Abstract
Background. The quantification of circulating endothelial cells (CECs) in whole blood is an increasingly recognized index of endothelial damage/dysfunction. Abnormal CECs have been linked to the severity of coronary artery disease (CAD).
Objective. We assessed the relationship of CECs to other markers of endothelial dysfunction (von Willebrand factor (vWF) and soluble E‐selectin (sEsel)) during exercise stress testing (EST) in a cohort of patients with suspected CAD.
Methods. We studied a cohort of patients referred to our chest pain clinic with a history of exertional chest pain. Treadmill EST was performed, using a full Bruce exercise protocol. Blood for CECs (immunobead method), vWF and sEsel (both ELISA) were collected immediately before (pre‐exercise), immediately following exercise, and at 30 minutes post‐EST.
Results. We studied 31 patients (84% male; mean (SD) age 58.4 (9.8) years). Of the entire cohort, 14 patients (45.2%) had a positive EST. Exercise led to significant increases in levels of CECs, sEsel, vWF, white blood cells (WBC), heart rate, mean and systolic blood pressure compared with base‐line (all P<0.05). There was a significant correlation between the change (Δ (immediate post–pre‐exercise)) in CECs and ΔvWF (r = 0.45; 95% CI 0.11–0.69: P = 0.01) and ΔsEsel (r = 0.41; 0.05–0.7: P = 0.02), as well as between ΔvWF and ΔsEsel (r = 0.55; 0.25–0.76: P = 0.001). Neither absolute nor ΔCEC counts were predictive of exercise work‐load/functional capacity, nor the presence of positive EST results.
Conclusion. EST led to a significant increase in endothelial markers (CECs, vWF, and sEsel) compared with base‐line levels. The rise in CECs correlated with the increases in other endothelial markers, but was not related to the either exercise work‐load/capacity or to the presence of a positive EST.
| Abbreviations | ||
| ELISA | = | enzyme‐linked immunosorbent assay |
| WBC | = | white blood cell |
| EDTA | = | ethylenediamine tetraacetic acid |
| FITC | = | fluorescein isothiocyanate |
| BMI | = | body mass index |
| ACE | = | angiotensin‐converting enzyme |
Introduction
Endothelial dysfunction and loss of endothelial homeostasis plays a key role in the pathogenesis of coronary artery disease (CAD) and its adverse clinical sequelae, including myocardial infarction (MI) and cardiovascular (CV)‐related death Citation1, Citation2. The hall‐marks in this process include adverse alterations in arterial vasomotor control, a propensity towards a pro‐inflammatory state, loss of fibrinolysis, and impaired control of the strict balance between cellular proliferation and death Citation3. Endothelial perturbation, as reflected by indices of endothelial damage/dysfunction, including impaired flow‐mediated dilatation (FMD) and elevated endothelial markers, such as soluble E‐selectin (sEsel) and von Willebrand factor (vWF), has been consistently demonstrated among patients with CAD compared with matched healthy controls Citation4–6.
Unlike sEsel and vWF, circulating endothelial cells (CECs) are the only direct cellular marker of endothelial injury/dysfunction Citation7. CECs are endothelial cells that have become mechanically detached from the endothelium in response to endothelial damage Citation8, Citation9. The relationship between increasing CECs and other well established markers of endothelial dysfunction, including impaired FMD, sEsel, and vWF, has been well described across a broad spectrum of CV disorders (see review) Citation7. Compared with very low numbers in healthy controls (0–6 cells/mL), elevated CECs have been described among a broad spectrum of CV disorders, such as with acute stroke, following elective coronary, with acute heart failure, and/or in acute coronary syndromes Citation7. More importantly, increasing CEC counts have been linked to increasing severity of CV disease, as well as worsening prognosis among patients presenting with myocardial infarction Citation8. For example, CEC counts are much higher amongst patients with unstable CAD versus stable CAD, suggesting a potential link between increasing CEC counts and disease burden Citation9, Citation10.
Exercise stress testing (EST) is an established screening method for the identification of patients with flow‐limiting CAD Citation11. Despite its recognized limitations, several exercise‐related parameters, such as the presence of significant exercise‐related ST depression, and reduced work‐load functional capacity, have all been consistently linked to adverse CV outcomes Citation12, Citation13. In this study, we sought to assess—for the first time—the influence of exercise stress testing (EST) on CEC counts and their relationship to other markers of endothelial damage/dysfunction among patients with suspected flow‐limiting CAD. Secondly, we aimed to see whether potential changes in CEC counts would be predictive of exercise work‐load/functional capacity and whether the EST was positive or negative.
Key messages
The quantification of circulating endothelial cells (CECs) in whole blood is an increasingly recognized index of endothelial damage/dysfunction.
We assessed the relationship of CECs to other markers of endothelial dysfunction (von Willebrand factor (vWF) and soluble E‐selectin (sEsel)) during exercise stress testing (EST) in a cohort of patients with suspected coronary artery disease.
EST led to a significant increase in endothelial markers (CECs, vWF, and sEsel) compared with base‐line levels.
The rise in CECs correlated with the increases in other endothelial markers, but was not related to the either exercise work‐load/capacity or to the presence of a positive EST.
Method
We performed an observational study of a cohort of patients (aged 40–75 years) referred to our rapid access chest pain clinic with a clinical history compatible with flow‐limiting CAD (e.g. exertional angina) and physically able to perform a treadmill test, using a Bruce protocol. We excluded patients with any of the following: atrial fibrillation/flutter, previous coronary artery bypass surgery, a history of liver disease; dialysis or with a serum creatinine >200 µmol/L, malignancy; recent (<3 months) arterial/venous thromboembolic disease; patients with active infection and/or a history of inflammatory or connective tissue disorders; and patients with uncontrolled blood pressure (>200/120 mmHg) and/or with left ventricular hypertrophy with a strain pattern on resting 12‐lead electrocardiogram (ECG). We also excluded patients with unstable angina, acute coronary syndrome within the previous 6 weeks, congestive cardiac failure, valvular disease, or left/right bundle branch block on a resting ECG as well as patients with exercise‐limiting claudication. All patients underwent a full history and physical examination with base‐line blood glucose, total and high‐density lipoprotein (HDL) cholesterol, and full blood count measured, pre‐inclusion. All included patients were required to provide written informed consent to the study, which was approved by the West Birmingham Research and Ethics Committee.
Exercise test protocol
Venous blood samples were taken from supine patients immediately pre‐exercise, immediately post‐exercise and at 30 minutes post‐exercise. The order of sampling was exactly the same for all blood draws, which was performed via clean venepuncture, using a 21‐G needle and vacutainer (Becton Dickinson, UK). At least the first 4 mL of blood was discarded in order to reduce the influence of initial needle passage through the endothelium on measured endothelial markers Citation14. Maximal symptom‐limited graded treadmill exercise testing was performed according to a standard Bruce protocol. Real‐time 12‐lead and computer‐averaged ECG recordings were taken during and after exercise testing. Blood pressure was taken immediately pre‐procedure and at 3‐minute intervals until complete recovery following the EST. The EST was considered positive in patients with exercise‐related ECG changes diagnostic of ischemia (⩾1 mm horizontal or down‐sloping ST‐segment depression or elevation for ⩾60–80 ms after the end of the QRS, significant arrhythmia, systolic blood pressure decrease of ⩾10 mmHg or significant symptoms Citation11. The total estimated work‐load in metabolic equivalents (METs), maximal oxygen consumption/acrobic capacity (VO2 max), the Duke treadmill score, and peak double‐product (maximal heart rate×maximal systolic blood pressure) were calculated by appropriate formulae for each exercise protocol Citation15.
Laboratory
For vWF and sEsel determination, all samples were collected on ice and were separated by centrifugation at 3,000 rpm (1,000 g) for 20 min at 4°C to obtain citrated plasma (for vWF) and serum (sEsel) respectively, which was then stored at −70°C to allow later batch analysis. The vWF levels were measured in duplicate by enzyme‐linked immunosorbent assay (ELISA) using commercial reagents (Dako‐Patts, Ely, United Kingdom). Soluble Esel was measured by ELISA with R&D Systems reagents (Abingdon, United Kingdom), with a minimal sensitivity of 1.6 ng/mL. The intra‐ and inter‐assay coefficients of variation for vWF were <5% and 10%, and for sEsel were <5% and <12%, respectively.
Isolation of circulating endothelial cells
Our detailed method of CEC isolation has been previously validated and published Citation14. For CEC analysis blood was collected into EDTA tubes (stored at room temperature) and was processed within 1 hour of collection. In short, 1 mL of venous whole blood is incubated with CD146 (endothelial‐associated marker) conjugated immunomagnetic beads and suspended in 1 mL of phosphate‐buffered saline. After mixing for ⩾30 minutes CD146+ cells are separated out using several washing steps in a magnet; 100 µL of fluorescein isothiocyanate (FITC)‐labelled Ulex Europeus lectin (an endothelial‐specific marker) is added to the remaining cell/bead suspension with a further mixing (⩾30 minutes) step in darkness. The cells are then washed again and suspended in 125 µL of phosphate‐buffered saline and viewed under fluorescence microscopy in a counting chamber. CECs were defined as CD146‐rosetted cells, bearing ⩾4 beads, sized approximately 10–50 µm in diameter with positive staining for FITC‐labelled Ulex Europeus lectin. The intra‐ and inter‐observer coefficient of variation is 17.0% and 24.9% respectively Citation14. The operator was blinded to the sample order.
Power calculation
Previous studies have demonstrated significant changes in CEC counts (n = 13) Citation10, soluble sEsel (⩾20%; n = 20) Citation16, and vWF (>10%; n = 13) Citation17 with exercise. Based on these studies we calculated that a sample size of 30 patients would provide >90% power to detect a >50% increase (immediate post‐EST versus pre‐EST levels) in CECs, >15% in sEsel and >8% in vWF at a significance level (alpha) of 0.05 (two‐tailed). Power calculations were performed using GraphPad StatMate version 2.00 (www.graphpad.com).
Statistical analysis
Data were analysed using GraphPad InStat version 3.05 (GraphPad Software, San Diego California USA; www.graphpad.com). Data normality was normality was assessed using both visual inspection and the Kolmogorov‐Smirnov test (for normality). All continuous data are presented as mean (SD, standard deviation; or 95% CI, confidence interval) or median (IQR, inter‐quartile range). To assess the effects of time on endothelial markers and other exercise parameters we performed a repeated measures analysis of variance assessment (ANOVA). Unpaired t‐tests and Mann‐Whitney tests were used for unpaired two‐group comparisons of continuous parametric and non‐parametric data, respectively. Fisher's exact test was used for all categorical comparisons. All correlations were assessed using Pearson correlation method. Multiple linear regression analysis was performed to determine the influence of several factors on the dependent variable of change in CEC counts with exercise. A two‐tailed P‐value <0.05 was considered statistically significant for all comparisons.
Results
We studied 31 patients (84% male; mean (SD) age 58.4 (9.8) years) (see Table ), with 14 patients (45.2%) demonstrating a positive EST. Exercise led to significant increases in CECs, sEsel, vWF, white blood cells (WBC), heart rate, mean and systolic blood pressure compared with base‐line values (see Table and Figure ). This rise was most marked at the immediate post‐exercise time point and was then followed by a significant fall by 30 minutes post‐exercise. Soluble Esel levels still remained significantly elevated at 30 minutes, compared with base‐line values.
Figure 1 Relationship of exercise to endothelial markers. A: Circulating endothelial cell (CEC) counts. B: Soluble E‐selectin (sEsel). C: von Willebrand Factor (vWF). (Pre = pre‐exercise; IP = immediate post; 30P = 30 minutes post‐exercise).
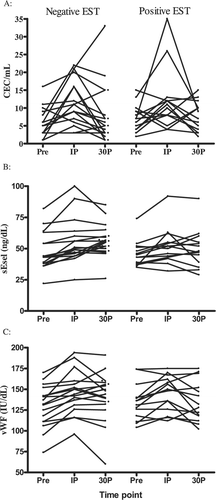
Table I. Base‐line characteristics of entire patient cohort.
Table II. The effects of exercise on blood and exercise‐related parameters.
Interrelationships of endothelial markers
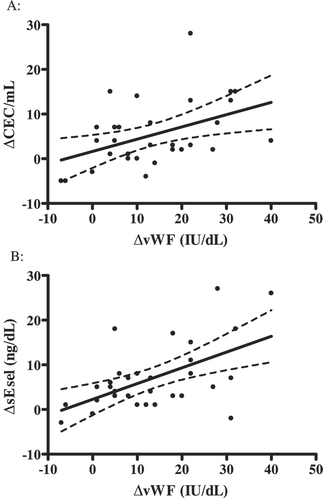
There was a significant correlation between the change (Δ (immediate post–pre‐exercise)) in CECs and ΔvWF (Pearson, r = 0.45; 95% CI 0.11–0.69: P = 0.01 (Figure )) and ΔsEsel (r = 0.41; 0.05–0.7: P = 0.02). There was also a significant correlation between ΔvWF and ΔsEsel (r = 0.55; 0.25–0.76: P = 0.001), but no relationship between ΔCEC and ΔWBC count.
Endothelial markers and exercise test result
On comparing the patients with a positive versus those with a negative EST, there were no significant differences in comparative base‐line or change (immediate post–pre) in CECs, sEsel, vWF, and WBC with exercise (Table ). Furthermore, there were no significant differences in comparative base‐line (e.g. age, sex, and previous cardiac history) and/or exercise‐related demographics (e.g. total work‐load, exercise time) between the two groups (Table ). As expected the Duke score was significantly lower in those with a positive test (P<0.0001).
Table III. Relationship between endothelial markers and exercise stress test (EST) result.
There were no significant correlations between either base‐line or ΔCEC (immediate post–pre) and total exercise time, VO2 max, METs, double‐product, Δheart rate, Δsystolic blood pressure, Δdiastolic blood pressure, and Δmean arterial blood pressure (data not shown).
Subgroup analyses
From the cohort of 31 patients, we identified 12 patients without known CAD, diabetes mellitus, and/or hypertension at inclusion, who were of similar age (57.3 (10.1) versus 59.2 (9.2) years; P = 0.61), sex (91% versus 79% males; P = 0.63), and body mass index (27.9 (4.2) versus 26.9 (3.3) kg/m2; P = 0.47) to the rest (n = 19) of the cohort, respectively. Among the 19 patients with cardiovascular disease (and its risk factors), there was a non‐significant trend to a rise (immediate post‐ versus pre‐EST) in CECs (6.5 (8.4) versus 3.4 (4.6) cells/mL; P = 0.23) and vWF (16.5 (12.8) versus 8.6 (8.4) IU/dL; P = 0.067) with no significant change in sEsel (6.1 (3.4) versus 7.6 (9.1) ng/mL; P = 0.60), when compared with those without known CAD, diabetes mellitus, and/or hypertension at inclusion.
The use of statins and/or angiotensin‐converting enzyme inhibitors did not influence the rise in CECs (data not shown). There was a non‐significant trend to a lower increase (immediate post–pre‐EST) in CEC counts only (but not vWF and sEsel) among patients pre‐treated with beta‐blockers compared with those without (2.0 (−2 to 4) versus 7.0 (3–13) cells/mL; P = 0.07).
Multivariate analysis
We performed multiple linear regression analysis to assess the relationship between ΔvWF (immediate post–pre) and ΔsEsel (immediate post–pre) on the dependent variable of ΔCEC (immediate post–pre) after adjusting for age, sex, BMI, and exercise time. Whilst the overall relationship remained significant (overall R2 = 41.2%; P = 0.03), only ΔvWF significantly contributed to the model (P = 0.015).
Discussion
This is the first study to investigate the comparative changes in CECs with other circulating endothelial markers with exercise. We found that EST led to a significant rise in all three studied endothelial markers (CECs, vWF, and sEsel) as well as a significant rise in WBC count. There was a moderate, yet significant, correlation between changing ΔCEC counts, and ΔvWF and ΔsEsel. We found no relationship between base‐line or changing CEC counts and exercise burden (VO2 max, METs, change in blood pressure/heart rate, or the double‐product), and CEC counts were not predictive of the EST result nor did they correlate with the Duke score.
There has been only one previous study that has investigated the influence of exercise on CEC counts. Mutin et al. (1999) studied 13 patients with exertional angina and quantified CEC counts (using the immunobead technique) before, immediately after, and at 4 hours following a 3‐minute bicycle EST Citation10. The authors noted an increase in CECs from 0/mL pre‐exercise, rising to 2/mL immediately after exercise, and falling to base‐line levels at four hours post‐exercise. Their study not only included a far smaller sample size than the present one, the authors did not investigate the relationship between CECs and other endothelial markers or the EST result.
The results of this study need to be considered in the context of any previous studies that have attempted to assess interrelationships between endothelial damage/dysfunction, and exercise work‐load/functional capacity. Kuvin et al. Citation18 noted a significant relationship between brachial artery FMD and the presence/absence of CAD, as defined by exercise myocardial perfusion imaging, in a cohort of low‐ to intermediate‐risk subjects, with a significant correlation between FMD and exercise time only. Patel et al. (2005) noted a significant, yet weak (r = 0.3) correlation between FMD and exercise time in a cohort of 105 women without CAD Citation19. Similarly, Mizia‐Stec et al. Citation16 demonstrated significant increases (pre‐ versus immediately post‐exercise) in sEsel levels following EST (Bruce protocol) among patients with stable CAD (n = 27) and separately, among 20 age‐ and sex‐matched healthy controls; however, the absolute post‐EST levels were higher among the disease group. Conversely, Jilma et al. found non‐significant and minor elevations in sEsel following bicycle ergometry (11%) amongst a cohort of healthy untrained men Citation20.
Collins et al. noted significant increases in vWF following maximal treadmill exercise among both healthy patients (n = 20) and among patients with intermittent claudication (IC, n = 20), with a return towards base‐line levels at 1 hour post‐exercise Citation21. In a very similar study, Signorelli et al. noted significant increases in both WBC counts and sEsel in both patients with IC (n = 20) and among healthy controls (n = 20), although the post‐treadmill concentrations of sEsel were much higher in the IC group Citation22. As with the study by Collins et al. (2006) Citation21, the relationships to exercise work‐load were not presented. Musumeci et al. observed a significant increase in vWF with exercise among 16 healthy subjects, but no correlations were observed between their increase and exercise‐related parameters such as change in heart rate/blood pressure nor exercise work‐load Citation23. Jilma et al. noted a 61% increase in vWF antigen following a strenuous bicycle exercise test among 13 healthy volunteers, but again its relationship with exercise capacity/work‐load was not examined Citation17. Finally, Lee et al. Citation24 demonstrated a significant increase in vWF with incremental shuttle walking among 53 patients with CAD and separately among 19 age‐ and sex‐matched healthy controls. Again, there was no correlation between changes in vWF and exercise work‐load.
Whilst we observed a significant correlation between ΔCECs and both ΔvWF and ΔsEsel with EST, quantification of CECs does not appear to provide an additional role in predicting either exercise work‐load/functional capacity or in determining EST results. The results of this study would tend to suggest that the degree of change in CECs and other endothelial markers, with exercise, are somewhat unpredictable and not determined by base‐line or exertion‐related factors. Despite its obvious utility, EST on its own is an imperfect test for diagnosing flow‐limiting CAD, with a reported sensitivity and specificity of for the detection of significant CAD ranging between 23% and 100% (mean 68%), and 17% and 100% (mean 77%), respectively Citation11. Its diagnostic accuracy is influenced by a number of factors, including the pre‐test probability, as well as the definition used to define a positive test. Hence, it cannot be excluded that there could have been obvious ischemia during stress imaging without ECG changes, and obvious ECG changes without ischemia. In our study, we only included patients with a history suggestive of CAD based on a good clinical history of exertional chest pain.
The entire premise of any argument regarding the utility of CECs to predict the presence of flow‐limiting CAD depends on several complex fundamental concepts that must be elucidated. Firstly, it is still not known whether the observed increases in CECs in patients with unstable CAD (versus stable CAD) actually reflect the local release/detachment of endothelial cells from the coronary vasculature or whether they reflect more wide‐spread generalized systemic endothelial release Citation7, Citation25. Also, the relationship between culprit coronary lesions, flow‐limiting CAD, and the total and/or coronary atherosclerotic burden is not an exact science Citation26.
It might be hypothesized that increasing CECs with worsening clinical severity of CAD may represent the total atherosclerotic burden (and culmination of CV risk factors) to the patient, rather than merely reflecting focal coronary release. In patients with stable CAD, plasma sEsel levels appear to reflect the severity and cumulative nature of systemic risk factors rather than coronary atherosclerosis per se Citation27. Moreover, the exact origins of CECs (arterial versus venous, coronary versus peripheral release), their mechanisms of detachment, and potential transcapillary passage have not been fully established.
Our study has several additional limitations that must be acknowledged. We have measured three time points and cannot be certain exactly when the peak rise in endothelial markers may have occurred. Secondly, the kinetics of the various markers studied are likely to be different, potentially limiting a full appreciation of the observed interrelationships between the various markers across arbitrary time points. Nevertheless, the increase in WBC count with EST is in keeping with the published literature Citation28, Citation29. The inclusion of a matched cohort of healthy controls, subjected to a similar exercise protocol, would have been preferable but would not necessarily have excluded the presence of asymptomatic CAD. However, our data from subgroup analysis are suggestive of greater endothelial activation/damage (and greater rise in CECs) with exercise among patients with CVD compared with those without. Finally, quantification of CECs in whole blood is a difficult technique and relies upon subjective interpretation of fluorescent microscopic images following a multistep preparation. In the present study, all CEC counts were performed blinded to the sample order, and the method used in this paper is current and is supported by a recent pan‐European consensus Citation30.
In conclusion, we have shown for the first time that EST leads to a significant increase in CECs and other blood biomarkers of endothelial dysfunction (vWF and sEsel), compared with base‐line levels, among a cohort of patients with suspected flow‐limiting CAD. This rise in CECs correlated with the rise in other endothelial markers but was not related to other exercise‐related parameters (including exercise work‐load or functional capacity) or to the positivity of the EST.
References
- Zhang X., Zhao S. P., Li X. P., Gao M., Zhou Q. C. Endothelium‐dependent and ‐independent functions are impaired in patients with coronary heart disease. Atherosclerosis 2000; 149: 19–24
- Jambrik Z., Venneri L., Varga A., Rigo F., Borges A., Picano E. Peripheral vascular endothelial function testing for the diagnosis of coronary artery disease. Am Heart J 2004; 148: 684–9
- Brandes R. P., Fleming I., Busse R. Endothelial aging. Cardiovasc Res 2005; 66: 286–94
- Blann A. D., Amiral J., McCollum C. N. Circulating endothelial cell/leucocyte adhesion molecules in ischaemic heart disease. Br J Haematol 1996; 95: 263–5
- Soman P., Dave D. M., Udelson J. E., Han H., Ouda H. Z., Patel A. R., et al. Vascular endothelial dysfunction is associated with reversible myocardial perfusion defects in the absence of obstructive coronary artery disease. J Nucl Cardiol 2006; 13: 756–60
- Senen K., Ileri M., Alper A., Yetkin F., Atak R., Hisar I., et al. Increased levels of soluble adhesion molecules E‐selectin and P‐selectin in patients with cardiac syndrome X. Angiology 2005; 56: 273–7
- Boos C. J., Blann A. D., Lip G. Y. H. Circulating Endothelial Cells in Cardiovascular Disease. J Am Coll Cardiol 2006; 48: 1538–47
- Blann A. D., Woywodt A., Bertolini F., Bull T. M., Buyon J. P., Clancy R. M., et al. Circulating endothelial cells. Biomarker of vascular disease. Thromb Haemost 2005; 93: 228–35
- Lee K. W., Lip G. Y., Tayebjee M., Foster W., Blann A. D. Circulating endothelial cells, von Willebrand factor, interleukin‐6, and prognosis in patients with acute coronary syndromes. Blood 2005; 105: 526–32
- Mutin M., Canavy I., Blann A., Bory M., Sampol J., Dignat‐George F. Direct evidence of endothelial injury in acute myocardial infarction and unstable angina by demonstration of circulating endothelial cells. Blood 1999; 93: 2951–8
- Fox K., Garcia M. A., Ardissino D., Buszman P., Camici P. G., Crea F., et al. Task Force on the Management of Stable Angina Pectoris of the European Society of Cardiology; ESC Committee for Practice Guidelines (CPG). Guidelines on the management of stable angina pectoris: executive summary: the Task Force on the Management of Stable Angina Pectoris of the European Society of Cardiology. Eur Heart J 2006; 27: 1341–81
- Mark D. B., Shaw L., Harrell F. E Jr., Hlatky M. A., Lee K. L., Bengtson J. R., et al. Prognostic value of a treadmill exercise score in outpatients with suspected coronary artery disease. N Engl J Med 1991; 325: 849–53
- Snader C. E., Marwick T. H., Pashkow F. J., Harvey S. A., Thomas J. D., Lauer M. S. Importance of estimated functional capacity as a predictor of all‐cause mortality among patients referred for exercise thallium single‐photon emission computed tomography: report of 3,400 patients from a single center. J Am Coll Cardiol 1997; 30: 641–8
- Boos C. J., Lane D. A., Kang D., Goon P. K. Y., Blann A. D., Lip G. Y. H. Temporal and venepuncture‐related decline in Circulating Endothelial Cell capture from mixed venous blood. J Thromb Thrombolysis 2006; 22: 125–31
- Shaw L. J., Peterson E. D., Shaw L. K., Kesler K. L., DeLong E. R., Harrell F. E Jr., et al. Use of a prognostic treadmill score in identifying diagnostic coronary disease subgroups. Circulation 1998; 98: 1622–30
- Mizia‐Stec K., Zahorska‐Markiewicz B., Mandecki T., Janowska J., Szulc A., Jastrzebska‐Maj E. Serum levels of selected adhesion molecules in patients with coronary artery disease. Int J Cardiol 2002; 83: 143–50
- Jilma B., Dirnberger E., Eichler H. G., Matulla B., Schmetterer L., Kapiotis S., et al. Partial blockade of nitric oxide synthase blunts the exercise‐induced increase of von Willebrand factor antigen and of factor VIII in man. Thromb Haemost 1997; 78: 1268–71
- Kuvin J. T., Patel A. R., Sliney K. A., Pandian N. G., Rand W. M., Udelson J. E., et al. Peripheral vascular endothelial function testing as a noninvasive indicator of coronary artery disease. J Am Coll Cardiol 2001; 38: 1843–9
- Patel A. R., Kuvin J. T., Sliney K. A., Rand W. M., Chiang J. C., Udelson J. E., et al. Gender‐based differences in brachial artery flow‐mediated vasodilation as an indicator of significant coronary artery disease. Am J Cardiol 2005; 96: 1223–6
- Jilma B., Eichler H. G., Stohlawetz P., Dirnberger E., Kapiotis S., Wagner O. F., et al. Effects of exercise on circulating vascular adhesion molecules in healthy men. Immunobiology 1997; 197: 505–12
- Collins P., Ford I., Ball D., Macaulay E., Greaves M., Brittenden J. A preliminary study on the effects of exercising to maximum walking distance on platelet and endothelial function in patients with intermittent claudication. Eur J Vasc Endovasc Surg 2006; 31: 266–73
- Signorelli S. S., Mazzarino M. C., Di Pino L., Malaponte G., Porto C., Pennisi G., et al. High circulating levels of cytokines (IL‐6 and TNFalpha), adhesion molecules (VCAM‐1 and ICAM‐1) and selectins in patients with peripheral arterial disease at rest and after a treadmill test. Vasc Med 2003; 8: 15–9
- Musumeci V., Cardillo C., Baroni S., Zuppi C., Zappacosta B., Tutinelli F., et al. Effects of calcium channel blockers on the endothelial release of von Willebrand factor after exercise in healthy subjects. J Lab Clin Med 1989; 113: 525–31
- Lee K. W., Blann A. D., Ingram J., Jolly K., Lip G. Y. H., BRUM Investigators. Incremental shuttle walking is associated with activation of haemostatic and haemorheological markers in patients with coronary artery disease: the Birmingham rehabilitation uptake maximization study (BRUM). Heart 2005; 91: 1413–7
- Boos C. J., Jaumdally R. J., Macfayden R. J., Varma C., Lip G. Y. H. Circulating endothelial cells and von Willebrand factor as indices of endothelial damage/dysfunction in coronary artery disease: a comparison of central vs peripheral levels and effects of coronary angioplasty. J Thromb Haemost 2007; 5: 630–2
- Naghavi M., Falk E., Hecht H. S., Jamieson M. J., Kaul S., Berman D., et al. From vulnerable plaque to vulnerable patient—Part III: Executive summary of the Screening for Heart Attack Prevention and Education (SHAPE) Task Force report. Am J Cardiol 2006; 98: 2H–15H
- Nasuno A., Matsubara T., Hori T., Higuchi K., Imai S., Nakagawa I., et al. Levels of soluble E‐selectin and ICAM‐1 in the coronary circulation of patients with stable coronary artery disease: association with the severity of coronary atherosclerosis. Jpn Heart J 2002; 43: 93–101
- Jae S. Y., Fernhall B., Lee M., Heffernan K. S., Lee M. K., Choi Y. H., et al. Exaggerated blood pressure response to exercise is associated with inflammatory markers. J Cardiopulm Rehabil 2006; 26: 145–9
- Ramel A., Wagner K. H., Elmadfa I. Acute impact of submaximal resistance exercise on immunological and hormonal parameters in young men. J Sports Sci 2003; 21: 1001–8
- Woywodt A., Blann A. D., Kirsch T., Erdbruegger U., Banzet N., Haubitz M. Isolation and enumeration of circulating endothelial cells by immunomagnetic isolation: proposal of a definition and a consensus protocol. J Thromb Haemost 2006; 4: 671–7