Abstract
Background. Different soluble molecules involved in inflammation, endothelial damage, or hemostasis are recognized as potential cardiovascular risk markers. Studies to assess the role of these markers in the atherosclerotic process by evaluating their relationship to carotid intima‐media thickness (C‐IMT) tend to provide contrasting results.
Purpose. To perform a review of studies addressing the association between C‐IMT and soluble markers and to investigate whether the observed inconsistencies could be explained by the characteristics of the patients included in different studies, for example prevalence of atherosclerotic disease (atherosclerotic burden), gender, age, or occurrence of specific vascular risk factors (VRFs).
Data sources. PubMed and Embase (January 1990 to March 2006).
Study selection. Articles in English reporting original cross‐sectional studies.
Data extraction. Two authors independently extracted data on study design, population, sample size, ultrasonic methodology, and statistical approach.
Data synthesis. Despite the marked heterogeneity of results presented in the literature, meta‐analysis established that studies showing positive associations between C‐IMT and plasma levels of C‐reactive protein (CRP) or fibrinogen are in the majority. Funnel plot analyses suggested the absence of an important publication bias. Data on the relationships between C‐IMT and other soluble markers are by contrast scanty, contradictory, or unconfirmed by multivariate (as opposed to univariate) analyses, and the freedom from publication bias here cannot be vouched for. The degree of atherosclerotic burden in the population studied does not account for the heterogeneity of findings reported. Gender, noninsulin‐dependent diabetes mellitus (NIDDM) and hypercholesterolemia influence the association between C‐IMT and CRP. Blood pressure and hypercholesterolemia influence the association between C‐IMT and fibrinogen. For all the other soluble markers considered, the number of groups was too small for this kind of statistical considerations.
Limitations. Heterogeneity in ultrasound methodologies and in statistical approach limited comparability between studies. For most soluble markers, publication bias of positive results cannot be excluded.
Conclusions. Only CRP and fibrinogen seem to be unequivocally related to C‐IMT. For all the other soluble markers considered, no clear‐cut conclusions can be drawn.
| Abbreviations | ||
| Bif | = | bifurcation |
| BMI | = | body mass index |
| CAD | = | coronary artery disease |
| CC | = | common carotid |
| C‐IMT | = | carotid intima‐media thickness |
| CRP | = | C‐reactive protein |
| CVD | = | cardiovascular disease |
| FH | = | familial hypercholesterolemia |
| ICA | = | internal carotid artery |
| ICAM | = | intercellular adhesion molecule |
| IL | = | interleukin |
| LDL‐C | = | low‐density lipoprotein cholesterol |
| MCP‐1 | = | monocyte chemoattractant protein‐1 |
| MMP | = | matrix metalloproteinases |
| NIDDM | = | noninsulin‐dependent diabetes mellitus |
| PAI‐1 | = | plasminogen activator inhibitor‐1 |
| SAA | = | serum amyloid A |
| TFPI | = | tissue factor pathway inhibitor |
| TIMP | = | tissue inhibitors of metalloproteinases |
| TNFα | = | tumor necrosis factor α |
| t‐PA | = | tissue plasminogen activator |
| VCAM | = | vascular cell adhesion molecule |
| VRFs | = | vascular risk factors |
Introduction
A number of soluble markers of inflammation, endothelial damage and hemostasis have been linked with the atherosclerotic process Citation1. On the basis of the relationship of their plasma levels with the occurrence/extent of atherosclerosis, these molecules have been evaluated as predictors of cardiovascular events, as promoters of disease progression, and as determinants of the effectiveness of cardiovascular therapies Citation2. These associations could arise from the involvement of these soluble markers in basic phenomena of the atherogenic process. Activated monocytes adhere to the endothelium through intercellular and vascular adhesion molecules (ICAM and VCAM) and migrate through the endothelial layer, a process facilitated by E‐selectin and monocyte chemoattractant protein‐1 (MCP‐1). In the subendothelial space, monocytes differentiate into macrophages, subsequently filled with modified cholesterol‐rich lipoproteins to become foam cells. This process is amplified by cytokines and acute phase reactants and leads to fatty streak development, the first step in atherosclerotic plaque formation. Increased platelet adherence/aggregation plays an important role in this process Citation3.
Besides the association of soluble markers with angiographic measures of atherosclerosis or with clinical vascular events, their role in the atherosclerotic process may be estimated by investigating their relationship with subclinical atherosclerosis assessed by noninvasive techniques. One of most favored markers of atherosclerosis is the thickness of the intima‐media complex in carotid arteries, the IMT Citation4. On the basis of 20 years of research, carotid IMT (C‐IMT) is now widely accepted as a surrogate marker of carotid/coronary atherosclerosis and a predictor of cardiovascular events Citation5–7.
Carotid IMT increases naturally with aging Citation8, but conventional and nonconventional atherosclerosis risk factors may accelerate the process Citation9. More than a hundred studies have been published so far associating C‐IMT and these soluble markers, but opposing data have also been reported.
The present review summarizes these findings and investigates whether the dissimilar prevalence of atherosclerotic disease (atherosclerotic burden) in patients included in the different studies could explain the observed inconsistencies. For this purpose, data from studies in groups with increasingly higher atherosclerotic burdens were compared. The literature was further explored to search for other potential characteristics of patients that might influence the C‐IMT‐soluble markers associations.
Key messages
C‐reactive protein and fibrinogen are the soluble markers most frequently related significantly to subclinical atherosclerosis as measured by carotid intima‐media thickness (C‐IMT).
The atherosclerotic burden of the groups investigated does not account for the heterogeneity of reported findings relating to associations between soluble markers and C‐IMT.
Studies using highly standardized protocols for C‐IMT measurement and rigorous multivariate statistical approaches are needed to elucidate the still controversial relationship between soluble markers and C‐IMT.
Methods
PubMed and Embase from 1990 to March 2006 were searched for articles in English, using the following keywords: IMT OR intima media thickness OR intimal medial thickness AND the full name or abbreviations of all soluble markers listed in Table , one at a time. Synonyms of soluble markers were identified in the iHOP web page and included in the literature search.
Only cross‐sectional studies examining the relationship between C‐IMT and soluble markers of low‐grade inflammation, endothelial damage, and hemostasis were included. References of included articles were also examined for relevant studies.
Articles considered of low methodological quality were excluded. The key criteria of quality were: reproducibility of ultrasound method, appropriateness of control group, and clarity of definition of inclusion and exclusion criteria.
Methods and results of all studies selected were tabulated. Data from studies reporting univariate or multivariate correlation/associations were included in the analyses.
A Fisher's exact test was first performed to assess whether the number of significant results among the published studies was different from expected according to the null hypothesis of no correlation between each soluble marker and C‐IMT. Moreover, a funnel plot was used to evaluate publication bias Citation10. For this analysis the correlation coefficients were normalized (i.e. transformed in order to obtain a variable with a normal distribution) according to the formula of Fisher Citation11.
In order to assess whether the atherosclerotic profile of the population studied affects the association between C‐IMT and soluble markers, the results from studies performed in healthy subjects, general populations, patients with vascular risk factors (VRFs) (e.g. hyperlipidemia, hypertension, or type 2 diabetes), and patients with overt atherosclerotic cardiovascular disease (CVD) were compared, assuming that the disease prevalence, herein termed ‘atherosclerosis burden’, progressively increase from the first to the last group.
Age, gender, and the prevalence of dyslipidemia, obesity, diabetes mellitus, or hypertension in the different studies were considered as further potential sources of inconsistency in the reported results.
Results
The search strategy identified 370 articles; 136 were rejected after reading the title and abstract, because they: 1) included patients with acute or chronic inflammatory diseases (e.g. rheumatoid arthritis) or inflammatory conditions (e.g. chronic renal failure), 2) included only children, 3) reported only relationships with genotypes, 4) were ex vivo studies, 5) were reviews, 6) were intervention studies not reporting baseline data, 7) described protocols of studies not yet performed, or 8) were published after March 2006 unless e‐publication was available before this date.
Among the 239 full articles retrieved, 131 were rejected because they: 1) were longitudinal studies not reporting baseline data, 2) were studies performed only after meals, 3) did not report correlations or associations between C‐IMT and soluble markers, 4) assessed atherosclerosis in arteries other than carotids or used ultrasonic measurements other than IMT, 5) did not clearly describe the patient recruitment method, 6) did not provide data on ultrasound reproducibility, 7) did not discuss the valid comparability of groups or did not describe inclusion or exclusion criteria clearly, and 8) did not determine C‐reactive protein (CRP) by a highly sensitive method.
A total of 107 articles were finally considered for this review, including one progression study Citation12 which also reported baseline cross‐sectional data. Table summarizes the characteristics of the patient groups included in the studies herein reviewed.
Table I. Characteristics of studies reviewed.
Markers of low‐grade inflammation
C‐reactive protein
C‐reactive protein (CRP) is an acute phase protein that is synthesized by the liver during inflammation in response to interleukin (IL)‐6 and other proinflammatory cytokines as well as by vascular smooth muscle cells Citation13, Citation14. An active role of this protein in atherogenesis has been suggested Citation15. The plasma concentration of CRP is also considered an independent predictor of the incidence and progression of cardiovascular disease (CVD) that is even more powerful than conventional VRFs such as low‐density lipoprotein cholesterol (LDL‐C) Citation16.
The relationship between plasma levels of CRP and C‐IMT was investigated in 65 groups within 54 studies Citation17–70. A significant univariate correlation/association was found in 39 groups (60%) within 29 studies Citation19, Citation23, Citation25, Citation27, Citation32, Citation33, Citation35, Citation37–40, Citation42, Citation44–46, Citation48, Citation49, Citation52, Citation55, Citation57–60, Citation62, Citation66–70, whereas fewer than 4 (5%) were expected by chance if there were no relationship between the two variables (P<0.001 by Fisher's exact test) and assuming no publication bias.
Figure shows the funnel plot analysis of univariate correlations between plasma levels of CRP and C‐IMT obtained by plotting correlation coefficients versus the sample size of the groups. The overall result (dashed line in the figure) supports a significant positive correlation between C‐IMT and CRP concentrations. Although the distribution is not perfectly symmetrical, the absence of publication bias is suggested by the lack of change in the overall effect after studies with fewer than 100 or even 200 patients (data not shown) are excluded.
Figure 1 Funnel plots of univariate correlations between C reactive protein(CRP) and carotid‐intima media thickness (C‐IMT) obtained by plotting univariate correlation coefficients against the sample size of groups studied. Dashed lines indicate the overall effect observed in the meta‐analysis. Publication bias would be suspected if there were a cluster of small studies on the upper left side of the graph not balanced by a similar cluster in the lower left side. Numbers within (or very close to) markers are references. VRF, vascular risk factors. CVD, cardiovascular diseases.
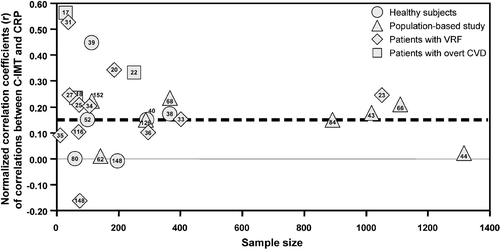
The number of groups with significant univariate associations was significantly higher than expected in population‐based cohorts (19 out of 20; versus 1 expected by chance alone, P<0.0001 by Fisher's exact test) (32,33,35,37–40,42,44–46,48) and in groups of patients with VRFs (11 out of 28; versus 1.4, P<0.0001) Citation23, Citation26, Citation30, Citation32–37. Although in healthy subjects Citation38–41 and in patients with overt CVD Citation17–19 the number of significant associations (4 out of 11, and 3 out of 3, respectively) was also higher than <1 expected by chance, the number of groups considered was too small for statistical analysis.
In the 59 groups in which multivariate analyses have been performed Citation17, Citation19, Citation20, Citation22–34, Citation36–38, Citation40–42, Citation44, Citation46–49, Citation51, Citation53, Citation55, Citation57, Citation58, Citation60–62, Citation64, Citation65, the association between CRP and C‐IMT was significant in 20 groups Citation29, Citation30, Citation32, Citation33, Citation36, Citation42–48 (versus 3 expected by chance, P<0.001 by Fisher's exact test).
No significant multivariate relationships were observed in studies performed in healthy subjects (n = 10) Citation24, Citation29, Citation38–41, Citation49–52, whereas significant associations were observed in 8 out of 24 groups with VRFs (versus 1 expected, P<0.03) Citation29, Citation30, Citation32, Citation33, Citation36, Citation7, in 11 out of 23 from general population groups (versus 1 expected, P<0.002) Citation42–47, and in the single study performed in subjects with overt CVD Citation48. No trend between the atherosclerotic burden as defined above and the prevalence of significant associations between CRP and C‐IMT was observed.
Studies showing significant univariate associations had a higher proportion of males (51.3%; P<0.0001), whereas females were more represented in negative studies (52%; P<0.01). The age or the prevalence of hypertension was similar in studies showing or not showing significant associations. Among patients with VRF, a higher prevalence of significant univariate associations was found in noninsulin‐dependent diabetes mellitus (NIDDM) (6 out of 12 versus <1 expected just by chance, P<0.0001) but not in hyperlipidemics (1 out of 3) or hypertensives (1 out of 4).
The mean value of total and LDL‐cholesterol of groups showing univariate associations was significantly higher than in groups showing no associations (5.59±0.48 versus 5.13±0.73 mmol/L, P = 0.013; and 3.6±0.37 versus 3.12±0.52 mmol/L, P = 0.002, respectively).
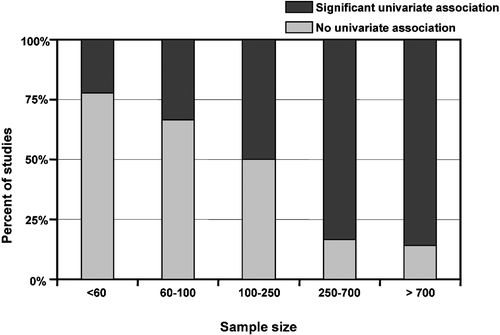
Figure shows the proportion of significant univariate associations between C‐IMT and CRP according to the sample size. Beyond showing the general statistical concept that the smaller the relation between two variables, the larger the sample size required to prove it as significant, the lack of an excess of positive associations in small studies (first bars) further suggests the absence of an important publication bias.
Figure 2 Percentage of studies with and without univariate association between C reactive protein(CRP) and carotid‐intima media thickness (C‐IMT).
With multivariate analyses, we found only a higher concentration of fasting glucose among groups showing positive associations (7.66±2.05 versus 5.92±1.34 mmol/L, P = 0.002).
The relationship between C‐IMT and CRP in obese subjects (body mass index≥30) was investigated in five studies only Citation32, Citation49, Citation50, Citation53, Citation54, and all failed to show multivariate associations between the two variables. However, stratifying all the groups considered in this review into tertiles of body mass index (BMI) (whenever this information was available), a higher proportion of studies showing a significant multivariate association between CRP and C‐IMT was observed in the highest BMI tertile.
Tumor necrosis factor α
Tumor necrosis factor α (TNFα) is a proinflammatory cytokine predominantly produced by monocytes/macrophages, endothelial cells, and smooth muscle cells Citation55. It acts via TNF receptors on target cells. Subjects with elevated TNFα levels are at increased risk of coronary death and recurrent myocardial infarction, independently of other VRFs Citation56.
A few studies have so far investigated the role of TNFα as a determinant of C‐IMT Citation25, Citation31, Citation38, Citation57–60. One out of two studies performed in healthy men Citation57, Citation61 showed a significant univariate association, not, however, confirmed after data adjustment for metabolic risk indicators for coronary artery disease Citation57. Forcing TNFα into the model, TNFα accounted for 6% of C‐IMT variability Citation57. In two population‐based studies Citation58, Citation62, no correlation between C‐IMT and TNFα was found either before or after data adjustment for VRF.
Among studies performed in patients with VRFs Citation25, Citation31, Citation59, no correlation was found either in first‐degree relatives of patients with NIDDM Citation31 or in patients with hyperlipidemia Citation25. A significant correlation was observed, however, in patients with NIDDM after data adjustment for age Citation59. In patients with manifest or suspected coronary artery disease (CAD) no correlation was found Citation60.
Interleukins
Interleukins are a class of cytokines that play an important role in the initiation and control of inflammatory processes Citation63. Interleukins are involved in cell activation, differentiation, chemotaxis, and proliferation in a broad range of cell types Citation64. Different interleukins are thought to play a role in the atherosclerotic process Citation63. Associations with C‐IMT have been studied for IL‐1β, IL‐2, IL‐6, IL‐8, and IL‐18. Among three studies performed in healthy men Citation53, Citation61, Citation65, only one reported a univariate correlation between IL‐6 and C‐IMT Citation53. In population‐based studies, no association between either IL‐1β or IL‐6 and C‐IMT was observed in multivariate analyses Citation44, Citation62, Citation66–68. The correlation between C‐IMT and IL‐18 was observed in one Citation68 but not in another study Citation67. A positive and significant association between IL‐2 and C‐IMT was also reported, which persisted after data adjustment for VRF Citation62.
Several studies have investigated patients with VRFs. In dyslipidemic patients, C‐IMT was significantly correlated with IL‐6 in univariate but not multivariate analyses Citation25, Citation69. In hypertensive patients, the correlation with IL‐6 was not significant Citation20, Citation27 even after data adjustment for VRF Citation27. In NIDDM patients, both a significant correlation Citation33 and no correlation Citation53 between C‐IMT and IL‐6 were observed. A univariate correlation with C‐IMT was found for IL‐18 in three studies Citation33, Citation65, Citation70, but in none of these were the results confirmed by multivariate analyses.
Two studies in patients with overt CVD showed no correlation between IL‐6, IL‐8 or IL‐18 and C‐IMT Citation60, Citation71, even though a negative correlation between IL‐6 and carotid lumen diameter—another index of carotid atherosclerosis—was observed in multivariate analysis Citation60.
Other inflammatory markers
Inflammatory molecules such as monocyte chemoattractant protein‐1 (MCP‐1), CD40 ligand (CD40L), and serum amyloid A (SAA) have also been considered as predictors of CVD Citation72–74, but their relationship with C‐IMT has not been extensively investigated. To the best of our knowledge, only one study provided data in healthy subjects showing a significant correlation between C‐IMT and SAA Citation53. In a general population, MCP‐1 was related to C‐IMT in univariate analysis Citation75.
In patients with VRFs, two studies in NIDDM patients have been reported. In one, SAA did not correlate with maximum C‐IMT in univariate analysis but correlation was significant after data adjustment for age, sex, smoking, and body mass index Citation53. In the other, no correlation between C‐IMT and MCP‐1 was found Citation76. Finally, a single study carried out in patients with overt CVD reported that C‐IMT was not correlated with MCP‐1 or SAA after data adjustment for VRF Citation60.
Markers of endothelial damage
Adhesion molecules
Adhesion molecules are responsible for the attraction and adhesion of monocytes to the activated endothelium and for their transendothelial migration. Both intercellular adhesion molecule‐1 (ICAM‐1) and vascular adhesion molecule‐1 (VCAM‐1) have been associated with atherosclerosis development Citation77, Citation78. E‐selectin is produced by endothelial cells and mediates endothelial rolling of leukocytes. The role of E‐selectin in CVD is less clear, although it has been suggested to be particularly significant in diabetics Citation79.
Among the six studies performed in healthy subjects Citation49, Citation53, Citation57, Citation61, Citation80, Citation81, three found no correlation between C‐IMT and ICAM or VCAM in multivariate analysis Citation49, Citation61, Citation81, whereas one reported a significant multivariate correlation with VCAM Citation53. Two further studies found no correlation in univariate analyses Citation57, Citation80. In healthy subjects, no correlation between C‐IMT and E‐selectin was found in multivariate analyses Citation49, Citation53, Citation61; in the unique study in which E‐selectin was the strongest predictor of C‐IMT, the association was lost when TNFα was added as a forced variable into the multivariate model Citation57.
Several population‐based studies have investigated the correlation between C‐IMT and ICAM or VCAM. Three studies did not identify any correlation Citation44, Citation82, Citation83, but two other studies showed correlations between both soluble markers and mean C‐IMT Citation84, Citation85 even after data adjustment for VRF Citation85. VCAM also correlated with maximum C‐IMT, but the correlation was lost after data adjustment for age Citation85.
For patients with VRFs, both significant Citation28, Citation30, Citation86, Citation87 and nonsignificant correlations Citation12, Citation49, Citation53, Citation88–91 were found between C‐IMT and ICAM. In mildly hypercholesterolemic patients, ICAM was related to C‐IMT in both univariate and multivariate analyses Citation87. Two studies in members of familial hypercholesterolemia (FH) families provided contrasting results Citation88, Citation92: Paiker et al. Citation88 found no correlation between C‐IMT and ICAM, VCAM or E‐selectin, whereas Karasek et al. Citation92 found such a relationship with ICAM but not VCAM; however, no multivariate analyses were reported. Contradictory data were also found in hypertensives Citation28, Citation89, Citation93. No relationship was observed in NIDDM patients between C‐IMT and ICAM, VCAM or E‐selectin Citation49, Citation53, Citation90. In two studies, one performed in patients at risk of NIDDM Citation30 and one in NIDDM patients and healthy subjects pooled together, Citation94 multivariate analysis confirmed the correlation between C‐IMT and ICAM Citation30 and VCAM Citation94.
In the only study carried out in patients with overt CVD, no correlation between C‐IMT and ICAM or VCAM was detected by multivariate analysis Citation60.
Von Willebrand factor
Von Willebrand factor (vWF) is a glycoprotein produced by endothelial cells that is involved in thrombus formation during vascular injury and is regarded as a well established marker of endothelial dysfunction Citation95. In addition, it has been shown to be related to the progression of CVD Citation96.
Among studies performed in healthy subjects Citation40, Citation57, Citation97–99, only one provided evidence of univariate correlation between vWF and C‐IMT Citation57. Three out of the seven population‐based studies found no univariate or multivariate correlation Citation83, Citation100, Citation101. Among the others, one reported only a univariate correlation Citation84, one a multivariate correlation Citation102, and two a multivariate correlation confined to women Citation103, Citation104.
In patients with VRFs, both a correlation Citation99, Citation105 and no correlation Citation36, Citation57, Citation91 have been reported. No correlation between C‐IMT and vWF was found in hypertensive patients with peripheral vascular disease Citation93.
MMPs and TIMPs
Matrix metalloproteinases (MMPs) are a family of proteolytic enzymes, mainly secreted by macrophages and smooth muscle cells, which regulate the physiological remodeling of vascular extracellular matrix. Their activity is inhibited by tissue inhibitors of metalloproteinases (TIMPs), also produced by macrophages. Their involvement in various steps of atherogenesis is well established Citation106.
Although more than 20 members of the MMP and 4 of the TIMP families are known, only MMP‐3, MMP‐9, TIMP‐1, and TIMP‐2 have been investigated for their relationships with C‐IMT. To the best of our knowledge, no studies in healthy subjects have been performed. In a population‐based study, TIMP‐1, MMP‐3, and MMP‐9 were not found to be associated with C‐IMT in multivariate analysis Citation107. With respect to studies including patients with VRFs, no association was found between MMP‐3, MMP‐9, TIMP‐1 and TIMP‐2 and C‐IMT in dyslipidemic patients Citation108. In this study, however, MMP‐3 and TIMP‐1 concentrations were significantly associated with obstructive carotid arterial lesions (10%–25% luminal obstruction). Studies in patients with overt CVD were not found.
Markers of hemostasis
Fibrinogen
Fibrinogen, a large glycoprotein produced by the liver, plays a role in platelet aggregation, endothelial cell injury, and plasma viscosity. A high fibrinogen plasma concentration is associated with increased cardiovascular risk and increases the prediction of cardiovascular events Citation109.
The relationship between fibrinogen and C‐IMT was investigated in 59 groups within 39 studies Citation8, Citation20, Citation25, Citation32, Citation36, Citation38–40, Citation42, Citation45, Citation50, Citation51, Citation56, Citation57, Citation63, Citation69, Citation94, Citation107–111, Citation113, Citation119–134. A significant univariate correlation/association was found in 33 groups (55.9%) Citation8, Citation20, Citation25, Citation32, Citation38–40, Citation42, Citation45, Citation56, Citation57, Citation69, Citation107, Citation109–111, Citation113, Citation120, Citation122, Citation124–130, Citation132, Citation133, when fewer than 3 (5%) were expected by chance alone, according to the null hypothesis of no correlation between the two variables (P<0.0001 by Fisher's exact test) and assuming no publication bias.
Figure shows the funnel plot analysis of univariate correlations between fibrinogen and C‐IMT. The overall results support a significant positive correlation between C‐IMT and fibrinogen, and no relevant publication bias can be detected.
Figure 3 Funnel plots of univariate correlations between fibrinogen and carotid‐intima media thickness (C‐IMT) (see legend of Figure ). VRF, vascular risk factors. CVD, cardiovascular diseases.
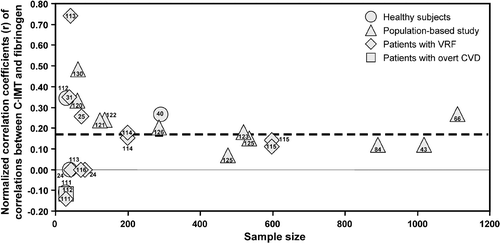
The number of groups with significant univariate associations between fibrinogen and C‐IMT Citation8, Citation32, Citation38–40, Citation42, Citation45, Citation111, Citation113, Citation120, Citation122, Citation125, Citation126, Citation128, Citation129 was higher than expected in population‐based cohorts (17 out of 21; versus 1 expected by chance, P<0.0001 by Fisher's exact test) and in patients with VRF Citation31, Citation32, Citation97, Citation113–115 (9 out of 20 versus 1 expected by chance, P<0.0001 by Fisher's exact test). Although in healthy subjects the number of positive associations (3 out of 8) Citation40, Citation99, Citation110 was also higher than the 5% expected by chance alone, the number of groups considered was too small for statistical consideration. In the single study performed in patients with overt CVD, no significant association was found Citation112.
At least four studies Citation8, Citation32, Citation101, Citation119 showed a correlation between fibrinogen and C‐IMT in men but not in women; in addition, females were more represented in negative studies (52.1%; P<0.0001). The mean age was similar in positive and negative studies, whereas the mean systolic and diastolic blood pressures were higher in positive than in negative studies (+12.7 and +6.8 mmHg, P = 0.003 and P = 0.025, respectively). The mean value of total and fasting glucose and BMI of groups showing univariate or multivariate associations was not significantly different from that of groups showing no association, whereas LDL‐cholesterol was higher in groups showing multivariate association (3.59±0.38 mmol/L versus 4.18±0.5 mmol/L, P = 0.018).
In contrast to CRP, the probability of finding a significant association was not related to sample size.
In the 49 groups within 29 studies Citation8, Citation20, Citation25, Citation32, Citation36, Citation38, Citation40, Citation42, Citation45, Citation56, Citation57, Citation69, Citation94, Citation107, Citation109–111, Citation113, Citation119–122, Citation125, Citation127–130, Citation132, Citation133 in which multivariate analyses was performed, the association between fibrinogen and C‐IMT was significant in 23 groups Citation8, Citation25, Citation32, Citation38, Citation42, Citation57, Citation69, Citation107, Citation109–111, Citation113, Citation119, Citation122, Citation125, Citation127–129, Citation133 (versus 2.4 expected by chance alone, P<0.0001 by Fisher's exact test). A significant association was observed in 2 Citation40, Citation99 out of 4 Citation24, Citation40, Citation99, Citation110 groups of healthy subjects, in 13 Citation8, Citation43, Citation47, Citation101, Citation104, Citation119–123 out of 27 groups Citation8, Citation43, Citation45, Citation47, Citation66, Citation83, Citation101, Citation104, Citation119–127 from the general population (versus 1.35 expected, P<0.001), and in 6 Citation32, Citation97, Citation113, Citation115 out of 14 groups Citation24, Citation31, Citation32, Citation97, Citation99, Citation113, Citation115 with VRFs. No studies with overt CVD patients were available.
Other markers of hemostasis
Other soluble markers of hemostasis have been studied in relation to C‐IMT, i.e. tissue plasminogen activator (t‐PA), plasminogen activator inhibitor‐1 (PAI‐1), tissue factor pathway inhibitor (TFPI), prothrombin fragment F1+2 (F1+F2), factor VII, factor VIII, and antithrombin. All these markers may play a role in the development of CVD by modulating the balance between coagulation and fibrinolysis Citation128.
In healthy subjects, a correlation was found between F1+2 and C‐IMT, explaining 6.5% of IMT variance Citation129. No correlation was observed between C‐IMT and FVII Citation110. In population‐based studies, C‐IMT did not correlate with free TFPI, t‐PA, FVII, FVIII, PAI‐1 or antithrombin III Citation45, Citation100, Citation101, Citation103, Citation104, Citation119, Citation120, Citation124, Citation130. However, in one study C‐IMT correlated with PAI‐1, in men only Citation103. In combined hyperlipidemia, TFPI was a determinant of C‐IMT variance Citation25, whereas t‐PA and PAI‐1 were not Citation25, Citation111. In two studies in hypertensive patients, PAI‐1 Citation114, Citation131 and t‐PA Citation132 were related to C‐IMT; in other studies, no such association was found Citation28, Citation132. In patients with carotid disease, C‐IMT correlated with antithrombin III concentrations but not with factor VII or VIII Citation100. In coronary patients, t‐PA and PAI‐1 were not correlated to C‐IMT in univariate analysis Citation112.
Considerations on protocols used to measure C‐IMT
Table clearly shows substantial heterogeneity among the protocols used to measure C‐IMT in the studies reviewed. To evaluate whether this aspect could have influenced the relationship between C‐IMT and the soluble markers considered, we recalculated the prevalence of groups showing significant associations after stratifying them according to the following aspects of the protocol used for C‐IMT measurement: location (common carotid, bifurcation, internal carotid, or composite), outcomes (Mean‐IMT, Max‐IMT, or Mean Max‐IMT), arterial wall (far wall, near wall, or both), carotid side (left plus right, or only right) and exclusion/inclusion of plaques. In these analyses, the prevalence of significant associations between C‐IMT and fibrinogen was higher than expected when Mean‐IMT and Max‐IMT were utilized and much lower when Mean Max‐IMT was used (P<0.0001 by chi‐square). In addition, the prevalence of significant associations between C‐IMT and CRP was higher than expected, with a P‐value close to statistical significance (0.062 by chi‐square), in studies excluding atherosclerotic plaques. No other significant differences between strata were observed.
Discussion
This review of data from 107 studies, addressing the relationship between carotid intima‐media thickness and soluble markers of inflammation, endothelial damage or hemostasis, leads to the conclusion that C‐IMT associates unequivocally only with fibrinogen and CRP plasma concentrations. Despite the marked heterogeneity of results present in the literature, the meta‐analysis shows (dashed lines in the funnel plots) a predominance of studies with significant positive associations between C‐IMT and CRP or fibrinogen. The symmetry of funnel plots, the constancy of the overall effect in the meta‐analyses after exclusion of small studies, and the lack of an excess of positive associations in small studies (first bars of Figure ) exclude publication bias, for both CRP and fibrinogen, as a significant effect on the univariate results.
Reliable meta‐analysis could not be performed on multivariate results because of the variety of statistical methods used in each study to take confounding variables into account (partial correlation, multiple regression, covariance analyses, and others). However, independently of the statistical approach used, a multivariate association with C‐IMT was observed in 20 out of 58 groups for CRP and in 23 out 49 groups for fibrinogen. Even though in multivariate analyses a publication bias cannot be completely excluded, the prevalence of studies linking C‐IMT with both fibrinogen and CRP greatly exceeds the statistical threshold of 5% expected on the basis of the hypothesis of a null relationship between the variables considered and C‐IMT Citation133. In addition, the lack of an excess of positive multivariate associations in small studies strongly suggests that also in this case publication bias could have only marginally affected the results.
Available data about the relationship between C‐IMT and all other soluble markers are either scanty or mutually contradictory and in any case unconfirmed by multivariate analyses. In addition, a strong publication bias for all the variables considered was detected by funnel plot analyses (data not shown).
In an attempt to explain the incomplete agreement about the relationship between C‐IMT and CRP or fibrinogen and the failure to establish a link between C‐IMT and other soluble markers of atherogenic processes, we reexamined the literature, taking into account in addition the potential influence on these associations of the atherosclerotic burden and other characteristics of the patients included in the different studies.
We found no trend between the atherosclerotic burden, as defined in this study, and the prevalence of significant associations between C‐IMT and any of the variables considered. A similar conclusion was reached by Moussavi et al. Citation49 in diabetic patients, showing that plasma concentrations of soluble markers are not directly linked to the atherosclerotic burden.
Sample size seems to be one of the most important determinants of the probability to find a significant univariate association between IMT and CRP, with an insufficient power when groups under study include less than 100–250 patients (Figure ).
Among other patients' features that could potentially influence the correlation between C‐IMT and soluble markers (i.e. gender, prevalence of risk factors, etc.), a predominance of men and a higher serum concentrations of fasting glucose and cholesterol (both total and LDL) was observed in groups showing a positive and significant relationship between C‐IMT and CRP, thus suggesting a possible role of these variables in this association. However, the fact that a higher prevalence of significant associations was found in NIDDM patients but not in hyperlipidemics or hypertensives suggests that the IMT‐CRP relationship is not influenced by the high‐risk status per se, but that different risk factors may affect the relationship through specific pathways. Type 2 diabetes, for example, is known to be associated with both increased C‐IMT Citation134 and elevated hs‐CRP levels Citation135.
A number of cross‐sectional studies have shown an effect of obesity on both C‐IMT Citation136 and CRP concentrations Citation137, Citation138, and some authors even suggest that knowing the degree of obesity is essential for the interpretation of the relationship between CRP and severity of CAD Citation139. The literature reviewed here does not support this view; in fact, although a higher proportion of studies showed a significant multivariate association between C‐IMT and CRP in the highest BMI tertiles of normal‐weight groups, none of the studies specifically performed in obese patients detected multivariate associations between the two variables.
The higher proportion of women in studies showing no association between C‐IMT and fibrinogen, as well as the higher mean values of systolic and diastolic blood pressure and LDL‐C in studies reporting a significant association, suggest that gender, blood pressure, and cholesterol levels could affect the atherogenic role of fibrinogen.
Fibrinogen itself may influence the relationship between CRP and C‐IMT. This possibility is supported by a study showing that the relationship between CRP and C‐IMT disappears when fibrinogen is added into the multivariate model Citation43.
Besides the characteristics of the patients, other possible sources for the inconsistencies in conclusions about associations could be methodological. Since many factors may influence C‐IMT, in determining the importance of an individual factor one should perform the analysis after data adjustment for all variables known to influence both the C‐IMT and the variable. For instance, C‐IMT increases with age more in men than in women Citation8, and gender differences also exist for soluble markers Citation140; thus, adjustments at least for age and gender are mandatory. Similarly, smoking is an important life‐style determinant both of fibrinogen concentration Citation141 and of C‐IMT Citation142, and adjustment for this life‐style component is also vital. Unfortunately, as shown in Table , these adjustments have rarely been made in the studies reviewed.
Again, since some soluble markers seem to relate better to localized plaques or complex lesions than to C‐IMT Citation108, Citation126, Citation143, inconsistencies of reported results may depend on whether plaques are incorporated into the IMT measurement or not. For example, ICAM showed no correlation with C‐IMT when plaques were not included in the IMT measurement Citation90, but correlated with plaque score Citation91 and Max‐IMT but not with Mean‐IMT Citation53.
Another source for the inconsistent relationship between C‐IMT and soluble markers may be the site of the IMT measured in different studies. IMT is different in common carotid, bifurcation or internal carotid artery of the same individual Citation144. In addition, some VRFs are related to C‐IMT in one segment but not in others Citation145, Citation146. For example, since hypertension induces medial hypertrophy (generally not considered as atherosclerosis) mainly in the common carotid artery Citation93, it has been suggested that C‐IMT should be measured as an atherosclerosis surrogate in hypertensives just at the bifurcation, because of the smaller number of smooth muscle cells at this site Citation147. Similarly, the outcomes (Mean‐, Max‐ or Mean Max‐IMT) as well as the arterial wall (far wall, near wall or both) or the carotid side (left plus right or just right) selected could also have influenced the relationship between C‐IMT and inflammatory markers, endothelial damage, and hemostasis. This plausible possibility cannot be resolved by the data available.
A final issue is the validity of the funnel plot approach. The funnel plot has been advocated to scrutinize meta‐analyses for publication bias Citation154. A common interpretation of funnel plots is that, when the points distribute around the overall effect asymmetrically and the plot loses the expected shape of an inverted funnel, then a publication bias may be present. It is worth acknowledging, however, that other factors besides publication bias (i.e. the different definition of precision and/or measured effect) may affect the shape of the funnel plot Citation155, Citation156, thus raising concerns about the appropriateness of this statistical approach to exclude publication bias. Thus, in the absence of consensus on how the plot should be constructed, the existence of publication biases in the meta‐analyses performed in the present study may not be definitely ruled out.
Conclusions
The present systematic review of studies addressing the association between subclinical carotid atherosclerosis and soluble markers of inflammation, endothelial damage or hemostasis shows that plasma CRP and fibrinogen levels are the variables most consistently related to C‐IMT. No clear conclusions can be drawn for other soluble markers. Atherosclerotic burden does not appear to account for the heterogeneity of the findings reported in the literature. Among other patients' characteristics, gender, presence of NIDDM, and hypercholesterolemia were seen to influence the association between C‐IMT and CRP, whereas blood pressure and hypercholesterolemia seem to affect the association between C‐IMT and fibrinogen. For the other soluble markers considered, the number of groups was too small for adequate statistical treatment.
Further studies using highly standardized protocols for C‐IMT measurement and rigorous multivariate statistical approaches are needed to elucidate the still controversial relationship between soluble markers and C‐IMT.
Acknowledgements
The first two authors contributed equally to the review.
References
- Blake G. J., Ridker P. M. Inflammatory bio‐markers and cardiovascular risk prediction. J Intern Med 2002; 252: 283–94
- Maisel A. Cardiac biomarkers: a contemporary status report. Nat Clin Pract Cardiovasc Med 2006; 107: 24–34
- Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med 1999; 340: 115–26
- Pignoli P., Tremoli E., Poli A., Oreste P., Paoletti R. Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation 1986; 74: 1399–406
- Wofford J. L., Kahl F. R., Howard G. R., McKinney W. M., Toole J. F., Crouse J. R 3rd. Relation of extent of extracranial carotid artery atherosclerosis as measured by B‐mode ultrasound to the extent of coronary atherosclerosis. Arterioscler Thromb 1991; 11: 1786–94
- Bots M. L., Hoes A. W., Koudstaal P. J., Hofman A., Grobbee D. E. Common carotid intima‐media thickness and risk of stroke and myocardial infarction: the Rotterdam Study. Circulation 1997; 96: 1432–7
- Burke G. L., Evans G. W., Riley W. A., Sharrett A. R., Howard G., Barnes R. W., et al. Arterial wall thickness is associated with prevalent cardiovascular disease in middle‐aged adults. The Atherosclerosis Risk in Communities (ARIC) Study. Stroke 1995; 26: 386–91
- Stensland‐Bugge E., Bonaa K. H., Joakimsen O. Age and sex differences in the relationship between inherited and lifestyle risk factors and subclinical carotid atherosclerosis: the Tromso study. Atherosclerosis 2001; 154: 437–48
- Simon A., Gariepy J., Chironi G., Megnien J. L., Levenson J. Intima‐media thickness: a new tool for diagnosis and treatment of cardiovascular risk. J Hypertens 2002; 20: 159–69
- Dickersin K. B. J. Meta‐analysis: state‐of‐the‐science. Epidemiol Rev 1992; 14: 154–76
- Snedecor G. W., Cochran W. G. Statistical methods. The Iowa State University Press, AmesUSA 1980; 185–8
- Kondo K., Kitagawa K., Nagai Y., Yamagami H., Hashimoto H., Hougaku H., et al. Associations of soluble intercellular adhesion molecule‐1 with carotid atherosclerosis progression. Atherosclerosis 2005; 179: 155–60
- Calabro P., Willerson J. T., Yeh E. T. Inflammatory cytokines stimulated C‐reactive protein production by human coronary artery smooth muscle cells. Circulation 2003; 108: 1930–2
- Jabs W. J., Theissing E., Nitschke M., Bechtel J. F., Duchrow M., Mohamed S., et al. Local generation of C‐reactive protein in diseased coronary artery venous bypass grafts and normal vascular tissue. Circulation 2003; 108: 1428–31
- Ji S. R., Wu Y., Potempa L. A., Liang Y. H., Zhao J. Effect of modified C‐reactive protein on complement activation: a possible complement regulatory role of modified or monomeric C‐reactive protein in atherosclerotic lesions. Arterioscler Thromb Vasc Biol 2006; 26: 935–41
- Ridker P. M. Clinical application of C‐reactive protein for cardiovascular disease detection and prevention. Circulation 2003; 107: 363–9
- Arroyo‐Espliguero R., Mollichelli N., Avanzas P., Zouridakis E., Newey V. R., Nassiri D. K., et al. Chronic inflammation and increased arterial stiffness in patients with cardiac syndrome X. Eur Heart J 2003; 24: 2006–11
- Orem C., Durmus I., Kilinc K., Baykan M., Gokce M., Orem A., et al. Plasma fibronectin level and its association with coronary artery disease and carotid intima‐media thickness. Coron Artery Dis 2003; 14: 219–24
- Benbir G., Bozluolcay M., Ince B. Is the level of C‐reactive protein correlated with the extent of carotid atherosclerosis?. Acta Neurol Belg 2005; 105: 73–80
- Choi H., Cho D. H., Shin H. H., Park J. B. Association of high sensitivity C‐reactive protein with coronary heart disease prediction, but not with carotid atherosclerosis, in patients with hypertension. Circ J 2004; 68: 297–303
- Magyar M. T., Szikszai Z., Balla J., Valikovics A., Kappelmayer J., Imre S., et al. Early‐onset carotid atherosclerosis is associated with increased intima‐media thickness and elevated serum levels of inflammatory markers. Stroke 2003; 34: 58–63
- Kojima S., Funahashi T., Maruyoshi H., Honda O., Sugiyama S., Kawano H., et al. Levels of the adipocyte‐derived plasma protein, adiponectin, have a close relationship with atheroma. Thromb Res 2005; 115: 483–90
- Blackburn R., Giral P., Bruckert E., Andre J. M., Gonbert S., Bernard M., et al. Elevated C‐reactive protein constitutes an independent predictor of advanced carotid plaques in dyslipidemic subjects. Arterioscler Thromb Vasc Biol 2001; 21: 1962–8
- Halenka M., Vaverkova H., Hutyra M., Karasek D., Slavik L., Novotny D., et al. Detection of early atherosclerosis using the ultrasound parameter of the intima‐media thickness of the common carotid artery in families with familial combined hyperlipidemia. Int Angiol 2004; 23: 230–7
- Sebestjen M., Zegura B., Videcnik V., Keber I. Determinants of endothelial dysfunction and carotid intima‐media thickness in combined hyperlipidemia. Coron Artery Dis 2005; 16: 175–80
- Takiuchi S., Kamide K., Miwa Y., Tomiyama M., Yoshii M., Matayoshi T., et al. Diagnostic value of carotid intima‐media thickness and plaque score for predicting target organ damage in patients with essential hypertension. J Hum Hypertens 2004; 18: 17–23
- Manabe S., Okura T., Watanabe S., Higaki J. Association between carotid haemodynamics and inflammation in patients with essential hypertension. J Hum Hypertens 2005; 19: 787–91
- Zavaroni I., Ardigo D., Zuccarelli A., Pacetti E., Piatti P. M., Monti L., et al. Insulin resistance/compensatory hyperinsulinemia predict carotid intimal medial thickness in patients with essential hypertension. Nutr Metab Cardiovasc Dis 2006; 16: 22–7
- Hak A. E., Stehouwer C. D., Bots M. L., Polderman K. H., Schalkwijk C. G., Westendorp I. C., et al. Associations of C‐reactive protein with measures of obesity, insulin resistance, and subclinical atherosclerosis in healthy, middle‐aged women. Arterioscler Thromb Vasc Biol 1999; 19: 1986–91
- Balletshofer B. M., Haap M., Rittig K., Stock J., Lehn‐Stefan A., Haring H. U. Early carotid atherosclerosis in overweight non‐diabetic individuals is associated with subclinical chronic inflammation independent of underlying insulin resistance. Horm Metab Res 2005; 37: 331–5
- Ahmad J., Ahmed F., Siddiqui M. A., Hameed B., Ahmad I. Inflammation, insulin resistance and carotid IMT in first degree relatives of north Indian type 2 diabetic subjects. Diabetes Res Clin Pract 2006; 73: 205–10
- Festa A., D'Agostino R Jr., Williams K., Karter A. J., Mayer‐Davis E. J., Tracy R. P., et al. The relation of body fat mass and distribution to markers of chronic inflammation. Int J Obes Relat Metab Disord 2001; 25: 1407–15
- Esposito K., Giugliano D., Nappo F., Marfella R. Regression of carotid atherosclerosis by control of postprandial hyperglycemia in type 2 diabetes mellitus. Circulation 2004; 110: 214–9
- Kang E. S., Kim H. J., Kim Y. M., Lee S., Cha B. S., Lim S. K., et al. Serum high sensitivity C‐reactive protein is associated with carotid intima‐media thickness in type 2 diabetes. Diabetes Res Clin Pract 2004; 66: S115–20
- Takebayashi K., Aso Y., Matsutomo R., Wakabayashi S., Inukai T. Association between the corrected QT intervals and combined intimal‐medial thickness of the carotid artery in patients with type 2 diabetes. Metabolism 2004; 53: 1152–7
- Tajiri Y., Mimura K., Umeda F. High‐sensitivity C‐reactive protein in Japanese patients with type 2 diabetes. Obes Res 2005; 13: 1810–6
- Kang E. S., Kim H. J., Ahn C. W., Park C. W., Cha B. S., Lim S. K., et al. Relationship of serum high sensitivity C‐reactive protein to metabolic syndrome and microvascular complications in type 2 diabetes. Diabetes Res Clin Pract 2005; 69: 151–9
- Hulthe J. F. B. Circulating oxidized LDL is associated with subclinical atherosclerosis development and inflammatory cytokines (AIR study). Arterioscler Thromb Vasc Biol 2002; 22: 1162–7
- Saijo Y., Kiyota N., Kawasaki Y., Miyazaki Y., Kashimura J., Fukuda M., et al. Relationship between C‐reactive protein and visceral adipose tissue in healthy Japanese subjects. Diabetes Obes Metab 2004; 6: 249–58
- Beloqui O., Paramo J. A., Orbe J., Benito A., Colina I., Monasterio A., et al. Monocyte cyclooxygenase‐2 overactivity: a new marker of subclinical atherosclerosis in asymptomatic subjects with cardiovascular risk factors?. Eur Heart J 2005; 26: 153–8
- Choi S. H., Kim H. C., Ahn C. W., Cho H. K., Cha B. S., Chung Y., et al. Is high‐sensitivity C‐reactive protein associated with carotid atherosclerosis in healthy Koreans?. Eur J Card Prev Rehab 2005; 12: 548–54
- Markus H., Kapozsta Z., Ditrich R., Wolfe C., Ali N., Powell J., et al. Increased common carotid intima‐media thickness in UK African Caribbeans and its relation to chronic inflammation and vascular candidate gene polymorphisms. Stroke 2001; 32: 2465–71
- Sitzer M., Markus H. S., Mendall M. A., Liehr R., Knorr U., Steinmetz H. C‐reactive protein and carotid intimal medial thickness in a community population. J Cardiovasc Risk 2002; 9: 97–103
- van der Meer I. M., de Maat M. P., Bots M. L., Breteler M. M., Meijer J., Kiliaan A. J., et al. Inflammatory mediators and cell adhesion molecules as indicators of severity of atherosclerosis: the Rotterdam Study. Arterioscler Thromb Vasc Biol 2002; 22: 838–42
- de Maat M. P., Bladbjerg E. M., Drivsholm T., Borch‐Johnsen K., Moller L., Jespersen J. Inflammation, thrombosis and atherosclerosis: results of the Glostrup study. J Thromb Haemost 2003; 1: 950–7
- Devynck M. A., Simon A., Pernollet M. G., Chironi G., Gariepy J., Rendu F., et al. Plasma cGMP and large artery remodeling in asymptomatic men. Hypertension 2004; 44: 919–23
- McDonald S. P., Maguire G. P., Duarte N., Wang X. L., Hoy W. E. Carotid intima‐media thickness, cardiovascular risk factors and albuminuria in a remote Australian Aboriginal community. Atherosclerosis 2004; 177: 423–31
- Winbeck K., Kukla C., Poppert H., Klingelhofer J., Conrad B., Sander D. Elevated C‐reactive protein is associated with an increased intima to media thickness of the common carotid artery. Cerebrovasc Dis 2002; 13: 57–63
- Moussavi N., Renier G., Roussin A., Mamputu J. C., Buithieu J., Serri O. Lack of concordance between plasma markers of cardiovascular risk and intima‐media thickness in patients with type 2 diabetes. Diabetes Obes Metab 2004; 6: 69–77
- Bowden D. W., Lange L. A., Langefeld C. D., Brosnihan K. B., Freedman B. I., Carr J. J., et al. The relationship between C‐reactive protein and subclinical cardiovascular disease in the Diabetes Heart Study (DHS). Am Heart J 2005; 150: 1032–8
- de Vries R., Dallinga‐Thie G. M., Smit A. J., Wolffenbuttel B. H., van Tol A., Dullaart R. P. Elevated plasma phospholipid transfer protein activity is a determinant of carotid intima‐media thickness in type 2 diabetes mellitus. Diabetologia 2006; 49: 398–404
- Lo J., Dolan S. E., Kanter J. R., Hemphill L. C., Connelly J. M., Lees R. S., et al. Effects of obesity, body composition, and adiponectin on carotid intima‐media thickness in healthy women. J Clin Endocrinol Metab 2006; 91: 1677–82
- Leinonen E. S., Hiukka A., Hurt‐Camejo E., Wiklund O., Sarna S. S., Mattson Hulten L., et al. Low‐grade inflammation, endothelial activation and carotid intima‐media thickness in type 2 diabetes. J Intern Med 2004; 256: 119–27
- Berneis K., Jeanneret C., Muser J., Felix B., Miserez A. R. Low‐density lipoprotein size and subclasses are markers of clinically apparent and non‐apparent atherosclerosis in type 2 diabetes. Metabolism 2005; 54: 227–34
- Barath P., Fishbein M. C., Cao J., Berenson J., Helfant R. H., Forrestor J. S. Detection and localization of tumor necrosis factor in human atheroma. Am J Cardiol 1990; 65: 297–302
- Ridker P. M., Rifai N., Pfeffer M., Sacks F., Lepage S., Braunwald E. Elevation of tumor necrosis factor‐alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation 2000; 101: 2149–53
- Skoog T., Dichtl W., Boquist S., Skoglund‐Andersson C., Karpe F., Tang R., et al. Plasma tumour necrosis factor‐alpha and early carotid atherosclerosis in healthy middle‐aged men. Eur Heart J 2002; 23: 376–83
- Elkind M. S., Cheng J., Boden‐Albala B., Rundek T., Thomas J., Chen H., et al. Tumor necrosis factor receptor levels are associated with carotid atherosclerosis. Stroke 2002; 33: 31–7
- Matsuda M., Kawasaki F., Yamada K., Kanda Y., Saito M., Eto M., et al. Impact of adiposity and plasma adipocytokines on diabetic angiopathies in Japanese Type 2 diabetic subjects. Diabet Med 2004; 21: 881–8
- Larsson P. T., Hallerstam S., Rosfors S., Wallen N. H. Circulating markers of inflammation are related to carotid artery atherosclerosis. Int Angiol 2005; 24: 43–51
- Hulthe J., Wikstrand J., Mattson‐Hulten L., Fagerberg B. Circulating ICAM‐1 (intercellular cell‐adhesion molecule 1) is associated with early stages of atherosclerosis development and with inflammatory cytokines in healthy 58‐year‐old men: the Atherosclerosis and Insulin Resistance (AIR) study. Clin Sci (Lond) 2002; 103: 123–9
- Elkind M. S., Rundek T., Sciacca R. R., Ramas R., Chen H. J., Boden‐Albala B., et al. Interleukin‐2 levels are associated with carotid artery intima‐media thickness. Atherosclerosis 2005; 180: 181–7
- von der Thusen J. H., Kuiper J., van Berkel T. J., Biessen E. A. Interleukins in atherosclerosis: molecular pathways and therapeutic potential. Pharmacol Rev 2003; 55: 133–66
- Young J. L., Libby P., Schonbeck U. Cytokines in the pathogenesis of atherosclerosis. Thromb Haemost 2002; 88: 554–67
- Nakamura A., Shikata K., Hiramatsu M., Nakatou T., Kitamura T., Wada J., et al. Serum interleukin‐18 levels are associated with nephropathy and atherosclerosis in Japanese patients with type 2 diabetes. Diabetes Care 2005; 28: 2890–5
- Chapman C. M., Beilby J. P., McQuillan B. M., Thompson P. L., Hung J. Monocyte count, but not C‐reactive protein or interleukin‐6, is an independent risk marker for subclinical carotid atherosclerosis. Stroke 2004; 35: 1619–24
- Chapman C. M., McQuillan B. M., Beilby J. P., Thompson P. L., Hung J. Interleukin‐18 levels are not associated with subclinical carotid atherosclerosis in a community population The Perth Carotid Ultrasound Disease Assessment Study (CUDAS). Atherosclerosis 2006; 189: 414–9
- Yamagami H., Kitagawi K., Hoshi T., Furukado S., Hougaku H., Nagai Y., et al. Associations of serum IL‐18 levels with carotid intima‐media thickness. Arterioscler Thromb Vasc Biol 2005; 25: 1458–62
- Okopien B., Hyper M., Kowalski J., Belowski D., Madej A., Zielinski M., et al. A new immunological marker of atherosclerotic injury of arterial wall. Res Commun Mol Pathol Pharmacol 2001; 109: 241–8
- Aso Y., Okumura K., Takebayashi K., Wakabayashi S., Inukai T. Relationships of plasma interleukin‐18 concentrations to hyperhomocysteinemia and carotid intima‐media thickness in patients with type 2 diabetes. Diabetes Care 2003; 26: 2622–7
- Jurcut R., Arsenescu I., Puscariu T., Uscatescu V., Jurcut C., Apetrei E., et al. Is interleukin‐18 correlated with endothelial dysfunction and platelet activation in patients with unstable angina?. Rom J Intern Med 2005; 43: 199–209
- Hoogeveen R. C., Morrison A., Boerwinkle E., Miles J. S., Rhodes C. E., Sharrett A. R., et al. Plasma MCP‐1 level and risk for peripheral arterial disease and incident coronary heart disease: Atherosclerosis Risk in Communities study. Atherosclerosis 2005; 183: 301–7
- Schonbeck U., Varo N., Libby P., Buring J., Ridker P. M. Soluble CD40L and cardiovascular risk in women. Circulation 2001; 104: 2266–8
- O'Brien K. D., Chait, A. Serum amyloid A: the ‘other’ inflammatory protein. Curr Atheroscler Rep 2006; 8: 62–8
- Tabara Y., Kohara K., Yamamoto Y., Igase M., Nakura J., Kondo I., et al. Polymorphism of the monocyte chemoattractant protein (MCP‐1) gene is associated with the plasma level of MCP‐1 but not with carotid intima‐media thickness. Hypertens Res 2003; 26: 677–83
- Takebayashi K., Matsumoto S., Aso Y., Inukai T. Association between circulating monocyte chemoattractant protein‐1 and urinary albumin excretion in nonobese Type 2 diabetic patients. J Diabetes Complications 2006; 20: 98–104
- Pradhan A. D., Rifai N., Ridker P. M. Soluble intercellular adhesion molecule‐1, soluble vascular adhesion molecule‐1, and the development of symptomatic peripheral arterial disease in men. Circulation 2002; 106: 820–5
- Yamamoto H., Uemura S., Tomoda Y., Fujimoto S., Hashimoto T., Okuchi K. Transcardiac gradient of soluble adhesion molecules predicts progression of coronary artery disease. Int J Cardiol 2002; 84: 249–57
- Roldan V., Marin F., Lip G. Y., Blann A. D. Soluble E‐selectin in cardiovascular disease and its risk factors. A review of the literature. Thromb Haemost 2003; 90: 1007–20
- Holmlund A., Hulthe J., Millgard J., Sarabi M., Kahan T., Lind L. Soluble intercellular adhesion molecule‐1 is related to endothelial vasodilatory function in healthy individuals. Atherosclerosis 2002; 165: 271–6
- Su T. C., Jeng J. S., Wang J. D., Torng P. L., Chang S. J., Chen C. F., et al. Homocysteine, circulating vascular cell adhesion molecule and carotid atherosclerosis in postmenopausal vegetarian women and omnivores. Atherosclerosis 2006; 184: 356–62
- Ahluwalia N., Drouet L., Ruidavets J. B., Perret B., Amar J., Boccalon H., et al. Metabolic syndrome is associated with markers of subclinical atherosclerosis in a French population‐based sample. Atherosclerosis 2006; 186: 345–53
- Bongard V., Elias A., Bal dit Sollier C., Ruidavets J., Boccalon H., Drouet L., et al. Soluble intercellular adhesion molecule‐1 is associated with carotid and femoral atherosclerosis but not with intima‐media thickness in a population‐based sample. Atherosclerosis 2002; 164: 297–304
- Amar J., Ruidavets J. B., Sollier C. B., Bongard V., Boccalon H., Chamontin B., et al. Relationship between C reactive protein and pulse pressure is not mediated by atherosclerosis or aortic stiffness. J Hypertens 2004; 22: 349–55
- Rohde L. E., Lee R. T., Rivero J., Jamacochian M., Arroyo L. H., Briggs W., et al. Circulating cell adhesion molecules are correlated with ultrasound‐based assessment of carotid atherosclerosis. Arterioscler Thromb Vasc Biol 1998; 18: 1765–70
- Hashimoto H., Kitagawa K., Kuwabara K., Hougaku H., Ohtsuki T., Matsumoto M., et al. Circulating adhesion molecules are correlated with ultrasonic assessment of carotid plaques. Clin Sci (Lond) 2003; 104: 521–7
- Bemelmans W. J., Lefrandt J. D., Feskens E. J., Broer J., Tervaert J. W., May J. F., et al. Change in saturated fat intake is associated with progression of carotid and femoral intima‐media thickness, and with levels of soluble intercellular adhesion molecule‐1. Atherosclerosis 2002; 163: 113–20
- Paiker J. E., Raal F. J., Veller M., von Arb M., Chetty N., Naran N. H. Cell adhesion molecules—can they be used to predict coronary artery disease in patients with familial hypercholesterolaemia?. Clin Chim Acta 2000; 293: 105–13
- Malmqvist K., Wallen H. N., Held C., Kahan T. Soluble cell adhesion molecules in hypertensive concentric left ventricular hypertrophy. J Hypertens 2002; 20: 1563–9
- Kawamura T., Umemura T., Kanai A., Uno T., Matsumae H., Sano T., et al. The incidence and characteristics of silent cerebral infarction in elderly diabetic patients: association with serum‐soluble adhesion molecules. Diabetologia 1998; 41: 911–7
- Troseid M., Hjerkinn E. M., Seljeflot I., Klemsdal T. O., Bergengen L., Breivik L., et al. Comparison of biochemical, functional and structural aspects of arterial wall properties in elderly men. Scand J Clin Lab Invest 2006; 66: 137–45
- Karasek D., Vaverkova H., Halenka M., Budikova M., Novotny D. Soluble cell adhesion molecules s‐VCAM‐1 and s‐ICAM‐1 in subjects with familial combined hyperlipidemia. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2005; 149: 101–8
- De Caterina R., Basta G., Lazzerini G., Dell'Omo G., Petrucci R., Morale M., et al. Soluble vascular cell adhesion molecule‐1 as a biohumoral correlate of atherosclerosis. Arterioscler Thromb Vasc Biol 1997; 17: 2646–54
- Otsuki M., Hashimoto K., Morimoto Y., Kishimoto T., Kasayama S. Circulating vascular cell adhesion molecule‐1 (VCAM‐1) in atherosclerotic NIDDM patients. Diabetes 1997; 46: 2096–101
- Lip G. Y., Blann A. von Willebrand factor: a marker of endothelial dysfunction in vascular disorders?. Cardiovasc Res 1997; 34: 255–65
- Lee K. W., Blann A. D., Lip G. Y. Plasma markers of endothelial damage/dysfunction, inflammation and thrombogenesis in relation to TIMI risk stratification in acute coronary syndromes. Thromb Haemost 2005; 94: 1077–83
- Agewall S., Wikstrand J., Suurkula M., Tengborn L., Fagerberg B. Carotid artery wall morphology, haemostatic factors and cardiovascular disease. An ultrasound study in men at high and low risk for atherosclerotic disease. Blood Coagul Fibrinolysis 1994; 5: 895–904
- Ramsis N., El‐Hawary A. A., Ismail E. Relation between carotid intima‐media thickness, platelet surface activation and endothelial cell markers. Haemostasis 1998; 28: 268–75
- Metcalf P. A., Folsom A. R., Davis C. E., Wu K. K., Heiss G. Haemostasis and carotid artery wall thickness in non‐insulin dependent diabetes mellitus. Diabetes Res Clin Pract 2000; 47: 25–35
- Wu K. K., Folsom A. R., Heiss G., Davis C. E., Conlan M. G., Barnes R. Association of coagulation factors and inhibitors with carotid artery atherosclerosis. Early results of the Atherosclerosis Risk in Communities (ARIC) Study. Ann Epidemiol 1992; 2: 471–80
- Lee A. J., Mowbray P. I., Lowe G. D., Rumley A., Fowkes F. G., Allan P. L. Blood viscosity and elevated carotid intima‐media thickness in men and women: the Edinburgh Artery Study. Circulation 1998; 97: 1467–73
- Wang T. J., Nam B. H., Wilson P. W., Wolf P. A., Levy D., Polak J. F., et al. Association of C‐reactive protein with carotid atherosclerosis in men and women: the Framingham Heart Study. Arterioscler Thromb Vasc Biol 2002; 22: 1662–7
- Sakata T., Mannami T., Baba S., Kokubo Y., Kario K., Okamoto A., et al. Potential of free‐form TFPI and PAI‐1 to be useful markers of early atherosclerosis in a Japanese general population (the Suita Study): association with the intimal‐medial thickness of carotid arteries. Atherosclerosis 2004; 176: 355–60
- Folsom A. R., Wu K. K., Shahar E., Davis C. E. Association of hemostatic variables with prevalent cardiovascular disease and asymptomatic carotid artery atherosclerosis. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. Arterioscler Thromb 1993; 13: 1829–36
- Paramo J. A., Beloqui O., Colina I., Diez J., Orbe J. Independent association of von Willebrand factor with surrogate markers of atherosclerosis in middle‐aged asymptomatic subjects. J Thromb Haemost 2005; 3: 662–4
- Galis Z. S., Khatri J. J. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res 2002; 90: 251–62
- Zureik M., Beaudeux J. L., Courbon D., Benetos A., Ducimetiere P. Serum tissue inhibitors of metalloproteinases 1 (TIMP‐1) and carotid atherosclerosis and aortic arterial stiffness. J Hypertens 2005; 23: 2263–8
- Beaudeux J. L., Burc L., Imbert‐Bismut F., Giral P., Bernard M., Bruckert E., et al. Serum plasma pregnancy‐associated protein A: a potential marker of echogenic carotid atherosclerotic plaques in asymptomatic hyperlipidemic subjects at high cardiovascular risk. Arterioscler Thromb Vasc Biol 2003; 23: e7–10
- Maresca G., Di Blasio A., Marchioli R., Di Minno G. Measuring plasma fibrinogen to predict stroke and myocardial infarction: an update. Arterioscler Thromb Vasc Biol 1999; 19: 1368–77
- Sosef M. N., Bosch J. G., van Oostayen J., Visser T., Reiber J. H., Rosendaal F. R. Relation of plasma coagulation factor VII and fibrinogen to carotid artery intima‐media thickness. Thromb Haemost 1994; 72: 250–4
- Lavrencic A., Kosmina B., Keber I., Videcnik V., Keber D. Carotid intima‐media thickness in young patients with familial hypercholesterolaemia. Heart 1996; 76: 321–5
- Vrtovec B., Keber I., Gadzijev A., Bardorfer I., Keber D. Carotid intima‐media thickness of young coronary patients. Coron Artery Dis 1999; 10: 407–11
- Montecchi F. R., Menzinger G., Lala A. Carotid intima‐media thickness in patients with Type 2 diabetes and hypercholesterolemia. Diabetes Nutr Metab 2001; 14: 58–61
- Marchesi E., Martignoni A., Tinelli C., Ravetta V., Resasco T., Piredda M., et al. Plasminogen activator inhibitor‐1 and carotid intima‐media thickening in patients with newly detected primary hypertension. J Cardiovasc Risk 1999; 6: 363–9
- Temelkova‐Kurktschiev T., Koehler C., Henkel E., Hanefeld M. Leukocyte count and fibrinogen are associated with carotid and femoral intima‐media thickness in a risk population for diabetes. Cardiovasc Res 2002; 56: 277–83
- Takebayashi K., Suetsugu M., Matsutomo R., Wakabayashi S., Aso Y., Inukai T. Correlation of high‐sensitivity C‐reactive protein and plasma fibrinogen with individual complications in patients with type 2 diabetes. South Med J 2006; 99: 23–7
- Raal F. J., Pilcher G. J., Waisberg R., Buthelezi E. P., Veller M. G., Joffe B. I. Low‐density lipoprotein cholesterol bulk is the pivotal determinant of atherosclerosis in familial hypercholesterolemia. Am J Cardiol 1999; 83: 1330–3
- Rossl A., Baldo‐Enzi G., Ganzaroli C., Coscetti G., Calabro A., Baiocchi M. R., et al. Relationship of early carotid artery disease with lipoprotein (a), apolipoprotein B, and fibrinogen in asymptomatic essential hypertensive patients and normotensive subjects. J Investig Med 2001; 49: 505–13
- Ebrahim S., Papacosta O., Whincup P., Wannamethee G., Walker M., Nicolaides A. N., et al. Carotid plaque, intima media thickness, cardiovascular risk factors, and prevalent cardiovascular disease in men and women: the British Regional Heart Study. Stroke 1999; 30: 841–50
- Joensuu T., Salonen R., Winblad I., Korpela H., Salonen J. T. Determinants of femoral and carotid artery atherosclerosis. J Intern Med 1994; 236: 79–84
- Poredos P., Orehek M., Tratnik E. Smoking is associated with dose‐related increase of intima‐media thickness and endothelial dysfunction. Angiology 1999; 50: 201–8
- Martinez‐Vila E., Paramo J. A., Beloqui O., Orbe J., Irimia P., Colina I., et al. Independent association of fibrinogen with carotid intima‐media thickness in asymptomatic subjects. Cerebrovasc Dis 2003; 16: 356–62
- Paramo J. A., Beloqui O., Roncal C., Benito A., Orbe J. Validation of plasma fibrinogen as a marker of carotid atherosclerosis in subjects free of clinical cardiovascular disease. Haematologica 2004; 89: 1226–31
- Folsom A. R., Pankow J. S., Williams R. R., Evans G. W., Province M. A., Eckfeldt J. H. Fibrinogen, plasminogen activator inhibitor‐1, and carotid intima‐media wall thickness in the NHLBI Family Heart Study. Thromb Haemost 1998; 79: 400–4
- Ferrieres J., Elias A., Ruidavets J. B., Cantet C., Bongard V., Fauvel J., et al. Carotid intima‐media thickness and coronary heart disease risk factors in a low‐risk population. J Hypertens 1999; 17: 743–8
- Muscari A., Martignani C., Bastagli L., Poggiopollini G., Tomassetti V., Baldini L., et al. A comparison of acute phase proteins and traditional risk factors as markers of combined plaque and intima‐media thickness and plaque density in carotid and femoral arteries. Eur J Vasc Endovasc Surg 2003; 26: 81–7
- Mitusch R., Luedemann J., Wood W. G., Berger K., Schminke U., Suter M., et al. Asymptomatic carotid atherosclerosis is associated with circulating chlamydia pneumoniae DNA in younger normotensive subjects in a general population survey. Arterioscler Thromb Vasc Biol 2005; 25: 386–91
- Haverkate F. Levels of haemostatic factors, arteriosclerosis and cardiovascular disease. Vascul Pharmacol 2002; 39: 109–12
- Paramo J. A., Orbe J., Beloqui O., Benito A., Colina I., Martinez‐Vila E., et al. Prothrombin fragment 1+2 is associated with carotid intima‐media thickness in subjects free of clinical cardiovascular disease. Stroke 2004; 35: 1085–9
- Mavri A., Stegnar M., Sentocnik J. T., Videcnik V. Impact of weight reduction on early carotid atherosclerosis in obese premenopausal women. Obes Res 2001; 9: 511–6
- Diamantopoulos E. J., Andreadis E. A., Vassilopoulos C. V., Theodorides T. G., Giannakopoulos N. S., Chatzis N. A., et al. Increased plasma plasminogen activator inhibitor‐1 levels: a possible marker of hypertensive target organ damage. Clin Exp Hypertens 2003; 25: 1–9
- Jeng J. R. Left ventricular mass, carotid wall thickness, and angiotensinogen gene polymorphism in patients with hypertension. Am J Hypertens 1999; 12: 443–50
- Fisher R. A. Statistical methods for research workers. 14th ed. Oliver and Boyd, Edinburgh 1970
- Brohall G., Oden A., Fagerberg B. Carotid artery intima‐media thickness in patients with Type 2 diabetes mellitus and impaired glucose tolerance: a systematic review. Diabet Med 2006; 23: 609–16
- Pu L. J., Lu L., Xu X. W., Zhang R. Y., Zhang O., Zhang J. S., et al. Value of serum glycated albumin and high‐sensitivity C‐reactive protein levels in the prediction of presence of coronary artery disease in patients with type 2 diabetes. Cardiovasc Diabetol 2006; 5: 27
- Kotsis V. T., Stabouli S. V., Papamichael C. M., Zakopoulos N. A. Impact of obesity in intima media thickness of carotid arteries. Obesity (Silver Spring) 2006; 14: 1708–15
- Aronson D., Bartha P., Zinder O., Kerner A., Markiewicz W., Avizohar O., et al. Obesity is the major determinant of elevated C‐reactive protein in subjects with the metabolic syndrome. Int J Obes Relat Disord 2004; 28: 674–9
- Greenfield J. R., Samaras K., Jenkins A. B., Kelly P. J., Spector P. D., Gallimore J. R., et al. Obesity is an important determinant of baseline serum C‐reactive protein concentration in monozygotic twins, independent of genetic influences. Circulation 2004; 109: 3022–8
- Aronson D., Goldberg A., Roquin A., Petcherski S., Rimer D., Gruberg L., et al. Effect of obesity on the relationship between plasma C‐reactive protein and coronary stenosis in patients with stable angina. Atherosclerosis 2006; 185: 137–42
- Kannel W. B., Wolf P. A., Castelli W. P., D'Agostino R. B. Fibrinogen and risk of cardiovascular disease. The Framingham Study. JAMA 1987; 258: 1183–6
- Folsom A. R. Epidemiology of fibrinogen. Eur Heart J 1995; 16: 21–3
- Tell G. S., Polak J. F., Ward B. J., Kittner S. J., Savage P. J., Robbins J. Relation of smoking with carotid artery wall thickness and stenosis in older adults. The Cardiovascular Health Study. The Cardiovascular Health Study (CHS) Collaborative Research Group. Circulation 1994; 90: 2905–8
- Makita S., Nakamura M., Hiramori K. The association of C‐reactive protein levels with carotid intima‐media complex thickness and plaque formation in the general population. Stroke 2005; 36: 2138–42
- Mackinnon A. D., Jerrard‐Dunne P., Sitzer M., Buehler A., von Kegler S., Markus H. S. Rates and determinants of site‐specific progression of carotid artery intima‐media thickness: the carotid atherosclerosis progression study. Stroke 2004; 35: 2150–4
- Schott L. L., Wildman R. P., Brockwell S., Simkin‐Silverman L. R., Kuller L. H., Sutton‐Tyrrell K. Segment‐specific effects of cardiovascular risk factors on carotid artery intima‐medial thickness in women at midlife. Arterioscler Thromb Vasc Biol 2004; 24: 1951–6
- Urbina E. M., Srinivasan S. R., Tang R., Bond M. G., Kieltyka L., Berenson G. S. Impact of multiple coronary risk factors on the intima‐media thickness of different segments of carotid artery in healthy young adults (The Bogalusa Heart Study). Am J Cardiol 2002; 90: 953–8
- Heath D., Smith P., Harris P., Winson M. The atherosclerotic human carotid sinus. J Pathol 1973; 110: 49–58
- Sigurdardottir V., Fagerberg B., Hulthe J. Preclinical atherosclerosis and inflammation in 61‐year‐old men with newly diagnosed diabetes and established diabetes. Diabetes Care 2004; 27: 880–4
- Folsom A. R., Pankow J. S., Tracy R. P., Arnett D. K., Peacock J. M., Hong Y., et al. Association of C‐reactive protein with markers of prevalent atherosclerotic disease. Am J Cardiol 2001; 88: 112–7
- Cao J. J., Thach C., Manolio T. A., Psaty B. M., Kuller L. H., Chaves P. H., et al. C‐reactive protein, carotid intima‐media thickness, and incidence of ischemic stroke in the elderly: the Cardiovascular Health Study. Circulation 2003; 108: 166–70
- Karvonen J., Paivansalo M., Kesaniemi Y. A., Horkko S. Immunoglobulin M type of autoantibodies to oxidized low‐density lipoprotein has an inverse relation to carotid artery atherosclerosis. Circulation 2003; 108: 2107–12
- Ashfaq S., Abramson J. L., Jones D. P., Rhodes S. D., Weintraub W. S., Hooper W. C., et al. The relationship between plasma levels of oxidized and reduced thiols and early atherosclerosis in healthy adults. J Am Coll Cardiol 2006; 47: 1005–11
- Beaudeux J. L., Giral P., Bruckert E., Bernard M., Foglietti M. J., Chapman M. J. Serum matrix metalloproteinase‐3 and tissue inhibitor of metalloproteinases‐1 as potential markers of carotid atherosclerosis in infraclinical hyperlipidemia. Atherosclerosis 2003; 169: 139–46
- Elvik R. Evaluating the statistical conclusion validity of weighted mean results in meta‐analysis by analysing funnel graph diagrams. Accid Anal Prev 1998; 30: 255–66
- Tang J. L., Liu J. L. Misleading funnel plot for detection of bias in meta‐analysis. J Clin Epidemiol 2000; 53: 477–84
- Lau J., Ioannidis J. P., Terrin N., Schmid C. H., Olkin I. The case of the misleading funnel plot. BMJ 2006; 333: 597–600