Abstract
Background. Oxidative stress has a role in the pathogenesis of gastroesophageal reflux disease (GERD).
Aim. To investigate the redox balance in proximal esophagus before and 6 and 48 months after antireflux surgery.
Methods. In 20 GERD patients and 9 controls oxidative stress by myeloperoxidase activity (MPO activity) and antioxidative capacity of esophageal mucosa by superoxide dismutase activity (SOD), and glutathione content (GSH) was measured from proximal esophageal samples.
Results. In proximal esophagus of GERD patients compared to controls', antioxidative capacity appearing as GSH level was significantly decreased (P<0.001) at all time points and as SOD levels preoperatively (P<0.001) and 4 years postoperatively (P = 0.01). MPO activity of patients was significantly lower than controls' preoperatively, and 6 months and 4 years postoperatively (P<0.05). MPO activity remained lower than that of the distal esophagus at 6 months and 4 years (P<0.01 for both).
Conclusions. In GERD patients, proximal esophageal mucosal antioxidative defense is defective before and after antireflux surgery. Antireflux surgery seems not to change the level of oxidative stress in proximal esophagus, suggesting that defective mucosal antioxidative capacity plays a role in development of oxidative damage to the esophageal mucosa in GERD.
Introduction
Reactive oxygen species (ROS), extremely reactive chemical species, have been linked to the pathogenesis of esophageal mucosal damage in gastroesophageal reflux disease (GERD) Citation1, Citation2. GERD can lead to esophagitis and Barrett's esophagus Citation3, which are associated with increased risk for esophageal adenocarcinoma Citation4, Citation5. This is also increased by low intake of antioxidants Citation6.
Oxidative stress related to chronic inflammation is now considered to be of pathogenic significance in numerous disease processes Citation7. In the defense mechanism against ROS, the glutathione redox system and superoxide dismutase (SOD) may play important roles Citation8, Citation9. Glutathione (GSH) can scavenge free radicals by itself and is an essential part in the reduction of H2O2Citation10–12. Myeloperoxidase (MPO), ROS‐generating enzyme, is mostly produced by neutrophils—hence it is a good marker for their presence, and by that of inflammation Citation10. The activated inflammatory cells, the hypoxanthine‐xanthine oxidase system, the disrupted mitochondrial electron transport system, the metabolism of arachidonate via the lipoxygenase pathway, and vascular endothelial cells all are potential sources of ROS Citation13. At sites of inflammation, MPO functions as a major catalyst for initiation of lipid peroxidation and, therefore, amplifies the oxidative stress Citation14.
Overall, oxidative damage arises either from the overproduction of free radicals, from deficiency of antioxidant defense mechanisms, or both. Wetscher et al. showed in 1995 the connection of ROS to the development of esophagitis and Barrett's esophagus Citation10. In malignant transformation of Barrett's esophagus, the pathogenesis has been suggested to be driven mainly by increased production of free radicals. An impaired antioxidant defense seen as low GSH content may, however, be relevant in the increased risk for development of esophageal adenocarcinoma as well Citation15. Persistent stimulation on Barrett's epithelium will result in a series of genetic and epigenetic changes such as inactivation of glutathione pathway Citation16. Therefore, the suppression of defense mechanisms in Barrett's esophagus seems to be a secondary event.
Fundoplication is effective against symptoms of GERD Citation17, but it may not prevent the oxidative stress in the distal esophagus Citation18 or development of esophageal adenocarcinoma Citation19, Citation20. Proximal esophagus is far from the esophagogastric (EG) junction and not normally the target of gastroesophageal reflux even in patients with GERD. This mucosal area should, therefore, reveal the primary deficiencies in antioxidant defenses critical in the protection of epithelium against DNA damage.
The objective of the present study was to examine the status of oxidative stress and radical scavenger capacity in the proximal esophagus before and after fundoplication on patients with gastroesophageal reflux disease and Barrett's esophagus mainly to reveal primary abnormalities in the antioxidant defense mechanisms. In addition, we compared myeloperoxidase activity between proximal and distal esophagus. Knowledge as to whether GERD patients may have a deficiency in antioxidant defense even in their healthy proximal esophageal mucosa is lacking. Our hypothesis was that patients with severe esophageal reflux disease may have a primary defect in their antioxidant defense with impaired protection of epithelium to oxidative stress, DNA damage, and mutation leading to esophagitis and its sequelae Barrett's esophagus and adenocarcinoma.
Key messages
GERD patients seem to have defective antioxidative defense in the proximal esophageal mucosal before antireflux surgery.
Antireflux surgery seems not to change the level of oxidative stress in the proximal esophagus, suggesting that defective mucosal antioxidative capacity plays an important role in development of oxidative damage to the esophageal mucosa in GERD.
Patients and methods
Our patient group consisted of 20 consecutive Caucasians with typical symptoms of gastroesophageal reflux disease (GERD) who in 1998 underwent Nissen‐Rosetti fundoplication (mean age 51.2, range 27–66; male/female ratio 14/6). The control group was nine Caucasians with neither symptoms nor endoscopic evidence of esophageal pathology (mean age 51.2, range 25–76, male/female ratio 4/5). Controls had undergone gastroscopy for dyspeptic symptoms.
Preoperative evaluation
Each of the 29 underwent a normal clinical workup with manometry excluding disturbances in esophageal motility, 24‐h pH measurement (mean% of time pH<4±SD; 20.0±13.4%), and endoscopy. Endoscopy revealed by Savary‐Miller's grading system Citation21 nonerosive reflux disease (NERD) in 8 and erosive reflux disease (ERD) in 12: grade I in 1 patient, grade II in 4 patients, and grade IV esophagitis in 7 patients (in 6: Barrett's esophagus; in 1: esophageal ulcer) in the distal esophagus but no macroscopic changes in the proximal esophagus. All patients gave their written informed consent. The Ethics Committee of Tampere University Hospital, Tampere, Finland, approved this study.
Surgery
In 19 patients laparoscopic and in 1 patient open (with ventral hernia repair) Nissen‐Rosetti fundoplication was performed by the fashioning of a floppy 360° posterior 2‐cm long fundic wrap over a 48‐Fr esophageal bougie. This technique included posterior crural repair, and the wrap was anchored to the gastric cardia by three nonabsorbable braided polyester sutures Citation22. No postoperative mortality occurred. Two patients suffered minor complications (one ileus, treated conservatively, and one hemorrhage from a trocar site which was immediately operated on).
Sampling
Patients were advised to go without any acid‐suppressive treatment for 2 weeks before mucosal biopsies. All gastroscopies were performed by author TKR, and four biopsy samples for analysis of oxidative metabolism from the distal esophagus and proximal esophagus were taken at 5 cm and 20 cm above the esophagogastric junction before surgery, as well as at 6 months and 4 years after surgery. For analysis of oxidative metabolism, specimens were snap‐frozen and stored at −70°C. Biopsies for histological investigation were processed as usual.
Follow‐up
Patients were followed up as previously described Citation18. Briefly, at 6 months all 20 patients were symptomless; all underwent gastroscopy, and 19 of them 24‐h pH measurement. Esophageal acid exposure time had normalized, and erosive esophagitis in the distal esophagus had healed in all cases. No macroscopic changes could be discovered in the proximal esophagus. In one patient open refundoplication was performed 6 months after the primary operation to repair a poorly placed fundic wrap.
At 4 years, 16 patients agreed to undergo endoscopy, none of these showed either erosive esophagitis in the distal esophagus or GERD symptoms. The proximal esophagus of all patients was macroscopically normal. One experienced dysphagia, and manometry showed motility disturbances in the distal esophagus. The three interviewed patients who refused follow‐up endoscopy were asymptomatic.
Assay of superoxide dismutase and myeloperoxidase activities, and glutathione content
Myeloperoxidase activity (MPO activity) was estimated by modification of the method of Suzuki in which the enzyme catalyzes the oxidation of 3,3′, 5,5′‐tetramethylbenzidine by H2O2 to yield a blue chromogen with a maximum wavelength of 655 nm Citation23. MPO activity is expressed as units/milligram protein (U/mg protein). Superoxide dismutase (SOD) activity, as U/mg protein, was determined by the method of Laihia Citation24, in which xanthine/xanthine oxidase‐dependent chemiluminescence was enhanced by both lucigenin and linoleate. Glutathione (GSH) content, expressed as nmol/mg protein, was assessed by Saville's method Citation25.
Statistical methods
Values are expressed as median and range unless otherwise stated. Comparison between groups was performed by the Mann‐Whitney test. Comparison between distal and corresponding proximal biopsy samples was done by Friedman and Wilcoxon rank sum tests. Nonparametric methods were applied because of the distribution of the data. All reported P‐values were based on two‐tailed tests without adjustment for multiple comparisons. Statistical calculations were carried out with SPSS software.
Results
Results of determination of MPO activity, SOD activity, and GSH contents in the proximal esophagus of patients and controls are shown in Figure .
Figure 1 Myeloperoxidase activity(MPO activity), superoxide dismutase (SOD) and glutathione content (GSH) in proximal esophagus in controls (Prox control) and in patients preoperatively (Preop), at 6 months (6 mo), and at 4 years (4 yrs) of follow‐up. Median and interquartile in box, 10th and 90th percentile shown by horizontal bars.
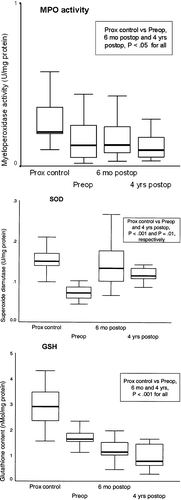
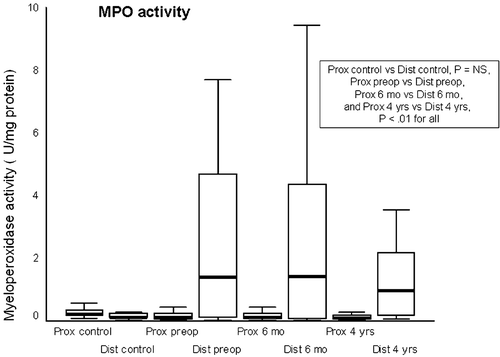
MPO activity in the proximal esophagus was significantly lower than that of controls before antireflux surgery, as well as 6 months and 4 years after surgery (P<0.05 for both). At all these time points, MPO activity of the distal esophagus was also significantly higher than that of the proximal esophagus in patients but not in controls (Figure ) (P<0.01, and P = NS, respectively).
Figure 2 Myeloperoxidase activity(MPO activity) in proximal and distal esophagus of controls (Prox control and Dist control) and patients preoperatively (Prox preop and Dist preop), at 6 months (Prox 6 mo and Dist 6 mo), and at 4 years (Prox 4 yrs and Dist 4 yrs) of follow‐up. Plots as in Figure .
GSH levels in the proximal esophagus before and 6 months or 4 years after antireflux surgery were significantly lower than were those of controls (P<0.001 at all time points). Compared to controls, SOD activity was significantly decreased preoperatively (P<0.001) and 4 years after surgery (P = 0.01).
Discussion
Based on our study, antioxidative capacity (GSH, SOD) of the proximal esophagus was diminished in GERD patients before and even 4 years after successful antireflux surgery. Oxidative stress (MPO activity) in the proximal esophagus was preoperatively lower than that of controls and remained so both 6 months and 4 years after fundoplication. It therefore seems that in patients with GERD the proximal esophageal mucosa is characterized by a low level of oxidative stress and a diminished antioxidative defense mechanism. This impairment in healthy esophageal mucosa could be a primary deficiency. With further suppression of antioxidant capacity, and with increased production of free radicals in esophagitis and in Barrett's epithelium, mucosa is exposed to amplified oxidative damage.
In the present study, MPO activity in the proximal esophagus was lower than that of the distal esophagus and that of controls both before and after successful surgery. This finding is supported by the Olyaee Citation2 and Wetscher Citation10 groups, who found fewer reactive oxygen species in the proximal than in the distal esophagus in patients with esophagitis or Barrett's esophagus. It is evident that gastroesophageal reflux as reflected by macroscopic changes in mucosa causes more oxidative stress in the distal esophagus. This is supported by findings of Potluri et al. Citation26, who found in their salivary pepsin assay that reflux into the proximal esophagus, even in GERD patients, is a rare event. For the continued difference in levels of oxidative stress between the distal and proximal esophagus even years after successful antireflux surgery several explanation are possible. First, higher MPO activity (MPA) in the distal esophagus may be an irreversible change after a long period of GERD. Second, the protective effects of saliva in the upper and the lower esophagus may differ. Third, this difference may reveal uneven myeloperoxidase activity of neutrophils in these parts of the esophagus in response to chemotactic stimuli, as is shown in gingival tissue Citation27. The reason for the lower MPO activity in the proximal esophagus than in controls, even before surgery, remains unclear. The volume, neutralizing capacity, or components of saliva might explain the difference between GERD patients and controls Citation28. Most important, our findings suggest that the impaired antioxidative capacity of the proximal esophagus may be caused not by increased oxidative stress but rather by changes in the antioxidative defense system itself. It can be speculated whether the cause of this phenomenon may be genetic.
The difference in vulnerability of the proximal esophagus to iatrogenic gastroesophageal reflux between patients with complicated GERD and without GERD comes up clearly when stomach has been used as an esophageal substitute. Only patients with Barrett's esophagus preoperatively tend to develop recurrent Barrett's metaplasia and even adenocarcinoma in their proximal esophagus a few years after esophagectomy Citation29, Citation30. In patients with esophageal atresia, intestinal metaplasia seems to be a rare consequence even after years of gastroesophageal reflux Citation31.
Regulation of GSH levels in the esophageal mucosa in GERD is unexplained. In rats, experimental esophagitis has both reduced and raised GSH levels Citation10. Similarly in humans, both mucosal reduction and increase in GSH have occurred in nonerosive and erosive esophagitis Citation32, Citation33. In Barrett's mucosa, low GSH has been apparent Citation32. In Barrett's esophagus, GSH‐dependent cellular defense mechanisms seem to be further impaired by low glutathione S‐transferase activity Citation15. Our GERD patients had depleted GSH levels in the proximal esophagus both before and after successful antireflux surgery. It therefore seems that the lack of chemotactic stimulus in the long run may reduce GSH levels. The lack of such an adaptative response to oxidative stress in Barrett's metaplasia has been suggested to contribute to increased risk for development of esophageal adenocarcinoma Citation15. In those patients with symptomatic GERD, impairment of GSH redox capacity in the entire esophagus may lead to an increased mucosal susceptibility to reflux‐related damage. In an animal model, antioxidants attenuated this depletion of GSH and improved the healing of esophagitis Citation1.
Regulation of SOD activity in the distal esophagus of GERD patients seems to be better understood than is regulation of GSH levels. In an experimental reflux model, SOD activity has decreased Citation33, a decrease apparently due to enzyme inactivation, because at the same time the inflammatory cytokines induced SOD expression Citation34. Similarly in humans with the severity of GERD, SOD expression has increased Citation33. SOD activity has, however, varied Citation10, Citation32, Citation33 with differences in SOD activity seeming at least in part to be due to differing grades of associated inflammation and possibly due to differing levels of enzyme inactivation Citation10, Citation32. In malignant transformation of Barrett's esophagus, a low level of SOD expression occurs Citation35. The reason for similarly decreased SOD activity in the proximal esophagus in GERD before and even 4 years after successful antireflux surgery remains unclear. A decrease in SOD leads to increased mucosal levels of ROS and may contribute to the esophageal damage and Barrett's esophagus in patients with esophageal reflux Citation33. The cause may be a genetic defect in mucosal oxidative defense predisposing these patients to harmful effects of gastric reflux. On the other hand, GERD patients seem to have increased production of saliva rich in nitrogen species Citation28 which in turn have been shown to inactivate SOD Citation33, Citation36. In animal models, exogenous administration of SOD has protected the esophageal mucosa from radiation‐induced damage and in a reflux model inhibited development of intestinal metaplasia Citation34, Citation37.
A limitation of our study is that the measured parameters provide only a limited picture of the redox state of a cell regulated by very complex mechanisms. In this human study, the ethical aspect regulated the amount of tissue taken from endoscopies for our analysis. We had to choose the methods to provide the best, practically achievable overview of the redox balance in the esophageal mucosa. In addition, we had to omit immunohistochemical stainings of chosen enzymes. SOD exists in isoforms, Cu/Zn‐SOD and Mn‐SOD, and the ratio between two isoforms of SOD would be useful to clarify. The total activity may, therefore, not reveal significant changes in these isoforms. A more detailed redox state of GSH pathway could be reached by determination together of GSH and its oxidized form, oxidized glutathione disulfide (GSSG). There is, however, convincing evidence that GSH alone gives a good picture of the redox state of the cell. Firstly, GSH serves as a substrate in reactions in which reactive oxygen species are detoxified Citation12. Secondly, low GSH values have been linked with various diseases including breast cancer, AIDS, lung cancer, colon cancer, diabetes, inflammatory diseases of lungs and bowel, and esophageal cancer Citation12. Thirdly, neuronal defense against H2O2 is mediated primarily by the glutathione system Citation12. Overall, in normal conditions total GSH (tGSH) is predominantly in its reduced form over the oxidized form with the ratio exceeding 100. Although this ratio can be temporarily lowered up to 10 in various models of oxidative stress, the consumed GSH is replaced by the cell in the long run in order to maintain a constant intracellular GSH concentration essential for the survival of the cell Citation12.
In conclusion, our study revealed a decreased level of SOD activity and lower GSH content in the proximal esophagus before and even 4 years postoperatively in a reflux‐free environment, regardless of low oxidative stress (MPO activity). Oxidative stress remains higher in the distal esophagus even after successful antireflux surgery. The fact that antioxidant defense seems to be defective in the proximal esophagus of GERD patients indicates that the defective antioxidative defense may have an important role in the development of complicated GERD.
Acknowledgements
The authors thank Yvonne Sundström for skillful secretarial assistance.
References
- Oh T. Y., Lee J. S., Ahn B. O., Cho H., Kim W. B., Kim Y. B., et al. Oxidative damages are critical in pathogenesis of reflux esophagitis: implication of antioxidants in its treatment. Free Radic Biol Med 2001; 30: 905–15
- Olyaee M., Sontag S., Salman W., Schnell T., Mobarhan S., Eiznhamer D., et al. Mucosal reactive oxygen species production in oesophagitis and Barrett's oesophagus. Gut 1995; 37: 168–73
- Campos G. M., DeMeester S. R., Peters J. H., Oberg S., Crookes P. F., Hagen J. A., et al. Predictive factors of Barrett esophagus: multivariate analysis of 502 patients with gastroesophageal reflux disease. Arch Surg 2001; 136: 1267–73
- Lagergren J., Bergstrom R., Lindgren A., Nyren O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999; 340: 825–31
- McArdle J. E., Lewin K. J., Randall G., Weinstein W. Distribution of dysplasias and early invasive carcinoma in Barrett's esophagus. Hum Pathol 1992; 23: 479–82
- Terry P., Lagergren J., Ye W., Nyren O., Wolk A. Antioxidants and cancers of the esophagus and gastric cardia. Int J Cancer 2000; 87: 750–4
- Andreoli T. E. Free radicals and oxidative stress. Am J Med 2000; 108: 650–1
- Ogino K., Oka S., Okazaki Y., Takemoto T. Gastric mucosal protection and superoxide dismutase. J Clin Gastroenterol 1988; 10: S129–32
- Hayes J. D., McLellan L. I. Glutathione and glutathione‐dependent enzymes represent a co‐ordinately regulated defence against oxidative stress. Free Radic Res 1999; 31: 273–300
- Wetscher G. J., Hinder P. R., Bagchi D., Perdikis G., Redmond E. J., Glaser K., et al. Free radical scavengers prevent reflux esophagitis in rats. Dig Dis Sci 1995; 40: 1292–6
- Wetscher G. J., Hinder R. A., Klingler P., Gadenstatter M., Perdikis G., Hinder P. R. Reflux esophagitis in humans is a free radical event. Dis Esophagus 1997; 10: 29–32, discussion 33
- Pastore A., Federici G., Bertini E., Piemonte F. Analysis of glutathione: implication in redox and detoxification. Clin Chim Acta 2003; 278: 42588–95
- Grisham M. B., Hernandez L. A., Granger D. N. Xanthine oxidase and neutrophil in filtration in intestinal ischemia. Am J Physiol 1986; 251: G567–74
- Zhang R., Brennan M. L., Shen Z., MacPherson J. C., Schmitt D., Molenda C. E., et al. Myeloperoxidase functions as a major enzymatic catalyst for initiation of lipid peroxidation at sites of inflammation. J Biol Chem 2002; 277: 46116–22
- Peters W. H., Roelofs H. M., Hectors M. P., Nagengast F. M., Jansen J. B. Glutathione and glutathione S‐transferase in Barrett's epithelium. Br J Cancer 1993; 67: 1413–7
- Lee O. J., Schneider‐Stock R., McChesney P. A., Kuester D., Roessner A., Vieth M., et al. Hypermethylation and loss of expression of glutathione peroxidase‐3 in Barrett's tumorigenesis. Neoplasia 2005; 7: 854–61
- Desai K. M., Frisella M. M., Soper N. J. Clinical outcomes after laparoscopic antireflux surgery in patients with and without preoperative endoscopic esophagitis. J Gastrointest Surg 2003; 7: 44–51, discussion 51
- Rantanen T. K., Rasanen J. V., Sihvo E. I., Ahotupa M. O., Farkkilä M. A., Salo J. A. The impact of antireflux surgery on oxidative stress of esophageal mucosa caused by gastroesophageal reflux disease: 4‐yr follow‐up study. Am J Gastroenterol 2006; 101: 222–8
- Spechler S. J., Lee E., Ahnen D., Goyal R. K., Hirano I., Ramirez F., et al. Long‐term outcome of medical and surgical therapies for gastroesophageal reflux disease: follow‐up of a randomized controlled trial. JAMA 2001; 285: 2331–8
- Corey K. E., Schmitz S. M., Shaheen N. J. Does a surgical antireflux procedure decrease the incidence of esophageal adenocarcinoma in Barrett's esophagus? A meta‐analysis. Am J Gastroenterol 2003; 98: 2390–4
- Savary M., Miller G. The esophagus. Handbook and Atlas of Endoscopy. Verlag Gassman AG, SolothurnSwitzerland 1978; 135–9
- Rantanen T. K., Salo J. A., Salminen J. T., Kellokumpu I. H. Functional outcome after laparoscopic or open Nissen fundoplication: a follow‐up study. Arch Surg 1999; 134: 240–4
- Suzuki K., Ota H., Sasagawa S., Sakatani T., Fujikura T. Assay method for myeloperoxidase in human polymorphonuclear leukocytes. Anal Biochem 1983; 132: 345–52
- Laihia J. K., Jansen C. T., Ahotupa M. Lucigenin and linoleate enhanced chemiluminescent assay for superoxide dismutase activity. Free Radic Biol Med 1993; 14: 457–61
- Saville B. A scheme for the colorimetric determination of microgram amounts of thiols. Analyst 1958; 83: 670–2
- Potluri S., Friedenberg F., Parkman H. P., Chang A., MacNeal R., Manus C., et al. Comparison of a salivary/sputum pepsin assay with 24‐hour esophageal pH monitoring for detection of gastric reflux into the proximal esophagus, oropharynx, and lung. Dig Dis Sci 2003; 48: 1813–7
- Kowolik M. J., Grant M. Myeloperoxidase activity in humangingival crevicular neutrophils. Arch Oral Biol 1983; 28: 293–5
- Kongara K. R., Soffer E. E. Saliva and esophageal protection. Am J Gastroenterol 1999; 94: 1446–52
- Wolfsen H. C., Hemminger L. L., DeVault K. R. Recurrent Barrett's esophagus and adenocarcinoma after esophagectomy. BMC Gastroenterol 2004; 4: 18
- Oberg S., Johansson J., Wenner J., Walther B. Metaplastic columnar mucosa in the cervical esophagus after esophagectomy. Ann Surg 2002; 235: 338–45
- Lindahl H., Rintala R. Long‐term complications in cases of isolated esophageal atresia treated with esophageal anastomosis. J Pediatr Surg 1995; 30: 1222–3
- Sihvo E. I., Salminen J. T., Rantanen T. K., Ramo O. J., Ahotupa M., Färkkilä M., et al. Oxidative stress has a role in malignant transformation in Barrett's oesophagus. Int J Cancer 2002; 102: 551–5
- Jimenez P., Piazuelo E., Sanchez M. T., Ortego J., Soteras F., Lanas A. Free radicals and antioxidant systems in reflux esophagitis and Barrett's esophagus. World J Gastroenterol 2005; 11: 2697–703
- Piazuelo E., Cebrian C., Escartin A., Jimenez P., Soteras F., Ortego J., et al. Superoxide dismutase prevents development of adenocarcinoma in a rat model of Barrett's esophagus. World J Gastroenterol 2005; 11: 7436–43
- Hermann B., Li Y., Ray M. B., Wo J. M., Martin R. C 2nd. Association of manganese superoxide dismutase expression with progression of carcinogenesis in Barrett esophagus. Arch Surg 2005; 140: 1204–9, discussion 1209
- McColl K. E. When saliva meets acid: chemical warfare at the oesophagogastric junction. Gut 2005; 54: 1–3
- Epperly M. W., Kagan V. E., Sikora C. A., Gretton J. E., Defilippi S. J., Bar‐Sagi D., et al. Manganese superoxide dismutase‐plasmid/liposome (MnSOD‐PL) administration protects mice from esophagitis associated with fractionated radiation. Int J Cancer 2001; 96: 221–31