Abstract
Background. Platelet activation is involved in the pathogenesis of the thrombotic complications of hypertension. Novel surrogate markers of platelet activation (mean platelet volume (MPV), mean platelet component (MPC, measure of platelet density), platelet component distribution width (PCDW, a marker of platelet shape change) and the number of platelet clumps) have been related to cardiovascular risk. We hypothesized a stepwise increase in these platelet activation indices between healthy controls (HC, n = 60), ‘high‐risk’ essential hypertensive subjects (HBP, n = 45) and treated, previously diagnosed patients with malignant phase hypertension (MHT, n = 45).
Methods. In a cross‐sectional study, we measured comparative platelet counts and indices of platelet activation (MPV, MPC, PCDW and the number of platelet clumps) using the Bayer ADVIATM haematology system, in our three study groups.
Results. There was a stepwise increase in MPV (P = 0.0002) and MPM (P = 0.03), and a stepwise decrease in the MPC (P = 0.03) and PCDW (P = 0.001) across the three study groups, despite similarities in platelet count. These differences were only significantly different (on post‐hoc analysis) between the healthy controls and the MHT group. On multivariate analysis, there was a significant relationship (R2 = 66.5%; P<0.0001) between the MPV and the PCDW (P<0.0001), systolic blood pressure (P = 0.008) and platelet count (P<0.0001).
Conclusion. There is a stepwise increase in platelet activation indices, despite similar platelet counts, with increasing severity of hypertensive disease. This may contribute to the pathogenesis of thrombosis‐related complications in hypertension.
Introduction
Despite the blood vessels being exposed to elevations in blood pressure, the complications of hypertension are paradoxically thrombotic (e.g. ischaemic stroke, myocardial infarction), rather than haemorrhagic Citation1,2. Platelet activation plays a key mechanistic role in the pathophysiology of hypertension‐related thrombotic events and its adverse clinical sequelae Citation1,2.
Platelet activation leads to platelet shape change (increasingly spherical), swelling (increase in platelet mass and volume), degranulation with consequent adhesion and aggregation (with increasing platelet‐clumping) Citation3. Degranulation is characterized by the translocation of α‐granules, containing P‐selectin (Psel, or CD62P, an adhesion molecule) to the platelet surface, with a consequent reduction in platelet density Citation3. Whilst a number of ex vivo markers of platelet activation exist (e.g. surface and soluble Psel and β‐thromboglobulin), they have not been widely adopted into everyday clinical practice. This relates, in part, to the prolonged and laborious processing involved in the derivation of these indices coupled with the recognized marked temporal variations in platelet activation indices with storage Citation4.
Automated blood cell counters have gained widespread clinical use and provide a number of novel platelet parameters in addition to traditional complete blood counts. This has created the advent of rapid assessment of platelet activation using whole blood, without the need for complicated specimen preparation. The ADVIATM 120 system generates a variety of derived platelet indices that are increasingly recognized as surrogate markers of platelet activation and include the mean platelet volume (MPV), mean platelet mass (MPM), the mean platelet component (MPC), the platelet component distribution width (PCDW) and the number of platelet clumps (a marker of platelet aggregation) Citation5.
Of these indices, the MPV has been the most extensively studied in the literature. MPV is increased in patients with a number of cardiovascular diseases, such as essential hypertension Citation6–8, diabetes mellitus Citation9 and stroke Citation10,11. Furthermore, increasing MPV has been linked to worsening cardiovascular outcomes Citation11,12.
Malignant phase hypertension (MHT) is a rare form of hypertension characterized by arteriolar fibrinoid necrosis, which carries a higher risk of intravascular thrombotic complications (e.g. stroke, myocardial infarction) than non‐malignant hypertension Citation13,14.
We hypothesized a stepwise increase in indices of platelet activation (MPV, MPC, PCDW and the number of platelet clumps) between healthy controls, ‘high‐risk’ essential hypertensive subjects and treated, previously diagnosed patients with malignant phase hypertension.
Key messages
Platelet activation is involved in the pathogenesis of the thrombotic complications of hypertension.
Novel surrogate markers of platelet activation (mean platelet volume (MPV), mean platelet component (MPC, measure of platelet density), platelet component distribution width (PCDW, a marker of platelet shape change) and the number of platelet clumps) have been related to cardiovascular risk.
In this study, there is a stepwise increase in platelet activation indices, despite similar platelet counts, with increasing severity of hypertensive disease.
Platelet abnormalities may contribute to the pathogenesis of thrombosis‐related complications in hypertension.
Methods
We performed a cross‐sectional study of ‘high‐risk’ subjects with essential hypertension (HBP, n = 45) and patients with treated, previously diagnosed malignant phase hypertension (MHT, n = 45), who were compared to healthy controls (HC, n = 60); all three groups were similar in relation to age, sex, ethnicity and smoking status. The two hypertensive groups were additionally matched for the use of antiplatelet therapy, systolic blood pressure as well as for the presence of associated cardiovascular disease
Healthy controls were defined following a detailed clinical history and physical examination, with normal baseline blood tests including full blood count, renal function, fasting glucose and lipid profile. For the HBP group, we included patients with treated and known HBP, who had one or more of the following cardiovascular risk factors: known coronary artery disease; diabetes mellitus; age >55 years; peripheral vascular disease; current smokers with a known family history of coronary artery disease. We also included a cohort of patients with previously diagnosed MHT; the latter was defined clinically by the presence of severe hypertension in association with bilateral retinal haemorrhages, cotton wool spots or exudates, with or without papilloedema on fundoscopy and retinal photography Citation13.
All HBP patients were clinically stable and treated (>3 months), and under regular follow‐up in either our specialist MHT clinic or general HBP clinic. We excluded patients with any of the following: a history of liver disease (liver enzymes >2× upper limit normal); dialysis or with a serum creatinine >200 µmol/L; any malignancy; recent (<3 months) arterial or venous thromboembolic disease; patients with active infections; a history of inflammatory or connective tissue disorders; and those with uncontrolled blood pressure greater than 200/120 mmHg. All study subjects provided written informed consent to the study, which was approved by the West Birmingham Research Ethics Committee.
Blood pressure was recorded using an automated blood pressure machine (Omron model 705CP) with the patient seated, and an average of the last two of three readings from the non‐dominant arm was used Citation15. Blood was collected via clean venepuncture using a 21‐G needle and Vacutainer (Becton Dickinson, UK) into glass K3 ethylenediaminetetraacetic acid (EDTA) tubes, with gentle mixing of the blood, prior to immediate processing at room temperature. The first 4 mL of blood was discarded to avoid the effects of traces of thrombin generation during venepuncture. All whole‐blood samples were processed with 20 minutes of collection.
Analysis of platelet indices
Analysis of platelet indices was performed using the flow cytometry capabilities of the ADVIATM120 haematology analyser (Bayer Diagnostics, USA). This analyser determines the volume and reflectivity index (RI) of platelets on a cell‐by‐cell basis. Platelet analysis is performed by the measurement of the intensity of scattered light at two discrete angular ranges of 2° to 3° and 5° to 15°, which is then converted to approximate measures of platelet volume and RI respectively Citation16 These are in turn converted to a variety of surrogate platelet activation indices, which include the MPV (derived from the platelet volume histogram), MPC (a measurement of platelet density, calculated directly from the RI). MPM (calculated from the platelet dry mass histogram = mean of platelet volume × platelet content/100), the platelet PCDW (a measure of the variation in platelet shape change = MPC × 100/standard deviation of MPC) and the number of platelet clumps Citation17. The PCDW is a measure of the variation of platelet size and the MPC is a measure of platelet density. The normal ranges for these values have been published previously Citation18. The coefficient of variation (COV) for duplicate analyses of platelet activation was <5% for all the platelet indices studied, with exception of the number of platelet clumps median (median COV 13%).
Power calculation
Coban et al. Citation6 demonstrated significantly increased MPV among patients with both essential (n = 36) and white coat hypertension (n = 36), compared with age and sex matched healthy controls (n = 36). Our group has previously demonstrated significant increases in MPV among 42 hypertensives compared with 30 age, sex and ethically matched controls Citation7. Based on these studies, we calculated that a sample size of 45 patients in each of the three patient groups would provide at least 80% power to detect a >5% increase in MPV on comparing healthy controls, HBP and MHT patient groups respectively.
Statistical analysis
Data was analysed using GraphPad InStat version 3.05 for Windows (GraphPad Software, San Diego California USA; www.graphpad.com). After determination of normality (using the Kolmogorov‐Smirnov test) for all continuous data sets, appropriate parametric and non‐parametric tests were utilized. All continuous data are presented as mean (SD, standard deviation) when normally distributed, or as the median (IQR, interquartile range) for non‐parametric data. The Kruskal‐Wallis (non‐parametric) and one‐way analysis of variance (ANOVA) tests were utilized for comparisons of more than two groups with appropriate post‐hoc corrections performed, when appropriate. The Mann‐Whitney and unpaired t‐test were used for unpaired comparisons between two groups of continuous data, as appropriate. For categorical variables, the chi‐square and Fisher's exact test were used where appropriate. Multiple linear regression analysis was performed to determine the influence of several baseline factors (based on the results of univariate analysis) on the dependent variable of MPV amongst the patients with hypertension. A two‐tailed P‐value <0.05 was considered statistically significant for all comparisons.
Results
We studied 60 healthy controls, 45 patients with HBP and 45 patients with treated previous MHT. The three groups were well matched for age, sex, ethnicity and smoking status (see ). The two hypertensive groups were also well matched for all baseline characteristics (including cardiovascular disease comorbidity) with the exception of serum creatinine, which was significantly higher in the MHT group (Table ).
Table I. Baseline demography and clinical features.
Platelet indices
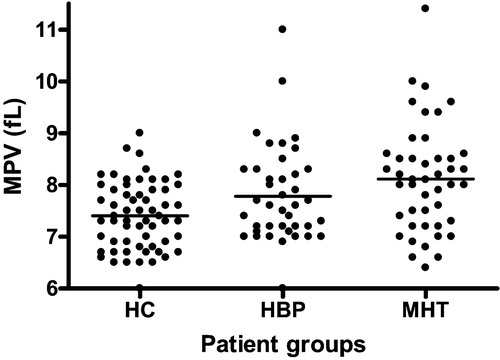
There was no significant difference in the platelet counts between the healthy controls and the two disease groups (). There was an overall significant increase in MPV () and MPM levels, and a significant decrease in the MPC and PCDW levels, across the three study cohorts (). On post‐hoc analysis, the difference between the groups was only significant for MPV, between the healthy controls and HBP groups and between the healthy controls and MHT groups. We performed additional post‐tests for linear trend between the three groups for all of the platelet activation indices. There were significant linear trends towards increases in the MPV and MPM and similar decreases in MPC and PCDW across the healthy controls, HBP and MHT patients respectively (P<0.0001) ().
Table II. Comparative platelet indices among the three patient groups.
Table III. Assessment for linear trend between the various platelet activation indices across the three patient groups.
Determinants of MPV
We performed univariate and multivariate regression analysis on the combined hypertensive patient group (n = 90) in order to determine the factors influencing the dependent variable of MPV. The variables found to be significantly associated with MPV on univariate analysis were the systolic blood pressure, the use of antiplatelet treatment, the total platelet count, MPC, MPM and the PCDW (see ).
Table IV. Univariate determinants of mean platelet volume.
We performed a multivariate analysis to assess the influence of platelet count, PCDW, systolic blood pressure and the effects of antiplatelet therapy on the MPV. We deliberately excluded MPC and MPM from the multivariate analysis, in order to avoid multicollinearity, as these variables are mathematically linked to the MPV. On multivariate analysis, there was a significant relationship between the MPV and the PCDW (P<0.0001), systolic blood pressure (P = 0.008) and platelet count (P<0.0001) (overall R2 = 66.5%; P<0.0001).
Discussion
We have shown increases in platelet activation indices across two differing hypertensive patient groups compared with matched healthy controls, despite similar platelet counts. Treated MHT patients had significantly higher MPV and MPM, with lower MPC and PCDW, compared to matched, healthy controls.
The exact mechanisms in support of increasing MPV as a surrogate for increasing platelet activation are not fully known. The increases MPV may relate to one or more of a combination of increased platelet swelling with activation, increased life‐span of larger platelets Citation19 and the increased production of larger and more active platelet precursors (platelet ‘left shift’) from the bone marrow Citation20. It is still not known whether an increasing MPV represents the collective platelet response to prothrombotic stimuli (clinical or subclincial) or whether it represents a greater pathophysiological role for the larger platelet in thrombogenesis. This ‘cause versus response’ question is somewhat academic, but may have important clinical implications. Indeed, the early detection of platelet activation might be useful in identifying patients at increased thrombotic risk prior to the onset of overt cardiovascular disease, such that early pre‐emptive strategies can be implemented.
The role of additional platelet markers such as the MPC, PCDW, MPM and the number of platelet clumps is being increasingly recognized. There are data to support their relationship to other established markers of platelet activation. Psel (CD62P) is a platelet α‐granule component of platelets that is redistributed to the plasma membrane during platelet activation and degranulation Citation4. Its expression has been shown to be inversely correlated with the PCDW Citation21 and the MPC Citation22–25 using the ADVIATM 120 analyser. Also, the density of the platelets decreases upon activation due to the release of Psel from α‐granules which are expressed on the surface and into the plasma Citation26,27. The relationship of platelet‐clumping to platelet activation has been less well studied, but Kratz et al. Citation17 noted significantly increased platelet‐clumping (a surrogate marker of aggregation) and decreasing MPC following endurance exercise, supporting a potential direct relationship between clumping and increased platelet activation.
Our results are in keeping with the three previous studies that have investigated the comparative differences in platelet activation indices among patients with HBP compared with healthy controls. They showed significant increases in MPM and MPV Citation6–8 and reductions in platelet density Citation8 amongst patients with hypertension versus healthy controls, despite similar platelet counts. Furthermore, the study by Nadar et al. Citation8 observed greater platelet activation (higher MPM and MPV and lower mean platelet density) in their subgroup of hypertensive patients with target organ disease compared with those without, again despite similar platelet counts.
This is the first paper to examine automated indices of platelet activation in a group of patients with MHT. Previous data have shown that despite early aggressive treatment, MHT is associated with a 20% risk of cardiovascular death at 5 years Citation13. Furthermore, it has been demonstrated that even treated MHT patients, at >11 years beyond their original diagnosis, have more evidence of endothelial damage/dysfunction, compared with matched non‐malignant hypertensives Citation14. This is important as endothelial damage/dysfunction is a critical step in the initiation and pathogenesis of vascular thrombosis.
The use of routine haematology analysers to measure platelet activation status circumvents many of the frequently encountered technical problems associated with traditional methods, such fluorescence microscopy (to detect activation specific markers), platelet aggregometry and β‐thromboglobulin/platelet factor 4 immunoassays Citation4. A profile of several potentially useful platelet activation indices, as well as a complete full blood count, can be gained within one minute of sample aspiration of whole blood. The ADVIATM 120 system is also an automated bench‐top system that provides reproducible and reliable results. However, whilst there does appear to be a relationship between a number of ADVIATM120‐derived platelet activation indices and increasing cardiovascular risk, data supporting their relationship to markers of platelet function (e.g. bleeding time, using platelet aggregometry or activated closure times) are lacking.
This study is limited by its cross‐sectional design. To fully appreciate the effects of disease activity on platelet activation, a longitudinal study would have been preferable, with additional investigation of the effects of medical treatment. This is potentially important as a number of drugs, such as angiotensin receptor blockers and statins, have been shown to have favourable effects on platelet activation Citation28,29. Secondly, whilst we observed overall significant differences in the markers of platelet activation across the three groups (with the exception of the number of platelet clumps) the source of these differences varied on post‐hoc analysis, and we cannot exclude the possibility that these differences might have reached statistical significance with a greater sample size. However, we do demonstrate significant linear trends across the three study groups. Given restrictions on sample size and the difficulty in recruiting acute, recent‐onset MHT patients, it is simply not possible to account for all of the possible potential confounders that may influence platelet activation indices. Finally, the demonstration of increased ex vivo markers of platelet activation, among patients with hypertension, does not necessarily equate to causation. Nevertheless, it does provide further potential pathophysiological insights into the links between platelet activation and hypertension.
In conclusion, we have observed a stepwise increase in platelet activation indices, derived from the ADVIATM 120 analyser, despite similar platelet counts, with increasing severity of hypertensive disease. This may contribute to the pathogenesis of thrombosis‐related complications in hypertension.
Acknowledgements
We acknowledge the financial support of the Sandwell and West Birmingham Hospitals NHS Trust Research and Development programme for the Haemostasis, Thrombosis and Vascular Biology Unit. We also wish to thank Dr A. Blann for statistical advice.
References
- Lip G. Y. H. Hypertension, platelets, and the endothelium: the “thrombotic paradox” of hypertension (or “Birmingham paradox”) revisited. Hypertension 2003; 41: 199–200
- Nadar S., Lip G. Y. The prothrombotic state in hypertension and the effects of antihypertensive treatment. Curr Pharm Des 2003; 9: 1715–32
- Ruf A., Patscheke H. Flow cytometric detection of activated platelets: comparison of determining shape change, fibrinogen binding, and P‐selectin expression. Semin Thromb Hemost 1995; 21: 146–51
- Gurney D., Lip G. Y., Blann A. D. A reliable plasma marker of platelet activation: does it exist?. Am J Hematol 2002; 70: 139–44
- Ahnadi C. E., Boughrassa F. F., Chapman‐Montgomery E. S., Poisson V., Gervais A., Okrongly D., et al. Comparison of two methods to assess variability of platelet response to anti‐platelet therapies in patients with acute coronary syndrome undergoing angioplasty. Thromb Haemost 2004; 92: 1207–13
- Coban E., Yazicioglu G., Berkant Avci A., Akcit F. The mean platelet volume in patients with essential and white coat hypertension. Platelets 2005; 16: 435–8
- Nadar S., Blann A. D., Lip G. Y. Platelet morphology and plasma indices of platelet activation in essential hypertension: effects of amlodipine‐based antihypertensive therapy. Ann Med 2004; 36: 552–7
- Nadar S., Blann A. D., Lip G. Y., Coban E., Yazicioglu G., Berkant Avci A., et al. Platelet indexes in relation to target organ damage in high‐risk hypertensive patients: a substudy of the Anglo‐Scandinavian Cardiac Outcomes Trial (ASCOT). J Am Coll Cardiol 2004; 44: 415–22
- Papanas N., Symeonidis G., Maltezos E., Mavridis G., Karavageli E., Vosnakidis T., et al. Mean platelet volume in patients with type 2 diabetes mellitus. Platelets 2004; 15: 475–8
- Nadar S. K., Lip G. Y., Blann A. D. Platelet morphology, soluble P selectin and platelet P‐selectin in acute ischaemic stroke. The West Birmingham Stroke Project. Thromb Haemost 2004; 92: 1342–8
- Greisenegger S., Endler G., Hsieh K., Tentschert S., Mannhalter C., Lalouschek W. Is elevated mean platelet volume associated with a worse outcome in patients with acute ischemic cerebrovascular events?. Stroke 2004; 35: 1688–91
- Martin J. F., Bath P. M., Burr M. L. Influence of platelet size on outcome after myocardial infarction. Lancet 1991; 338: 1409–11
- Lip G. Y., Beevers M., Beevers G. The failure of malignant hypertension to decline: a survey of 24 years' experience in a multiracial population in England. J Hypertens 1994; 12: 1297–305
- Lip G. Y., Edmunds E., Hee F. L., Blann A. D., Beevers D. G. A cross‐sectional, diurnal, and follow‐up study of platelet activation and endothelial dysfunction in malignant phase hypertension. Am J Hypertens 2000; 14: 823–8
- Williams B., Poulter N. R., Brown M. J., Davis M., McInnes G. T., Potter J. F., Sever P. S., et al. British Hypertension Society. Guidelines for management of hypertension: report of the fourth working party of the British Hypertension Society, 2004‐BHS IV. J Hum Hypertens 2004; 18: 139–85
- Harris N., Kunicka J., Kratz A. The ADVIA 2120 hematology system: flow cytometry‐based analysis of blood and body fluids in the routine hematology laboratory. Lab Hematol 2005; 11: 47–61
- Kratz A., Wood M. J., Siegel A. J., Hiers J. R., Van Cott E. M. Effects of marathon running on platelet activation markers: direct evidence for in vivo platelet activation. Am J Clin Pathol 2006; 125: 296–300
- Hamm C. W., Lorenz R. L., Bleifeld W., Kupper W., Wober W., et al. Biochemical evidence of platelet activation in patients with persistent unstable angina. J Am Coll Cardiol 1987; 10: 998–1006
- Thompson C. B., Love D. G., Quinn P. G., Valeri C. R. Platelet size does not correlate with platelet age. Blood 1983; 62: 487–94
- Tschoepe D., Roesen P., Esser J., Schwippert B., Nieuwenhuis H. K., Kehrel B., et al. Large platelets circulate in an activated state in diabetes mellitus. Semin Thromb Hemost 1991; 17: 433–8
- Lim Y. A., Hyun B. H. Evaluation of platelet parameters on the ADVIA 120 as the quality indicator for stored platelets. Clin Lab Haematol 2002; 24: 377–84
- Van Cott E. M., Fletcher S. R., Kratz A. Effects of the blood‐collection tube material and long‐term storage on platelet activation parameters on the ADVIA 120/2120 hematology system. Lab Hematol 2005; 11: 71–5
- Macey M. G., Carty E., Webb L., Chapman E. S., Zelmanovic D., Okrongly D., et al. Use of mean platelet component to measure platelet activation on the ADVIA 120 haematology system. Cytometry 1999; 38: 250–5
- Ahnadi C. E., Sabrinah Chapman E., Lepine M., Okrongly D., Pujol‐Moix N., Hernandez A., et al. Assessment of platelet activation in several different anticoagulants by the Advia 120 Hematology System, fluorescence flow cytometry, and electron microscopy. Thromb Haemost 2003; 90: 940–8
- Chapman E. S., Sorette M., Hetherington E., Zelmanovic D., Kling G., Dugailliez J., et al. A rapid, automated flow cytometric method to measure activated degranulated platelets by density determination. Thromb Haemost 2003; 89: 1004–15
- Corash L., Tan H., Gralnick H. R. Heterogeneity of human whole blood platelet subpopulations. I. Relationship between buoyant density, cell volume, and ultrastructure. Blood 1977; 49: 71–87
- Mezzano D., Hwang K., Catalano P., Aster R. H. Evidence that platelet buoyant density, but not size, correlates with platelet age in man. Am J Hematol 1981; 11: 61–76
- Chrysant S. G., Chrysant G. S. The pleiotropic effects of angiotensin receptor blockers. J Clin Hypertens (Greenwich) 2006; 8: 261–8
- Varughese G. I., Patel J. V., Lip G. Y., Varma C. Novel concepts of statin therapy for cardiovascular risk reduction in hypertension. Curr Pharm Des 2006; 12: 1593–609