Abstract
Aim. To study the association between helicobacters and chronic liver diseases and chronic inflammatory bowel diseases.
Patients and methods. Thirty‐two patients with various chronic liver diseases and 137 patients with inflammatory bowel disease were enrolled. Antibodies to H. pylori, H. hepaticus, H. bilis, and H. pullorum were measured by enzyme immunoassay (EIA), and sera positive in a non‐pylori helicobacter EIA were further examined by immunoblot assay. Detection of Helicobacter DNA in liver biopsies was done by denaturating gradient gel electrophoresis of PCR products (PCR‐DGGE) and DNA sequence analysis.
Results. Six inflammatory bowel disease patients, four with ulcerative colitis and two with Crohn's disease, and one liver disease patient with autoimmune cholangitis had antibodies to non‐pylori helicobacters by an immunoblot assay. Four immunoblot assay‐negative patients, three with autoimmune and one with non‐autoimmune liver disease, had Helicobacter DNA in liver biopsies; three of the polymerase chain reaction (PCR) products were closely related to non‐pylori helicobacters.
Conclusion. Evidence for non‐pylori helicobacters was scant in Finnish patients with inflammatory bowel disease or chronic but not end stage liver disease. We cannot, however, rule out their role in these diseases.
| Abbreviations | ||
| CAH | = | CHRONIC AUTOIMMUNE HEPATITIS |
| CD | = | Crohn's disease |
| EIA | = | enzyme immunoassay |
| IB | = | immunoblot assay |
| IBD | = | inflammatory bowel disease |
| PBC | = | primary biliary cirrhosis |
| PCR | = | polymerase chain reaction |
| PCR‐DGGE | = | denaturating gradient gel electrophoresis of PCR products |
| PSC | = | primary sclerosing cholangitis |
| UC | = | ulcerative colitis |
Introduction
Helicobacter pylori (H. pylori) causes lifelong gastritis in humans. Since its discovery, 30 other formally named Helicobacter species (List of Prokaryotic Names with Standing in Nomenclature (http://www.bacterio.cict.fr) accessed 18th May 2007) have been detected, some of which have been associated with hepatitis and colitis in animals Citation1,2. The chronic nature of H. pylori infection has prompted increasing interest in the possible association between the non‐pylori helicobacters and chronic bowel and hepatobiliary diseases in humans. On the other hand, data suggesting that H. pylori‐induced autoantibodies may play a crucial role in the pathogenesis of autoimmune type gastric atrophy Citation3 have led to an idea that other Helicobacter species might have a role in the etiology of autoimmune diseases Citation4.
The reports on the association between helicobacters and hepatobiliary diseases in humans have revealed contradictory results Citation5. In most of these studies Helicobacter DNA has been detected by polymerase chain reaction (PCR) which may not always be specific Citation6. There are, however, two reports on the culture of a Helicobacter isolate from liver Citation7,8 and several reports on the demonstration of helicobacters by immunohistochemistry in human liver tissue Citation9–11. The growth of H. pylori is known to be inhibited by bile salts in vitro (this may not be so in vivo in the case of biliary pathology) but some enterohepatic Helicobacter species, such as H. hepaticus, H. bilis, H. pullorum, are bile‐resistant Citation5.
In primary biliary cirrhosis (PBC) over 95% of patients have anti‐mitochondrial antibodies that are reactive with a sub‐unit of the pyruvate dehydrogenase complex, which is considered to be the major autoantigen in PBC Citation12,13. Several yeasts and bacteria express an epitope similar to that found in the pyruvate dehydrogenase complex, and this observation has led to a hypothesis that an infection may trigger PBC Citation12. In addition, another liver disease with unknown etiology, primary sclerosing cholangitis (PSC), is in most patients associated with ulcerative colitis, which may also be triggered by microbial factors Citation14. Although it has been tempting to speculate about the possible role of helicobacters in chronic inflammatory diseases of the liver, namely PBC, PSC, and chronic autoimmune hepatitis (CAH), the results are conflicting. In a Swedish study, almost all patients with cholestatic end stage PBC and PSC studied with Helicobacter PCR were positive Citation15, whereas this was shown only for one of 29 American patients suffering from PBC Citation16. Recently Boomkens et al. 2005 Citation17 found no differences in the presence of Helicobacter DNA in liver biopsies between patients with cholestatic and non‐cholestatic chronic liver diseases. Furthermore, serological studies based on EIAs have failed to show any association between different chronic liver diseases and either H. pyloriCitation18,19 or bile‐tolerant Helicobacter species Citation20. However, when sera were analysed by an immunoblot assay, H. hepaticus was found to be associated with alcoholic liver disease Citation21 as well as H. pullorum, H. bilis, and H. hepaticus with PSC, PBC and CAH Citation22,23.
The chronic inflammatory bowel diseases (IBDs), namely ulcerative colitis (UC) and Crohn's disease (CD), are chronic inflammatory diseases of unknown etiology. For both diseases there is a link between several environmental and microbial factors with pathogenesis Citation14. However, the PCR‐based studies have failed to show any association between helicobacters and IBDs Citation24,25, and H. pylori DNA in colonic biopsies has been found to occur with the same frequency in IBD as in non‐IBD patients Citation26–28. Oliveira et al. 2006 Citation29 detected with PCR H. pylori DNA more often in colonic biopsies in a subgroup of Crohn's disease than in non‐IBD patients, but using an EIA method they found H. pylori antibodies to be less common in Crohn's disease than in non‐IBD patients, confirming an earlier finding Citation30. In four studies non‐pylori helicobacter DNA has been detected by PCR in colonic biopsies, not only in IBD but also in non‐IBD patients Citation31–34. Thus, despite the attractive hypothesis of Helicobacter infection possibly triggering IBD or autoimmune liver diseases, the scientific evidence remains contradictory.
In this report, in order to assess the role of helicobacters in liver diseases with an emphasis on autoimmune diseases, the presence of Helicobacter DNA in liver specimens of patients with various benign chronic liver diseases was analysed by denaturating gradient gel electrophoresis of PCR products (PCR‐DGGE). The antibody response to immunogenic proteins of Helicobacter species was measured in sera from these patients and in patients with IBD to further study the association between bile‐tolerant helicobacters and these diseases.
Key messages
Extra‐gastric helicobacters are rare in patients with chronic inflammatory bowel disease or chronic liver disease.
Patients and methods
Patients
Percutaneous liver biopsies taken at Peijas Hospital (Hospital District of Helsinki and Uusimaa) between 1st January 1999 and 30th September 2003 were retrospectively reviewed. Biopsies from patients with malignancy, viral hepatitis, storage diseases of the liver, or known drug‐induced liver damage were excluded. Eighty‐one eligible patients with elevated liver enzymes were identified. From these, 32 patients (median age 56 years, range 27–75; 25 were females) gave serum and stool samples. The serum samples were drawn an average 1.5 years (range 0–8 years) after the liver biopsies had been taken. Twelve patients had PBC, whilst the remainder of the patients had the following clinical diagnoses: CAH in four patients, PSC in one, alcoholic cirrhosis in two, cryptogenic cirrhosis in one, fatty liver in three, focal nodular hyperplasia in one, cholestatic hepatopathy (including two with autoimmune cholangitis) in four, hepatitis with unknown etiology in three, and normal liver in one (in this patient the indication of liver biopsy was slightly elevated liver enzymes). Some characteristics of the patients with liver disease are shown in .
Table I. Characteristics of the 32 patients with liver diseases.
The presence of Helicobacter antibodies were studied in serum samples taken from 61 patients suffering from Crohn's disease (median age 37 years, range 17–75 years; median duration of CD 6.3 years, range 0–23 years) and 73 patients suffering from ulcerative colitis (median age 44 years, range 21–77 years; median duration of UC 11.4 years, range 0–44 years). According to the Vienna classification Citation35 the location of CD was upper gastrointestinal in 6 patients, ileum in 28 patients, ileocolic in 18, and colitis in 9 patients, and the behaviour of CD was penetrating in 12 patients, stricturing in 37 patients, and inflammatory in 12 patients. A total of 37 patients with UC had extensive colitis and 35 had left‐sided colitis.
The Ethics Committee of the Hospital District of Helsinki and Uusimaa approved the study, and all participants gave their informed consent.
Histology
One senior pathologist (JM) experienced in the field of liver pathology reviewed all the liver biopsies and assessed them according to the METAVIR scoring system Citation36. Standard diagnostic criteria were used to diagnose liver and bowel disease.
Detection of H. pylori and immunoblot assay (IB)
Serum samples were stored at ‐20°C before being analysed. The serum samples from the liver patients and IBD patients were examined by EIA as described earlier Citation37,38. In the latter H. pylori EIA units <25 were regarded as negative, ⩾35 as positive, and 25–35 as border‐line values. Serum samples from IBD patients with positive H. pylori EIA results were further studied by immunoblot assay (IB).
Stool specimens from the liver patients that were collected at the same time as the serum samples were examined with a polyclonal antibody‐based (Premier Platinum HpSA, Meridian Inc., Cincinnati, OH, USA) and monoclonal antibody‐based (Amplified IDEIA HpStAR test, formerly FemtoLab, Dako, Glostrup, Denmark) stool antigen tests according to the manufacturers' instructions as described earlier Citation39.
Enteric Helicobacter EIAs and IB
Sera tested for enteric Helicobacter by EIA were initially absorbed with lysed H. pylori cells. The EIAs were performed according to a method recently published Citation23 with minor modification to the cut‐off values, which were in the present study based on EIA results obtained with sera from 90 healthy Swedish blood donors. Cut‐off values (corresponding to the upper limit for the 95th percentile level of the reference standard and expressed as relative antibody activity) were for H. pullorum ⩾25, for H. bilis ⩾35, and for H. hepaticus ⩾25. Only sera giving positive/border‐line EIA results were further analysed by IB (after absorbing with lysed H. pylori cells) for detection of the three enteric Helicobacter species as described earlier Citation40.
DNA extraction and amplification conditions
DNA was extracted from paraffin‐embedded liver specimens available for 30 patients as described earlier Citation9,Citation15 using the QIAamp DNA Kit (QIAGEN, Hilden, Germany).
PCR detection of Helicobacter was done using a nested Helicobacter genus 16S rDNA PCR assay: The primers used in the first step were C97 and C05 Citation41, whereas in the second step they were HG16S 1F with GC‐clamp and HG16S 2R Citation42.
PCR‐DGGE analysis
Amplified PCR products were analysed in agarose gels containing ethidium bromide and visualized by ultraviolet light. Analysis of Helicobacter‐specific PCR products was done by the DGGE technique as described previously Citation43. Identification of Helicobacter PCR products was done by comparing the mobility pattern of unknown PCR products with the mobility pattern of PCR products amplified from the following Helicobacter strains: H. muridarum (CCUG 29262), H. bilis (CCUG 38995), H. pullorum (NCTC 12825), H. pylori (CCUG 17874), ‘H. rappini’ (CCUG 23435), H. hepaticus (CCUG 33637), and H. bizzozeronii (AF 53).
16S rDNA sequence analysis
The separated DNA fragments were cut from the DGGE gels and prepared for sequencing as described previously Citation43. Sequencing of both DNA strands was performed with an ABI 310 DNA sequencer (Applied Biosystems, Foster City, CA, USA), using the ABI PRISM BigDye Terminator Cycle Sequencing Ready Reaction Kit v3.0 (Applied Biosystems). The closest known relatives of the partial 16S rDNA sequences were identified by using BLASTN 2.2.1 (http://www.ncbi.nlm.nih.gov/BLAST/).
Results
Chronic liver disease
Twenty‐three (72%) of the 32 patients with liver disease were negative by H. pylori EIA and by the two different stool antigen tests. Of the nine H. pylori EIA‐positive patients, six were also positive in the two stool antigen tests; from one patient a stool specimen was not available. Positive EIA results were detected for H. bilis in eight patients, for H. hepaticus in nine, and for H. pullorum in seven, and only these were further studied by IB; only one specimen was positive for H. bilis from a patient with autoimmune cholangitis ().
Table II. Positive helicobacter polymerase chain reaction (PCR) results of liver biopsy specimens and serum antibody responses studied by immunoblot (IB) for non‐pylori helicobacters in five patients with different liver diseases. The sera were first screened by enzyme immunoassay (EIA) and only positive samples were further studied by IB.
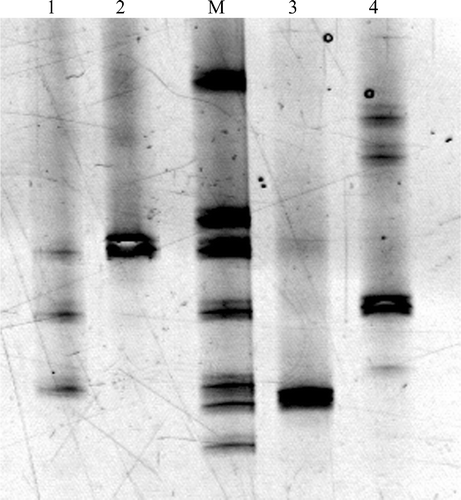
Of the 30 patients with liver disease with liver specimens suitable for PCR, four had positive Helicobacter PCR results as shown in . The PCR‐DGGE analysis is shown in . The patients with positive PCR results were negative in the H. pylori EIA and stool antigen tests, except for the patient positive for H. hepaticus, who was positive for H. pylori Immunoglobulin A (IgA) antibodies.
Figure 1. PCR‐DGGE analysis of 4 Helicobacter positive liver specimens. Lanes: M(mobility)‐Ladder, DNA amplified from reference Helicobacter spp. which consists from top to the bottom of PCR products amplified from H. muridarum (CCUG 29262), H. bilis (CCUG 38995), H. pullorum (NCTC 12825), H. pylori (CCUG 17875), ‘H. rappini’ (CCUG 23435), H. hepaticus (CCUG 33637) and H. bizzozeronii (AF 53). 1: F3A (H. pullorum), F3B (H. pylori), F3C (Helicobacter sp. ‘Flexispira taxon. 8’). 2: F4 (H. pullorum). 3: F11 (H. hepaticus). 4: F17 (H. pylori).
Chronic inflammatory bowel disease
Of the 134 IBD patients, 63 were positive in EIA for H. pylori, and 30 of them were also positive when studied further with IB. The positive EIA and IB results for non‐pylori helicobacters were the following: 24 and 3 for H. bilis, respectively, 48 and 1 for H. hepaticus, respectively, and 32 and 2 for H. pullorum, respectively. Of the CD patients, H. pylori antibodies were found in 13 (21%), H. bilis antibodies in 1 (1.6%), and H. hepaticus antibodies in 1 (1.6%) patient by IB. Of the UC patients, antibodies detected by IB were found in 17 (23%) patients for H. pylori, in 2 (2.7%) for H. bilis, and in 2 (2.7%) for H. pullorum. Certain characteristics of the IBD patients with positive non‐pylori Helicobacter findings are presented in .
Table III. Characteristics of six IBD patients with non‐pylori helicobacter antibodies demonstrated by immunoblot assay. In four of these patients also H. pylori antibodies were detected in the IB.
Discussion
In our study, evidence for non‐pylori Helicobacter species was only rarely found among patients with liver disease and IBDs. We studied the non‐pylori helicobacters in liver disease patients using both IB and PCR methods, and our results were conflicting. Of the 32 liver disease patients only 1 with autoimmune cholangitis had a positive IB result for H. bilis, and of the 30 liver specimens studied by PCR there were only 4 specimens positive for Helicobacters, and none of these patients showed Helicobacter antibodies by IB. Three of these particular patients had autoimmune disease, two had PBC, and one CAH. In the three non‐pylori Helicobacter‐positive cases (of all the four Helicobacter PCR‐positive cases) the PCR was positive for different Helicobacter species, and in one case for three different Helicobacters. Six of the 137 patients with IBD had positive IB results to non‐pylori helicobacters, two with Crohn's disease and four with ulcerative colitis.
Earlier IB‐based studies on non‐pylori helicobacters have shown a high prevalence of these species in patients with autoimmune and alcoholic liver disease Citation21,22. Although the number of patients in our study was small, all those with alcoholic liver disease, and all except one with autoimmune liver disease, had negative IB results for non‐pylori helicobacters. The limitation in our study is the lack of healthy control patients, but in Sweden, a neighbouring country with very similar infectious disease profiles, the prevalence of non‐pylori helicobacter antibodies among blood donors studied with IB has been shown to be very low. In 4 patients out of 80, non‐pylori helicobacter antibodies were found, and H. hepaticus antibodies were demonstrated in none out of 48 Citation21,22.
An earlier study showing non‐pylori helicobacter PCR products in liver samples from PBC and PSC patients included only patients with end stage liver disease (explanted livers), and an association was also found between the grade of cholestasis and the incidence of HelicobacterCitation15. However, in advanced liver disease, PCR products of several bacteria have often been found Citation44. In our study, most of the patients had incipient liver disease and the grade of cholestasis in our PBC patients was mild since alkaline phosphatase values were only slightly elevated. Two of the 19 patients with autoimmune liver disease (including patients with PBC, PSC, CAH, and autoimmune cholangitis) had non‐pylori helicobacter PCR products in their liver specimens as compared to 1 of the 13 patients with non‐autoimmune liver disease. In addition, among the PCR positive patients the PCR products belonged to different non‐pylori Helicobacter species. Thus, in accordance with several but not all study reports Citation15–17, our results do not support the association between Helicobacter species and autoimmune liver diseases.
An earlier serological study investigating the relationship of non‐pylori helicobacters and IBD showed by IB the presence of antibodies to H. pullorum in a subset of patients with UC (10/24) and CD (7/24) Citation45. However, the Helicobacter PCR studies using colonic biopsies have been negative both in IBD and control patients even for H. pylori PCR products Citation24,25, or have revealed H. pylori PCR products with almost the same frequency both in IBD and non‐IBD patients Citation26–28. Oliveira et al. 2006 Citation29 found colonic biopsies to be PCR‐positive for H. pylori more often in a subset of CD patients as compared to the non‐IBD patients. However, in three other studies non‐pylori Helicobacter DNA was found in either a small number of IBD or non‐IBD patients Citation31–33, the identified species were H. pullorum and H. fennelliae in the German study Citation32 and H. muridarum and H. cholecystus in the Swedish study Citation33. In line with these earlier studies we could find by IB antibodies to non‐pylori helicobacters (H. bilis, H. pullorum, or H. hepaticus) in two CD patients and in four UC patients and an association with neither the duration nor the behaviour of these diseases. Thus, both PCR and IB‐based studies have demonstrated only in a few cases a possible association between IBD and non‐pylori helicobacters.
In conclusion, evidence for H. bilis, H. pullorum, or H. hepaticus in patients with chronic but not end stage liver disease and chronic IBD was only rarely detected either by IB or PCR, but we cannot rule out their role in some patients with these diseases.
Acknowledgements
We greatly appreciate the skilful assistance of Pirjo Kosunen and Sari Karesvuori. We are also thankful to Timo Helske MD PhD and Tom Böhling MD PhD for their help during the study.
This study was supported by grants from the Swedish Science Research Council and Lund University (ALF). This study was partly supported by a grant from City of Helsinki to Lea Veijola. The authors have no financial or other conflicting interest relevant to the study.
References
- Fox J. G., Dewhirst F. E., Tully J. G., Paster B. J., Yan L., Taylor N. S., et al. Helicobacter hepaticus sp. nov., a microaerophilic bacterium isolated from livers and intestinal mucosal scrapings from mice. J Clin Microbiol 1994; 32: 1238–45
- Fox J. G., Handt L., Sheppard B. J., Xu S., Dewhirst F. E., Motzel S., et al. Isolation of Helicobacter cinaedi from colon, liver, and mesenteric lymph node of a rhesus monkey with chronic colitis and hepatitis. J Clin Microbiol 2001; 39: 1580–5
- Appelmelk B. J., Simoons‐Smit I., Negrini R., Moran A. P., Aspinall G. O., Forte J. G., et al. Potential role of molecular mimicry between Helicobacter pylori lipopolysaccharide and host Lewis blood group antigens in autoimmunity. Infect Immun 1996; 64: 2031–40
- Wadström T., Ljungh Å. Chronic Helicobacter infection of the human liver and bile are common and may trigger autoimmune disease. Curr Gastroenterol Rep 2002; 4: 349–50
- Leong R. W. L., Sung J. J. Y. Review article: Helicobacter species and hepatobiliary diseases. Aliment Pharmacol Ther 2002; 16: 1037–45
- On S. L. W., Hynes S., Wadström T. Extragastric Helicobacter species. Helicobacter 2002; 7(Suppl 1)63–7
- Queiroz D. M. M., Santos A. Isolation of a Helicobacter strain from the human liver. Gastroenterology 2001; 121: 1023–4
- Xuan S. Y., Li N., Qiang X., Zhou R. R., Shi Y. X., Jiang W. J. Helicobacter infection in hepatocellular carcinoma tissue. World J Gastroenterol 2006; 12: 2335–40
- Apostolov E., Abu Al‐Soud W., Nilsson I., Kornilovska I., Usenko V., Lyzogubov V., et al. Helicobacter pylori and other Helicobacter species in gallbladder and liver of patients with chronic cholecystitis detected by immunological and molecular methods. Scand J Gastroenterol 2005; 40: 96–102
- Stalke P., Abu Al‐Soud W., Bielawski K. P., Bakowska A., Trocha H., Stepinski J., et al. Detection of Helicobacter species in liver and stomach tissues of patients with chronic liver diseases using polymerase chain reaction‐denaturating gradient gel electrophoresis and immunohistochemistry. Scand J Gastroenterol 2005; 40: 1032–41
- Wadström T., Ljungh Å., Willen R. Primary biliary cirrhosis and primary sclerosing cholangitis are of infectious origin!. Gut 2001; 49: 454
- Poupon R., Chazouillères O., Poupon R. E. Chronic cholestatic diseases. J Hepatol 2000; 32(Suppl 1)129–40
- Sutton I., Neuberger J. Primary biliary cirrhosis: seeking the silent partner of autoimmunity. Gut 2002; 50: 743–6
- Ohkusa T., Nomura T., Sato N. The role of bacterial infection in the pathogenesis of inflammatory bowel disease. Intern Med 2004; 43: 534–9
- Nilsson H. ‐. O., Taneera J., Castedal M., Glatz E., Olsson R., Wadström T. Identification of Helicobacter pylori and other Helicobacter species by PCR, Hybridization, and partial DNA sequencing in the human liver samples from patients with primary sclerosing cholangitis or primary biliary cirrhosis. J Clin Microbiol 2000; 38: 1072–6
- Tanaka A., Prindiville T. P., Gish R., Solnick J. V., Coppel R. L., Keeffe E. B., et al. Are infectious agents involved in primary biliary cirrhosis? A PCR approach. J Hepatology 1999; 31: 664–71
- Boomkens S. Y., de Rave S., Pot R. G. J., Egberink H. F., Penning L. C., Rothuizen J., et al. The role of Helicobacter spp. in the pathogenesis of primary biliary cirrhosis and primary sclerosing cholangitis. FEMS Immunol Med Microbiol 2005; 44: 221–5
- Durazzo M., Pellicano R., Premoli A., Berrutti M., Leone N., Ponzetto A., et al. Helicobacter pylori seroprevalence in patients with autoimmune hepatitis. Dig Dis Sci 2002; 47: 380–3
- Durazzo M., Rosina F., Premoli A., Morello E., Fagoonee S., Innarella R., et al. Lack of association between seroprevalence of Helicobacter pylori infection and primary biliary cirrhosis. World J Gastroenterol 2004; 10: 3179–81
- Ananieva O., Nilsson I., Vorobjova T., Uibo R., Wadström T. Immune responses to bile‐tolerant Helicobacter species in patients with chronic liver diseases, a randomized population group, and healthy blood donors. Clin Diagn Lab Immunol 2002; 9: 1160–4
- Nilsson I., Lindgren S., Eriksson S., Wadström T. Serum antibodies to Helicobacter hepaticus and Helicobacter pylori in patients with chronic liver disease. Gut 2000; 46: 410–4
- Nilsson I., Kornilovs'ka I., Lindgren S., Ljungh Å., Wadström T. Increased prevalence of seropositivity for non‐gastric Helicobacter species in patients with autoimmune liver disease. J Med Microbiol 2003; 52: 949–53
- Vorobjova T., Nilsson I., Terjajev S., Granholm M., Lyyra M., Porkka T., et al. Serum antibodies to enterohepatic Helicobacter spp. in patients with chronic liver diseases and in a population with high prevalence of H. pylori infection. Dig Liver Dis 2006; 38: 171–6
- Bell S. J., Chisholm S. A., Owen R. J., Borriello S. P., Kamm M. A. Evaluation of Helicobacter species in inflammatory bowel disease. Aliment Pharmacol Ther 2003; 18: 481–6
- Huijsdens X. W., Linskens R. K., Koppes J., Tang Y. L., Meuwissen S. G. M., Vandenbroucke‐Grauls C. M. J. E., et al. Detection of Helicobacter species DNA by quantitative PCR in the gastrointestinal tract of healthy individuals and of patients with inflammatory bowel disease. FEMS Immunol Med Microbiol 2004; 41: 79–84
- Grehan M., Danon S., Lee A., Daskalopoulos G., Mitchell H. Absence of mucosa‐associated colonic Helicobacters in an Australian urban population. J Clin Microbiol 2004; 42: 874–6
- Oliveira A. G., Sanna M., Rocha G. A., Rocha A. M. C., Santos A., Dani R., et al. Helicobacter species in the intestinal mucosa of patients with ulcerative colitis. J Clin Microbiol 2004; 42: 384–6
- Streutker C. J., Bernstein C. N., Chan V. L., Riddell R. H., Croitoru K. Detection of species‐specific Helicobacter ribosomal DNA in intestinal biopsy samples from a population‐based cohort of patients with ulcerative colitis. J Clin Microbiol 2004; 42: 660–4
- Oliveira A. G., Rocha G. A., Rocha A. M., Sanna M. G., Moura S. B., Dani R., et al. Isolation of Helicobacter pylori from intestinal mucosa of patients with Crohn's disease. Helicobacter 2006; 11: 2–9
- Halme L., Rautelin H., Leidenius M., Kosunen T. U. Inverse correlation between Helicobacter pylori infection and inflammatory bowel disease. J Clin Pathol 1996; 49: 65–7
- Basset C., Holton J., Bazeos A., Vaira D., Bloom S. Are Helicobacter species and enterotoxigenic Bacteroides fragilis involved in inflammatory bowel disease?. Dig Dis Sci 2004; 49: 1425–32
- Bohr U. R. M., Glasbrenner B., Primus A., Zagoura A., Wex T., Malfertheiner P. Identification of enterohepatic Helicobacter species in patients suffering from inflammatory bowel disease. J Clin Microbiol 2004; 42: 2766–8
- Sturegård E., Hertervig E., Sjunnesson H., Wadström T. Helicobacter species in human colon biopsies (letter). Aliment Pharmacol Ther 2004; 19: 613–4
- Zhang L., Day A., McKenzie G., Mitchell H. Nongastric Helicobacter species detected in intestinal tract of children. J Clin Microbiol 2006; 44: 2274–9
- Gasche C., Scholmerich J., Brynskov J., D'Haens G., Hanauer S. B., Irvine E. J., et al. A simple classification of Crohn's disease: report of the working party for the World congresses of gastroenterology Vienna 1998. Inflamm Bowel Dis 2000; 6: 8–15
- Bedossa P., Poynard T., for the METAVIR cooperative study group. An algorithm for the grading of activity in chronic hepatitis C. Hepatology 1996; 24: 289–93
- Oksanen A., Veijola L., Sipponen P., Schauman K. O., Rautelin H. Evaluation of Pyloriset Screen, a rapid whole‐blood diagnostic test for Helicobacter pylori infection. J Clin Microbiol 1998; 36: 955–7
- Guruge J. L., Schalen C., Nilsson I., Ljungh Å., Tyszkiewicz T., Wikander M., et al. Detection of antibodies to Helicobacter pylori cell surface antigens. Scand J Infect Dis 1990; 22: 457–65
- Veijola L., Oksanen A., Löfgren T., Sipponen P., Karvonen A. L., Rautelin H. Comparison of three stool antigen tests in confirming Helicobacter pylori eradication in adults. Scand J Gastroenterol 2005; 40: 395–401
- Kornilovska I., Nilsson I., Utt M., Ljungh Å., Wadström T. Immunogenic proteins of Helicobacter pullorum, Helicobacter bilis and Helicobacter hepaticus identified by two‐dimensional gel electrophoresis and immunoblotting. Proteomics 2002; 2: 775–83
- Fox J. G., Dewhirst F. E., Shen Z., Feng Y., Taylor N. S., Paster B. J., et al. Hepatic Helicobacter species identified in bile and gallbladder tissue from Chileans with chronic cholecystitis. Gastroenterology 1998; 114: 755–63
- Goto K., Ohashi H., Takakura A., Itoh T. Current status of Helicobacter contamination of laboratory mice, rats, gerbils, and house musk shrews in Japan. Curr Microbiol 2000; 41: 161–6, 41:
- Abu Al‐Soud W., Bennedsen M., On S. L. W., Ouis I. S., Vandamme P., Nilsson H. O., et al. Assessment of PCR‐DGGE for the identification of diverse Helicobacter species, and application to faecal samples from zoo animals to determine Helicobacter prevalence. J Med Microbiol 2003; 52: 765–71
- Harada K., Tsuneyama K., Sudo Y., Masuda S., Nakanuma Y. Molecular identification of bacterial 16S ribosomal RNA gene in liver tissue of primary biliary cirrhosis: Is Propionibacterium acnes involved in granuloma formation?. Hepatology 2001; 33: 530–6
- Andersson R., Kornilovska I., Melin T., Ljungh Å. H. Helicobacter pullorum and Mycobacterium paratuberculosis in inflammatory bowel disease. Gut 2002; 51(Suppl 11)A74