyrosine kinase is a key signalling event for induction of mucin synthesis and goblet cell hyperplasia Citation63,64. Inhibitors of EGF‐R tyrosine kinase block these responses. One of these, gefitinib (Iressa), is in clinical trial for cancer, but not yet for asthma or COPD.
The p38 mitogen‐activated protein (MAP) kinase pathway, the MEK/ERK pathway, and the phosphatidylinositol 3‐kinase pathway are all involved, to a greater or lesser extent, in intracellular events leading to mucin synthesis and goblet cell hyperplasia Citation65–68. Inhibitors of these pathways inhibit mucus hypersecretory endpoints in experimental systems.
Calcium‐activated chloride (CLCA) channels appear to be critically involved in development of an airway hypersecretory phenotype Citation69. In mice, suppression of mCLCA3 inhibits goblet cell hyperplasia, whilst overexpression increases goblet cell number Citation70. Talniflumate is a small molecule putative inhibitor of hCLCA1 which is currently being developed as a mucoregulatory treatment for asthma and COPD Citation71.
Retinoic acid (vitamin A) acting on the RAR‐α receptor appears to be involved in mucin expression Citation72–74 and in the development and maintenance of a hypersecretory phenotype Citation74. RAR‐α antagonists, such as RO‐41‐5253, inhibit a number of these activities Citation75.
Antisense technology is also being explored as a new approach to inhibition of goblet cell hyperplasia. For example, an 18‐mer MUC antisense oligomer suppressed mucin gene expression and wood smoke‐induced epithelial metaplasia in rabbit airways Citation76.
Hyperplastic airway goblet cells in COPD models express the antiapoptotic factor Bcl‐2 Citation77. Reduction of Bcl‐2 expression by antisense oligonucleotides induces a dose‐dependent resolution of hyperplasia.
P2Y2 agonists and antagonists
Adenosine 5'‐triphosphate (ATP) and uridine triphosphate (UTP) increase airway mucin and water secretion via interaction with P2Y2 purinoceptors Citation78,79. Consequently, P2Y2 antagonists might inhibit airway hypersecretion Citation80. However, mucus hydration is associated with improved mucociliary clearance, and stimulation of water secretion may have greater therapeutic potential than inhibition of P2Y2‐mediated mucin secretion Citation6. Consequently, there is considerable interest in development of P2Y2 agonists Citation78.
Conclusions
Mucus secretion in the airways is a vital homeostatic mechanism that protects the respiratory tract from a barrage of inhaled insult. However, abnormal production of mucus can contribute to respiratory disease. Airway obstruction by mucus is a common feature of a number of severe respiratory conditions, including asthma, COPD and CF. These diseases share pulmonary inflammation and remodelling as a pathophysiological characteristic. They also each have a number of unique features that characterize their airway mucus obstruction. For example, mucus plug formation and mucus tethering are features of asthma, whereas submucosal gland hypertrophy with a disproportionate increase in the ratio of mucous to serous cells is a significant feature in COPD. Understanding of the relative importance of the differences and similarities in the pathophysiology of mucus obstruction between different respiratory diseases should lead to rational development of therapeutic interventions.
References
- Del Donno M., Bittesnich D., Chetta A., Olivieri D., Lopez‐Vidriero M. T. The effect of inflammation on mucociliary clearance in asthma: an overview. Chest 2000; 118: 1142–9
- Houtmeyers E., Gosselink R., Gayan‐Ramirez G., Decramer M. Regulation of mucociliary clearance in health and disease. Eur Respir J 1999; 13: 1177–88
- Maestrelli P., Saetta M., Mapp C. E., Fabbri L. M. Remodeling in response to infection and injury. Airway inflammation and hypersecretion of mucus in smoking subjects with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 164: S76–S80
- Davis P. B. Cystic fibrosis. Pediatr Rev 2001; 22: 257–64
- Prescott E., Lange P., Vestbo J. Chronic mucus hypersecretion in COPD and death from pulmonary infection. Eur Respir J 1995; 8: 1333–8
- Knowles M. R., Boucher R. C. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest 2002; 109: 571–7
- Davies J. R., Herrmann A., Russell W., Svitacheva N., Wickström C., Carlstedt I. Respiratory tract mucins: structure and expression patterns. Novartis Foundation Symposium 248. Mucus Hypersecretion in Respiratory Disease. John Wiley & Sons, Chichester 2002; pp. 76–93
- Rogers D. F. The airway goblet cell. Int J Biochem Cell Biol 2003; 35: 1–6
- Finkbeiner W. E. Physiology and pathology of tracheobronchial glands. Respir Physiol 1999; 118: 77–83
- Moniaux N., Escande F., Porchet N., Aubert J. P., Batra S. K. Structural organization and classification of the human mucin genes. Front Biosci 2001; 6: D1192–D1206
- Dekker J., Rossen J. W., Buller H. A., Einerhand A. W. The MUC family: an obituary. Trends Biochem Sci 2002; 27: 126–31
- Gum J. R., Jr., Crawley S. C., Hicks J. W., Szymkowski D. E., Kim Y. S. MUC17, a novel membrane‐tethered mucin. Biochem Biophys Res Commun 2002; 291: 466–75
- Wu G. J., Wu M. W., Wang S. W., Liu Z., Qu P., Peng Q., et al. Isolation and characterization of the major form of human MUC18 cDNA gene and correlation of MUC18 over‐expression in prostate cancer cell lines and tissues with malignant progression. Gene 2001; 279: 17–31
- Chen Y., Zhao Y. H., Kalaslavadi T. B., Hamati E., Nehrke K., Le A. D., et al. Genome‐wide search and identification of a novel gel‐forming mucin MUC19/Muc19 in glandular tissues. Am J Respir Cell Mol Biol 2004; 30: 155–65
- Higuchi T., Orita T., Nakanishi S., Katsuya K., Watanabe H., Yamasaki Y., et al. Molecular cloning, genomic structure, and expression analysis of MUC20, a novel mucin protein, up‐regulated in injured kidney. J Biol Chem 2004; 279: 1968–79
- Eapen S. S., Busse W. W. Asthma. Clin Allergy Immunol 2002; 16: 325–53
- Rogers D. F. Airway mucus hypersecretion in asthma: an undervalued pathology?. Curr Opin Pharmacol 2004; 241–50, 4
- Houston J. C., De Navasquez S., Trounce J. R. A clinical and pathological study of fatal cases of status asthmaticus. Thorax 1953; 8: 207–13
- Dunnill M. S. The pathology of asthma with special reference to changes in the bronchial mucosa. J Clin Pathol 1960; 13: 27–33
- Saetta M., Di Stefano A., Rosina C., Thiene G., Fabbri L. M. Quantitative structural analysis of peripheral airways and arteries in sudden fatal asthma. Am Rev Respir Dis 1991; 143: 138–43
- Aikawa T., Shimura S., Sasaki H., Ebina M., Takishima T. Marked goblet cell hyperplasia with mucus accumulation in the airways of patients who died of severe acute asthma attack. Chest 1992; 101: 916–21
- Ordonez C. L., Khashayar R., Wong H. H., Ferrando R., Wu R., Hyde D. M., et al. Mild and moderate asthma is associated with airway goblet cell hyperplasia and abnormalities in mucin gene expression. Am J Respir Crit Care Med 2001; 163: 517–23
- National Heart Lung and Blood Institute, World Health Organisation. Global initiative for chronic obstructive lung disease. Publication number 2701. Bethesda National Institutes of Health. 2001
- Reid L. Pathology of chronic bronchitis. Lancet 1954; 266: 274–8
- Aikawa T., Shimura S., Sasaki H., Takishima T., Yaegashi H., Takahashi T. Morphometric analysis of intraluminal mucus in airways in chronic obstructive pulmonary disease. Am Rev Respir Dis 1989; 140: 477–82
- Steiger D., Fahy J., Boushey H., Finkbeiner W. E., Basbaum C. Use of mucin antibodies and cDNA probes to quantify hypersecretion in vivo in human airways. Am J Respir Cell Mol Biol 1994; 10: 538–45
- Caramori G., Di Gregorio C., Carlstedt I., Casolari P., Guzzinati I., Adcock I. M., et al. Mucin expression in peripheral airways of patients with chronic obstructive pulmonary disease. Histopathology 2004; 45: 477–84
- Saetta M., Turato G., Baraldo S., Zanin A., Braccioni F., Mapp C. E., et al. Goblet cell hyperplasia and epithelial inflammation in peripheral airways of smokers with both symptoms of chronic bronchitis and chronic airflow limitation. Am J Respir Crit Care Med 2000; 161: 1016–21
- Mullen J. B., Wright J. L., Wiggs B. R., Pare P. D., Hogg J. C. Structure of central airways in current smokers and ex‐smokers with and without mucus hypersecretion: relationship to lung function. Thorax 1987; 42: 843–8
- Reid L. Measurement of the bronchial mucous gland layer: a diagnostic yardstick in chronic bronchitis. Thorax 1960; 15: 132–41
- Restrepo G., Heard B. E. The size of the bronchial glands in chronic bronchitis. J Pathol Bacteriol 1963; 85: 305–10
- Jeffery P. K. Differences and similarities between chronic obstructive pulmonary disease and asthma. Clin Exp Allergy 1999; 29 Suppl 2: 14–26
- Saetta M., Turato G., Maestrelli P., Mapp C. E., Fabbri L. M. Cellular and structural bases of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 163: 1304–9
- Djukanovic R. Airway inflammation in asthma and its consequences: implications for treatment in children and adults. J Allergy Clin Immunol 2002; 109 Suppl 6: S539–S548
- Barnes P. J. Mediators of chronic obstructive pulmonary disease. Pharmacol Rev 2004; 56: 515–48
- Lopez‐Vidriero M. T., Reid L. Chemical markers of mucous and serum glycoproteins and their relation to viscosity in mucoid and purulent sputum from various hypersecretory diseases. Am Rev Respir Dis 1978; 117: 465–77
- Charman J., Reid L. Sputum viscosity in chronic bronchitis, bronchiectasis, asthma and cystic fibrosis. Biorheology 1972; 9: 185–99
- Shimura S., Sasaki T., Sasaki H., Takishima T., Umeya K. Viscoelastic properties of bronchorrhoea sputum in bronchial asthmatics. Biorheology 1988; 25: 173–9
- Sheehan J. K., Richardson P. S., Fung D. C., Howard M., Thornton D. J. Analysis of respiratory mucus glycoproteins in asthma: a detailed study from a patient who died in status asthmaticus. Am J Respir Cell Mol Biol 1995; 13: 748–56
- Rogers D. F., Evans T. W. Plasma exudation and oedema in asthma. Br Med Bull 1992; 48: 120–34
- Shimura S., Andoh Y., Haraguchi M., Shirato K. Continuity of airway goblet cells and intraluminal mucus in the airways of patients with bronchial asthma. Eur Respir J 1996; 9: 1395–401
- Thornton D. J., Carlstedt I., Howard M., Devine P. L., Price M. R., Sheehan J. K. Respiratory mucins: identification of core proteins and glycoforms. Biochem J 1996; 316: 967–75
- Hovenberg H. W., Davies J. R., Herrmann A., Linden C. J., Carlstedt I. MUC5AC, but not MUC2, is a prominent mucin in respiratory secretions. Glycoconj J 1996; 13: 839–47
- Wickstrom C., Davies J. R., Eriksen G. V., Veerman E. C., Carlstedt I. MUC5B is a major gel‐forming, oligomeric mucin from human salivary gland, respiratory tract and endocervix: identification of glycoforms and C‐terminal cleavage. Biochem J 1998; 334: 685–93
- Sheehan J. K., Howard M., Richardson P. S., Longwill T., Thornton D. J. Physical characterization of a low‐charge glycoform of the MUC5B mucin comprising the gel‐phase of an asthmatic respiratory mucous plug. Biochem J 1999; 338: 507–13
- Kirkham S., Sheehan J. K., Knight D., Richardson P. S., Thornton D. J. Heterogeneity of airways mucus: variations in the amounts and glycoforms of the major oligomeric mucins MUC5AC and MUC5B. Biochem J 2002; 361: 537–46
- Chen Y., Zhao Y. H., Di Y. P., Wu R. Characterization of human mucin 5B gene expression in airway epithelium and the genomic clone of the amino‐terminal and 5′‐flanking region. Am J Respir Cell Mol Biol 2001; 25: 542–53
- Davies J. R., Carlstedt I. Respiratory tract mucins. Cilia and Mucus: from Development to Respiratory Defense., M Salthe. Marcel Dekker, Inc., New York 2001; p. 167–78, In
- Groneberg D. A., Eynott P. R., Lim S., Oates T., Wu R., Carlstedt I., et al. Expression of respiratory mucins in fatal status asthmaticus and mild asthma. Histopathology 2002; 40: 367–73
- Groneberg D. A., Eynott P. R., Oates T., Lim S., Wu R., Carlstedt I., et al. Expression of MUC5AC and MUC5B mucins in normal and cystic fibrosis lung. Respir Med 2002; 96: 81–6
- Davies J. R., Svitacheva N., Lannefors L., Kornfalt R., Carlstedt I. Identification of MUC5B, MUC5AC and small amounts of MUC2 mucins in cystic fibrosis airway secretions. Biochem J 1999; 344: 321–30
- Glynn A. A., Michaels L. Bronchial biopsy in chronic bronchitis and asthma. Thorax 1960; 15: 142–53
- Houtmeyers E., Gosselink R., Gayan‐Ramirez G., Decramer M. Effects of drugs on mucus clearance. Eur Respir J 1999; 14: 452–67
- Rogers D. F. The role of airway secretions in COPD: pathophysiology, epidemiology and pharmacotherapeutic options. COPD: J Chron Obstructive Pulm Dis 2005; 2: 341–53
- Rogers D. F. Mucociliary dysfunction in COPD: effect of current pharmacotherapeutic options. Pulm Pharmacol Ther 2005; 18: 1–8
- Rogers D. F. Mucoactive drugs for asthma and COPD: any place in therapy?. Expert Opin Investig Drugs 2002; 11: 15–35
- National Institute for Clinical Excellence (NICE). Chronic obstructive pulmonary disease: national clinical guideline on management of chronic obstructive pulmonary disease in adults in primary and secondary care. Thorax 2004; 59((Suppl 1))1–232
- Li Y., Martin L. D., Spizz G., Adler K. B. MARCKS Protein Is a Key Molecule Regulating Mucin Secretion by Human Airway Epithelial Cells in Vitro. J Biol Chem 2001; 276: 40982–90
- Singer M., Martin L. D., Vargaftig B. B., Park J., Gruber A. D., Li Y., et al. A MARCKS‐related peptide blocks mucus hypersecretion in a mouse model of asthma. Nat Med 2004; 10: 193–6
- Evans C., Kheradmand F., Corry D., Tuvim M., Densmore C., Waldrep C., et al. Gene therapy of mucus hypersecretion in experimental asthma. Chest 2002; 121((3 Suppl))90S–91S
- Foster K. A. A new wrinkle on pain relief: re‐engineering clostridial neurotoxins for analgesics. Drug Discov Today 2005; 10: 563–9
- Ford E., Cruttwell C., Nute E., Chaddock J., Barnes P., Sutton J., et al. Inhibition of mucin secretion from A549 cells using a re‐targeted clostridial endopeptidase. Proc Am Thor Soc 2005; 2: A219
- Burgel P. R., Nadel J. A. Roles of epidermal growth factor receptor activation in epithelial cell repair and mucin production in airway epithelium. Thorax 2004; 59: 992–6
- O'Donnell R. A., Richter A., Ward J., Angco G., Mehta A., Rousseau K., et al. Expression of ErbB receptors and mucins in the airways of long term current smokers. Thorax 2004; 59: 1032–40
- Longphre M., Li D., Li J., Matovinovic E., Gallup M., Samet J. M., et al. Lung mucin production is stimulated by the air pollutant residual oil fly ash. Toxicol Appl Pharmacol 2000; 162: 86–92
- Shim J. J., Dabbagh K., Takeyama K., Burgel P. R., Dao‐Pick T. P., Ueki I. F., et al. Suplatast tosilate inhibits goblet‐cell metaplasia of airway epithelium in sensitized mice. J Allergy Clin Immunol 2000; 105: 739–45
- Wang B., Lim D. J., Han J., Kim Y. S., Basbaum C. B., Li J. D. Novel cytoplasmic proteins of nontypeable Haemophilus influenzae up‐regulate human MUC5AC mucin transcription via a positive p38 mitogen‐activated protein kinase pathway and a negative phosphoinositide 3‐kinase‐Akt pathway. J Biol Chem 2002; 277: 949–57
- Atherton H. C., Jones G., Danahay H. IL‐13‐induced changes in the goblet cell density of human bronchial epithelial cell cultures: MAP kinase and phosphatidylinositol 3‐kinase regulation. Am J Physiol Lung Cell Mol Physiol 2003; 285: L730–L9
- Zhou Y., Shapiro M., Dong Q., Louahed J., Weiss C., Wan S., et al. A calcium‐activated chloride channel blocker inhibits goblet cell metaplasia and mucus overproduction. Mucus Hypersecretion in Respiratory Disease. Wiley, Chichester 2002; p. 150–65
- Nakanishi A., Morita S., Iwashita H., Sagiya Y., Ashida Y., Shirafuji H., et al. Role of gob‐5 in mucus overproduction and airway hyperresponsiveness in asthma. Proc Natl Acad Sci U S A 2001; 98: 5175–80
- Knight D. Talniflumate (Genaera). Curr Opin Investig Drugs 2004; 5: 557–62
- Guzman K., Gray T. E., Yoon J. H., Nettesheim P. Quantitation of mucin RNA by PCR reveals induction of both MUC2 and MUC5AC mRNA levels by retinoids. Am J Physiol 1996; 271: L1023–8
- Yoon J. H., Gray T., Guzman K., Koo J. S., Nettesheim P. Regulation of the secretory phenotype of human airway epithelium by retinoic acid, triiodothyronine, and extracellular matrix. Am J Respir Cell Mol Biol 1997; 16: 724–31
- Koo J. S., Jetten A. M., Belloni P., Yoon J. H., Kim Y. D., Nettesheim P. Role of retinoid receptors in the regulation of mucin gene expression by retinoic acid in human tracheobronchial epithelial cells. Biochem J 1999; 338: 351–7
- Apfel C., Bauer F., Crettaz M., Forni L., Kamber M., Kaufmann F., et al. A retinoic acid receptor alpha antagonist selectively counteracts retinoic acid effects. Proc Natl Acad Sci U S A 1992; 89: 7129–33
- Bhattacharyya S. N., Manna B., Smiley R., Ashbaugh P., Coutinho R., Kaufman B. Smoke‐induced inhalation injury: effects of retinoic acid and antisense oligodeoxynucleotide on stability and differentiated state of the mucociliary epithelium. Inflammation 1998; 22: 203–14
- Tesfaigzi Y., Fischer M. J., Martin A. J., Seagrave J. Bcl‐2 in LPS‐ and allergen‐induced hyperplastic mucous cells in airway epithelia of Brown Norway rats. Am J Physiol Lung Cell Mol Physiol 2000; 279: L1210–17
- Kellerman D. J. P2Y(2) receptor agonists: a new class of medication targeted at improved mucociliary clearance. Chest 2002; 121((5 Suppl))201S–05S
- Roger P., Gascard J. P., Bara J., de Montpreville V. T., Yeadon M., Brink C. ATP induced MUC5AC release from human airways in vitro. Mediators Inflamm 2000; 9: 277–84
- Jackson A. D. Airway goblet‐cell mucus secretion. Trends Pharmacol Sci 2001; 22: 39–45