Abstract
Background. Behcet's disease (BD) is characterized with remissions and exacerbations. However, to date, there is no study to investigate a possible association of disease activity (active versus inactive disease period) with cardiovascular complications.
Methods. Forty patients with BD were evaluated in both active and in inactive disease period. For the control group 45 healthy volunteers, age and sex matched, were registered. Subjects with at least a 15‐day lesion‐free period were regarded in inactive disease period, and subjects with any oral, skin, and/or genital lesion was regarded as in active disease period. In each subject coronary diastolic peak flow velocities (DPFV) were measured at baseline and after dipyridamole infusion (0.84 mg/kg over 6 minutes) using an Acuson Sequoia C256® echocardiography system. Coronary flow reserve (CFR) was defined as the ratio of hyperemic to baseline DPFV.
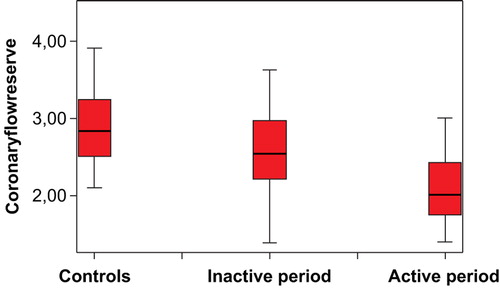
Results. CFR values were significantly lower in BD patients compared to the controls (2.57±0.50 versus 2.87±0.53, P = 0.006). In active disease period, basal DPFV (24.6±7.5 versus 27.3±6.6, P = 0.019) was significantly higher than in the inactive disease period. In the active disease period hyperemic DPFV (61.7±14.9 versus 56.8±16.7, P = 0.015) values decreased significantly. Therefore, in the active disease period CFR significantly decreased from 2.57±0.50 to 2.09±0.46, P<0.001. The only independent predictor of CFR within the active disease period was the disease duration (β = −0.384, P = 0.012).
Conclusion. Within the active disease period, coronary microvascular function is more prominently impaired in BD patients. Therefore, BD patients are possibly more vulnerable to cardiovascular manifestations when they are in an active disease period.
| Abbreviations | ||
| BD | = | Behcet's disease |
| TDHE | = | transthoracic second harmonic Doppler echocardiography |
| LAD | = | left anterior descending coronary artery |
| CFR | = | coronary flow reserve |
| hsCRP | = | high‐sensitivity C‐reactive protein |
| ESR | = | ERYTHROCYTE SEDIMENTATION RATE |
| BMI | = | body mass index |
| HDL | = | high‐density lipoprotein |
| LDL | = | low‐density lipoprotein |
| DPFV | = | diastolic peak flow velocity |
| NO | = | nitric oxide |
Introduction
Behcet's disease (BD) is a chronic, multisystem inflammatory disorder with relapsing oral aphthous ulcers, skin lesions, ocular lesions, and genital ulcers. Vascular involvement in BD is usually recognized as an unclassified vasculitis, and it involves both veins and arteries of all sizes. Furthermore, coronary vascular involvement resulting in acute myocardial infarction without coronary atherosclerosis has been reported Citation1–3. Several studies have suggested endothelial dysfunction in patients with BD through measurement of flow‐mediated dilation of brachial artery which is an indirect evidence of the initiation of coronary vascular atherosclerosis Citation4,5. Additionally, in BD patients with angiographically normal coronary arteries, increased prevalence of silent myocardial ischemia has been reported Citation6,7.
Oral aphthous ulcers, skin lesions, ocular lesions, and genital ulcers are the diagnostic determinants and primary symptoms of BD. However, to date, there is no study to investigate the possible association between skin and/or mucosal lesions and cardiovascular involvement. Because these lesions are indicatives of disease activity, it is possible that they might coincide with cardiovascular involvement.
Evolved recently, transthoracic second harmonic Doppler echocardiography (TDHE) is capable of measuring coronary blood flow velocity in the middle to distal portion of the left anterior descending coronary artery (LAD). Coronary flow reserve (CFR) is defined as the ratio of stimulated coronary blood flow velocity to baseline (resting). CFR measurement is used both to assess epicardial coronary arteries and to examine the integrity of coronary microvascular circulation Citation8,9.
We have hypothesized that an active BD period with oral, skin, genital, and ocular lesions might have more deteriorating effect on the cardiovascular system.
Key messages
During the active disease period, coronary microvascular function is more prominently impaired in Behcet's disease patients
Methods
Study population
For this study, subjects were consecutively registered from patients followed up and treated as BD according to the International Study Group Criteria Citation10 in our rheumatology outpatient clinic. Inclusion criteria were to be 18–50 years of age and having BD. Exclusion criteria were to have any identifiable reason that might cause coronary microvascular dysfunction such as diabetes mellitus, hypertension, renal dysfunction, hyperlipidemia, smoking, drinking alcohol, using any vasoactive drug, taking corticosteroid and/or methotrexate therapy. Additionally, patients newly diagnosed with Behcet's disease were excluded from the study to eliminate the possible effect of drugs that would be administered.
Fulfilling all inclusion and exclusion criteria, 40 patients with BD consecutively registered for the study. Each subject was evaluated two times, in both active and inactive disease period. Subjects with at least a 15‐day lesion‐free period were regarded in inactive disease period if they had high‐sensitivity C‐reactive protein (hsCRP) and erythrocyte sedimentation rate (ESR) values within normal limits, and subjects with any oral, skin, and/or genital lesion were regarded as in active disease period. Twenty‐five of the patients were in the active disease period, and 15 of them were in the inactive disease period in the first run of the study. For the control group, 40 age‐ and sex‐matched healthy subjects were recorded from our hospital staff and/or healthy volunteers. Age, gender, and body mass index (BMI) were recorded. Fasting blood glucose, total cholesterol, high‐density lipoprotein (HDL) cholesterol, low‐density lipoprotein (LDL) cholesterol, triglyceride levels, and ESR were recorded. Plasma levels of hsCRP were measured by use of a highly sensitive sandwich ELISA technique. Additionally homocysteine values were determined. Two‐dimensional, M‐mode TDHE examination was performed on each subject.
The study was conducted according to the recommendations set forth by the Declaration of Helsinki on Biomedical Research involving Human Subjects. Written informed consent was obtained from each subject, and the institutional ethics committee approved the study protocol.
Transthoracic coronary flow reserve measurement
In this study, TDHE examination was performed on each subject after at least 12 hours fast and caffeine‐containing beverages‐free period using an Acuson Sequoia C256® Echocardiography System (Acuson Corp, Mountain View, Calif, USA) equipped with a high‐resolution transducer with second harmonic capability (5V2c). Visualization of the distal LAD was performed using a modified, foreshortened, two‐chamber view obtained by sliding the transducer on the upper part and medially, from an apical two‐chamber view. Subsequently, coronary flow in the distal LAD was examined by color Doppler flow mapping over the epicardial part of the anterior wall, with the color Doppler velocity range set in the range of 9.0–24.0 cm/second. The acoustic window was around the midclavicular line, in the fourth and fifth intercostal spaces, with the subject in the left lateral decubitus position Citation8,9,Citation11–14. By placing the sample volume on the color signal, spectral Doppler of the LAD showed the characteristic biphasic flow pattern, with larger diastolic and smaller systolic components. Coronary diastolic peak flow velocities (DPFV) were measured at baseline and after dipyridamole infusion (0.84 mg/kg over 6 minutes). By averaging the three highest Doppler signals for each measurement, CFR was defined as the ratio of hyperemic to baseline diastolic peak velocities Citation13,14. One investigator, blinded to the study's parameters, performed all the measurements. Echocardiographic images were recorded on VHS videotapes. Two experienced echocardiographers, who had been blinded to the clinical data, analyzed the recordings. The interobserver, intraclass correlation coefficient for CFR measurement was 0.942.
Statistical analysis
The analyses were performed using SPSS 9.0 (SPSS for windows 9.0, Chicago, IL). Data was expressed as mean±SD. The comparison analyses between the BD patients and control group was performed using Student's t test. Measurements of BD patients while they were in active and inactive disease period were compared using paired samples t test. Pearson's correlation test was used to test the associations between CFR and the study variables. Stepwise linear regression analysis was performed to identify the independent associations of CFR in BD patients. P‐values less than 0.05 were considered significant.
Results
The demographic and baseline characteristics of BD patients and control groups are given in . The two groups were similar regarding age, BMI, blood pressure, glucose, total cholesterol, HDL cholesterol, LDL cholesterol, and hsCRP values (). Mitral E/A ratio was slightly higher in BD patients (1.48±0.31 versus 1.33±0.30, P = 0.052) (). Thirty‐seven of BD patients were on treatment with colchicines, and none of the patients was using statins or angiotensin‐converting enzyme inhibitors and/or any vasoactive drug. Resting DPFV values were similar between the BD and control groups (24.6±7.5 versus 23.6±3.6, P = 0.429). Hyperemic DPFV values tended to differ between the BD and control groups though this was not statistically significant (61.7±14.9 versus 67.4±13.3, P = 0.046). CFR values were significantly impaired in the BD group (2.57±0.50 versus 2.87±0.53, P = 0.006) (). In we reveal the results of comparison analysis of measurements of BD patients within active and inactive disease period. represents the CFR values of the controls and the BD patients while they were in inactive and in inactive disease period. The hsCRP and ESR values were significantly higher in the active disease period (). In the active disease period basal DPFV (27.3±6.6 versus 24.6±7.5, P = 0.019) was significantly higher than that in the inactive disease period. In the active disease period hyperemic DPFV values decreased significantly (from 61.7±14.9 to 56.8±16.7, P = 0.015). Therefore, in the active disease period, CFR significantly decreased from 2.57±0.50 to 2.09±0.46 (P<0.001). Additionally, the mitral E/A ratio significantly decreased from 1.48±0.31 to 1.31±0.29 (P = 0.004). The mitral E wave deceleration time significantly increased from 187.2±27.8 to 203.9±31.6 (P = 0.007).
Table I. Demographic, biochemical, and coronary flow measurements of the patients with Behcet's disease (BD) within inactive disease period and the control groups.
Table II. Comparison of coronary flow and echocardiographic measurements of patients with Behcet's disease (BD) while they were in the inactive and in the active disease period.
Figure 1. Coronary flow reserve values of the controls and Behcet's disease (BD) patients while they were in inactive and in active disease period.
Pearson's correlation analysis revealed that when the measurements taken in the inactive disease period were concerned, CFR correlated significantly with BD duration (r = 0.343, P<0.05), age (r = 387, P<0.05), and HDL cholesterol level (r = 0.297, P<0.05). When the measurements recorded within the active disease period were taken into account, disease duration (r = 452, P<0.05), age (r = 0.399, P<0.05), and hsCRP (r = 291, P<0.05) values were the significant correlates of CFR in the active disease period.
When CFR within the active disease period was entered as dependent, and disease duration, age, HDL cholesterol, hsCRP as independent variables in a multivariate model, linear regression analysis revealed that the only independent predictor of CFR within the active disease period was the disease duration (β = −0.384, P = 0.012).
Discussion
This study revealed that coronary microvascular function is impaired in BD patients; furthermore, when the disease is active the impairment in CFR is more prominent. Mitral E/A ratio, indicative of left ventricular diastolic function, was lower in the active disease period. Additionally, in this study we have found that disease duration is the only independent predictor of impaired CFR in the active disease period.
Vasculitic lesions in BD are characterized by perivascular lymphocyte and mononuclear cell infiltration, endothelial edema, degeneration of the elastic lamina interna, fibrinoid necrosis, and deposition of immune complexes within the vascular wall Citation15,16. The inflammation usually affects all layers of the vessel wall with very adherent thrombi in the lumen. Endothelial cell injury due to vasculitis seems to be a key event in the prethrombotic state of BD Citation17,18. In the active disease period, more prominent inflammatory changes in the vessel wall may be an explanation for the result of our study.
Chambers et al. Citation4 established endothelial dysfunction by the demonstration of impaired brachial artery flow‐mediated dilation, and they have observed that vitamin C administration might reverse the impaired flow‐mediated dilation. Therefore, they have suggested that oxidative stress might play an important role in endothelial dysfunction and in vascular involvement in BD Citation4. Additional theories have been suggested to explain the pathogenesis of vascular involvement in BD. Orem et al. Citation19 have suggested that increase in lipid and lipoprotein levels, lipid hydroperoxide, susceptibility of LDL to oxidation, autoantibodies against oxidized LDL, decrease of antioxidant enzyme activities, total antioxidant status, increased secretion of endothelially derived peptides including sICAM and PAI‐1, and their interactions, may be indications of tendency to atherothrombotic events in patients with Behcet's disease. Buldanlioglu et al. Citation20 have investigated nitric oxide (NO) levels and serum total antioxidant capacity in BD patients with active and inactive disease, and they have found that in BD patients with active disease, serum total NO levels are significantly decreased when compared to the inactive disease and control groups. We found that CFR was more prominently impaired in BD patients in active disease period. Decreased NO production in BD patients with active disease may be one of the reasons for coronary microvascular impairment.
Turkolmez et al. Citation7 have investigated the prevalence of silent myocardial ischemia in BD patients. They have found that exercise electrocardiography showed ischemic changes in 8 of 41 BD patients and in 1 of 35 control patients. Myocardial ischemia was documented in those 8 (19.5%) patients with BD and 1 (2.9%) in the control group, with thallium‐201 myocardial perfusion scintigraphy. In this study the mean duration of BD was higher in the patients with silent myocardial ischemia. In our study, confirming the result of Turkolmez et al., we found an association between BD duration and CFR in the active disease period. The possible basis for BD lesions is believed to be vasculitis, especially in the microvascular circulation Citation21. Involvement of the arteries and arterioles due to vasculitis in BD is characterized with narrowing of their lumen by focal fibrinoid deposition and fibroelastic proliferation in the wall of the small vessels Citation22. The pathophysiology of silent myocardial ischemia in BD patients is unclear. Our finding that BD patients in the active disease period have prominently impaired CFR compared to healthy controls may be an explanation for the silent myocardial ischemia. In their study, Turkolmez et al. Citation7 performed coronary angiography in patients with silent myocardial ischemia, but they did not find any significant coronary stenosis in patients with BD. Our results imply that the silent myocardial ischemia suggested by Turkolmez et al. might have been related to an impairment of coronary microvascular functions.
Several studies have suggested the prognostic significance of impaired CFR. Marks at al. Citation22 reported that reduced CFR due to unspecified microvascular dysfunction predicts increased mortality in a group with CFR values within normal range Citation23. The relationship between unfavorable prognosis and reduced CFR has been reported in patients with hypertrophic cardiomyopathy Citation23 and in patients with dilated cardiomyopathy Citation24,25. In a recently published study, Britten and co‐workers Citation26 suggested that CFR in normal to mildly diseased arteries is an independent predictor for long‐term prognosis of atherosclerosis within the next decade.
Our study demonstrated that coronary microvascular function and, therefore, CFR is more prominently impaired in BD patients within the active disease period. Accordingly, active inflammatory processes within the active disease period may take an important part in the development of coronary microvascular dysfunction and future cardiovascular manifestations. Therefore, closely monitoring and suppressing the exacerbations of the skin and mucosal lesions, which are the indicatives of disease activity, might result in more favorable cardiovascular prognosis in BD patients.
Study limitations
In this study we have excluded subjects with confounding factors which are common in the normal population, such as hypertension, diabetes mellitus, morbid obesity, and current smoking, for CFR to investigate the independent effect of disease activity on CFR. Therefore, the study does not provide information about BD effects on CFR in patients with risk factors for coronary heart disease.
In this study, for active disease evaluation, we evaluated the subjects while they had skin and/or mucosal lesions, which are the most easily detectable manifestations of disease activity; however, the determinants of disease activity are hsCRP and ESR values. Therefore, our results do not mean that BD patients are at risk for microvascular dysfunction only when they have mucosal and/or skin lesions.
References
- Koc Y., Gullu I., Akpek G., Akpolat T., Kansu E., Kiraz S., et al. Vascular involvement in Behcet's disease. J Rheumatol 1992; 19: 402–10
- Ehrlich G. E. Vasculitis in Behcet's disease. Int Rev Immunol 1997; 14: 81–8
- Kosar F., Sahin I., Gullu H., Cehreli S. Acute myocardial infarction with normal coronary arteries in a young man with the Behcet's disease. Int J Cardiol 2005; 99: 355–7
- Chambers J. C., Haskard D. O., Kooner J. S. Vascular endothelial function and oxidative stress mechanisms in patients with Behcet's syndrome. J Am Coll Cardiol 2001; 37: 517–20
- Ozdemir R., Barutcu I., Sezgin A. T., Acikgoz N., Ermis N., Esen A. M., et al. Vascular endothelial function and plasma homocysteinee levels in Behcet's disease. Am J Cardiol 2004; 94: 522–5
- Gullu I. H., Benekli M., Muderrisoglu H., Oto A., Kansu E., Kabakci G., et al. Silent myocardial ischemia in Behcet's disease. J Rheumatol 1996; 23: 323–7
- Turkolmez S., Gokcora N., Alkan M., Gorer M. A. Evaluation of myocardial perfusion in patients with Behcet's disease. Ann Nucl Med 2005; 19: 201–6
- Youn H. J., Foster E. Demonstration of coronary artery flow using transthoracic Doppler echocardiography. J Am Soc Echocardiogr 2004; 17: 178–85
- Dimitrow P. P. Transthoracic Doppler echocardiography—noninvasive diagnostic window for coronary flow reserve assessment. Cardiovasc Ultrasound 2003; 1: 4
- International Study Group for Behcet's Disease. Criteria for diagnosis of Behcet's disease., Lancet. 335. 1078–80
- Dimitrow P. P., Galderisi M., Rigo F. The non‐invasive documentation of coronary microcirculation impairment: role of transthoracic echocardiography. Cardiovasc Ultrasound 2005; 3: 18
- Saraste M., Koskenvuo J., Knuuti J., Toikka J., Laine H., Niemi P., et al. Coronary flow reserve: measurement with transthoracic Doppler echocardiography is reproducible and comparable with positron emission tomography. Clin Physiol 2001; 21: 114–22
- Lambertz H., Tries H. P., Stein T., Lethen H. Noninvasive assessment of coronary flow reserve with transthoracic signal‐enhanced Doppler echocardiography. J Am Soc Echocardiogr 1999; 12: 186–95
- Korcarz C. E., Stein J. H. Noninvasive assessment of coronary flow reserve by echocardiography: technical considerations. J Am Soc Echocardiogr 2004; 17: 704–7
- Atzeni F., Sarzi‐Puttini P., Doria A., Boiardi L., Pipitone N., Salvarani C. Behcet's disease and cardiovascular involvement. Lupus 2005; 14: 723–6
- al‐Dalaan A. N., al Balaa S. R., el Ramahi K., al‐Kawi Z., Bohlega S., Bahabri S., et al. Behcet's disease in Saudi Arabia. J Rheumatol 1994; 2: 658–61
- Haznedaroglu I. C., Ozcebe O. I., Ozdemir O., Celik I., Dundar S. V., Kirazli S. Impaired haemostatic kinetics and endothelial function in Behcet's disease. J Intern Med 1996; 240: 181–7
- Haznedaroglu I. C., Ozdemir O., Ozcebe O., Dundar S. V., Kirazli S. Circulating thrombomodulin as a clue of endothelial damage in Behcet's disease. Thromb Haemost 1996; 75: 974–5
- Orem A., Yandi Y. E., Vanizor B., Cimsit G., Uydu H. A., Malkoc M. The evaluation of autoantibodies against oxidatively modified low‐density lipoprotein (LDL), susceptibility of LDL to oxidation, serum lipids and lipid hydroperoxide levels, total antioxidant status, antioxidant enzyme activities, and endothelial dysfunction in patients with Behcet's disease. Clin Biochem 2002; 35: 217–24
- Buldanlioglu S., Turkmen S., Ayabakan H. B., Yenice N., Vardar M., Dogan S., et al. Nitric oxide, lipid peroxidation and antioxidant defence system in patients with active or inactive Behcet's disease. Br J Dermatol 2005; 153: 526–30
- Chajek T., Fainaru M. Behçet's disease. Report of 41 cases and a review of the literature. Medicine 1975; 54: 179–96
- Marks D. S., Gudapati S., Prisant L. M., Leir B., Donato‐Gonzalez C., Baller J. L., et al. Mortality in patients with microvascular disease. J Clin Hypertens 2004; 6: 304–09
- Rigo F., Cortigiani L., Gherardi S., Zanella C., Di Pede F., Pasanisi E., et al. The prognostic meaning of coronary flow reserve assessed by Doppler echocardiography in nonischemic dilated cardiomyopathy [abstract]. Circulation 2004; III–511, 110
- Rigo F., Pasanisi E., Gherardi S., Zanella C., Cutaia V., Cortigiani L., et al. Coronary microvascular dysfunction and prognosis in hypertrophic cardiomyopathy: a echocardiographic study [abstract]. Eur Heart J 2004; 25: 16
- Neglia D., Michelassi C., Trivieri M. G., Sambuceti G., Giorgetti A., Pratali L., et al. Prognostic role of myocardial blood flow impairment in idiopathic left ventricular dysfunction. Circulation 2002; 105: 186–93
- Britten M. B., Zeiher A. M., Schachinger V. Microvascular dysfunction in angiographically normal or mildly diseased coronary arteries predicts adverse cardiovascular long‐term outcome. Coron Artery Dis 2004; 15: 259–64