Abstract
Background. The populations in adjacent Russian Karelia and Finland are equally exposed to grain products and share partly the same ancestry, but live in completely different socioeconomic environments.
Aim. This creates an ideal epidemiological setting to study gene‐environmental interactions in pathogenesis of celiac disease.
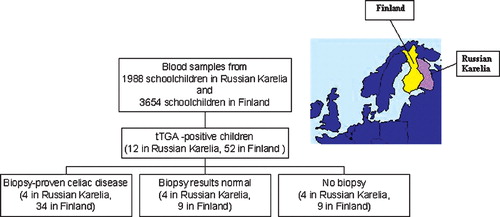
Methods. The prevalence of celiac disease and predisposing human leukocyte antigen (HLA) alleles was compared between Russian Karelia and Finland. Tissue transglutaminase antibodies and HLA‐DQ alleles were screened from 1988 schoolchildren from Karelia and 3654 children from Finland. Children with transglutaminase antibodies were invited to small‐bowel biopsy.
Results. Transglutaminase antibodies were less frequent in Russian Karelia than in Finland (0.6% versus 1.4%, P = 0.005). Immunoglobulin class G (IgG) antigliadin antibodies were also less frequent in Russian Karelia (10.2% versus 28.3%, P<0.0001). Celiac disease was confirmed by duodenal biopsy in four of the eight transglutaminase antibody‐positive Karelian children, giving a prevalence of 1 in 496 compared to 1 in 107 children in Finland. The same HLA‐DQ alleles were associated with celiac disease and transglutaminase antibody positivity in both populations.
Conclusions. The prevalence of transglutaminase antibodies and celiac disease is lower in Russian Karelia than in Finland. This may be associated with a protective environment characterized by inferior prosperity and standard of hygiene in Karelia.
Introduction
Celiac disease is an immune‐mediated disorder of the small intestine with a number of extraintestinal manifestations driven by ingestion of wheat gluten (gliadin) or related proteins from rye (secalins) and barley (hordeins). It is prevalent among Caucasians affecting on average approximately 1 in 100 individuals Citation1–4. The prevalence is high in many European countries, South America, USA, North Africa, Iran, and India, but considerably lower in Japan and China Citation5–8. There are no earlier publications on the prevalence of celiac disease in Russia. Celiac disease is quite common in Finland (1:100) while in neighboring Estonia the prevalence is 1:290 Citation9.
Celiac disease has long been considered a disease of childhood with classic symptoms of malabsorption. However, in recent years serological screening by antibody testing has made it possible to detect celiac disease in subjects without classic symptoms Citation10, Citation11. Subjects with silent and latent forms of the disease can be reliably identified by the presence of highly sensitive and specific tissue transglutaminase (tTGA) and endomysial antibodies (EMA) Citation12. Studies based on large‐scale screening of the background population for the presence of these antibodies have indicated that only 20%–50% of affected individuals have subjective symptoms Citation13. Gliadin antibodies are not such specific risk markers for celiac disease as tTGA Citation14, Citation15, but rather reflect immunization to dietary gliadin, which may have no pathogenetic relevance.
The pathogenesis of celiac disease is multifactorial, and human leukocyte antigen molecules (HLA) and certain other genes together with environmental factors contribute to the process Citation16–18. Around 90% of patients with celiac disease express the HLA‐DQ2 molecule encoded by the DQA1*05 and DQB1*02 genes (either in cis position in the DR3‐DQ2 haplotype or in trans position in the DR5‐DQ7/DR7‐DQ2 genotype). Half of the remaining patients carry the HLA‐DQ8 molecule encoded by DQA1*03 and DQB1*0302 genes Citation19, Citation20. However, it is known that only a small proportion of HLA‐DQ2 or DQ8‐positive individuals develop celiac disease, and additional genes may predispose to the disease. The concordance rate is about 90% among monozygotic twins and 30% among HLA‐identical siblings Citation21. Environmental factors other than gluten (e.g. gastrointestinal infections) may also modulate the disease risk Citation22.
The aim of this study was to compare the prevalence of celiac disease and immunological and genetic risk markers for the disease in two populations, living geographically close to each other in markedly different socioeconomic circumstances (Karelian Republic of Russia and adjacent Finland). In addition to identical geographical and climatic factors these populations have a comparable consumption of grain products Citation23, but due to the sharp welfare gradient across the border, the populations are exposed to completely different socioeconomic environments, reflected e.g. by marked differences in the frequency of microbial infections Citation24, Citation25 and early feeding in childhood Citation26. This creates an ideal setting to study the role of such environmental factors in the pathogenesis of celiac disease.
Key messages
There is a conspicuous difference in the prevalence of celiac disease between Russian Karelia and Finland.
The lower prevalence of celiac disease in Russian Karelia seems not to be due to differences in genetic predisposition or to the consumption of grain products, but may be associated with a protective environment characterized by poorer living conditions and standard of hygiene.
Methods
Subjects
Karelian cohort
Serum samples were obtained from 1988 schoolchildren (984 males) with a mean age of 11.6 years (range 6.2–18.3 years) in Russian Karelia during the period 1997–2001 as part of the type 1 diabetes‐related EPIVIR project (EU INCO‐Copernicus Programme, contract number IC15‐CT98‐0316). Samples were taken from randomly selected schoolchildren in different regions of Russian Karelia. In addition, an EDTA (ethylenediamine tetra‐acetic acid) whole blood sample was obtained from all children for genetic analyses. Serum and blood samples were stored at −20°C until analyzed. The ethnic background of the children was recorded according to the ancestry of both the mother and father and was classified as follows: 500 children with both parents having a Finnish or Karelian background, 382 with a Russian background, and 1106 children with a mixed or other ethnic background. The diagnosis of celiac disease was confirmed by upper gastrointestinal endoscopy and small‐bowel biopsy. Treatment of celiac disease was organized for the children with confirmed disease. All children had parental consent to participate in the study, and the study was approved by the Ministry of Health in the Karelian Republic of Russia.
Finnish cohort
The Finnish cohort comprised 3654 schoolchildren (1826 males) living in five municipalities in northern Finland and recruited during 1994. The mean age of these subjects was 11.7 years (range 7.0–18.0 years). All children had parental consent to take part in the study, and the study was approved by the ethical committee of the Faculty of Medicine, University of Oulu, Finland. The results of their tTGA and HLA screening have been reported earlier Citation6. The methods and laboratories used for that purpose are identical to those used for the Karelian cohort.
Antibody analyses
All schoolchildren from both Russian Karelia and Finland were screened for serum immunoglobulin class A (IgA)‐class antibodies to tTGA using an enzyme‐linked immunosorbent assay (ELISA)‐based Celikey assay (Pharmacia Diagnostics, Freiburg, Germany) according to the manufacturer's instructions, and the cutoff limit for tTGA positivity was set at 5 U/mL Citation6. Serum IgA‐class endomysial antibodies (EMA) were additionally analyzed from all tTGA‐positive subjects by indirect immunofluorescence as previously described Citation27. A characteristic staining pattern at serum dilution ⩾1:5 was considered positive. IgA‐ and IgG‐class antigliadin antibodies were analyzed using an in‐house ELISA method Citation28 in a subgroup of 265 Finnish and 265 Karelian children (median age 11.4±2.0 (SD) years in both groups), the latter being selected based on Finnish‐Karelian ethnicity in both parents. Values equal to or above 10 EU/mL for IgG‐class antigliadin antibodies and 0.2 EU/mL for IgA‐class antigliadin antibodies were considered positive. All antibody analyses were carried out at the Medical School, University of Tampere, Finland, using identical methods for both series.
Endoscopy and small intestinal biopsy
All subjects who were positive for serum tTGA were offered an option for upper gastrointestinal endoscopy and small intestinal mucosal biopsy to confirm the diagnosis of celiac disease.
A complete clinical examination was carried out, and a new serum sample was obtained at the time of endoscopy. In Russian Karelia the endoscopy was performed at the Municipal Children's Clinic in Petrozavodsk. In Finland the endoscopy was carried out as a part of an earlier study Citation6 at the Department of Pediatrics, Oulu University Hospital, Finland. For histological analysis four duodenal mucosal biopsy samples were prepared (three samples fixed in formalin and one snap‐frozen in liquid nitrogen for cryostat sections). Light microscopy and morphometric techniques were used to study the formalin‐fixed biopsy samples stained with hematoxylin and eosin. A ratio of villous height to crypt depth less than 2 was considered an indicator of celiac disease (i.e. villous atrophy with crypt hyperplasia). The frozen biopsy specimens were stained for intraepithelial lymphocytes bearing CD3+, α/β+ and γ/δ+ T‐cell receptors, and corresponding cell densities were determined as previously described Citation29. Small‐bowel mucosal tissue transglutaminase‐specific IgA deposits were investigated from frozen sections by direct immunofluorescence, and the intensity of the deposits was graded 0–3 as previously described Citation30, Citation31. In celiac disease a clear subepithelial IgA deposit can be found below the basement membrane along the villous and crypt epithelium and around mucosal vessels. This is in contrast to normal small‐bowel samples, where IgA is detected only inside the plasma and epithelial cells. All histological analyses were centralized to Tampere, Finland, and were performed without prior knowledge of disease history or laboratory findings.
HLA typing
HLA class II risk alleles were typed by polymerase chain reaction and microtiter well plate‐based hybridization with lanthanide‐labeled oligonucleotide probes as previously described Citation32. Samples that were positive for the HLA‐DQB1*02 allele were further analyzed for the presence of associated HLA alleles DQA1*0201 and DQA1*05. Samples from 1977 schoolchildren (99%) from Russian Karelia and from 3649 Finnish schoolchildren (99.9%) were available for genotyping.
Statistical analysis
Student's t‐test was used for the analyses of continuous variables and the chi‐square test for the analyses of distributions (SPSS, version 12.1, SPSS Inc., Chicago, IL, USA). The 95% confidence intervals (CI) were calculated with the exact method. A two‐tailed P‐value of 0.05 was considered to indicate statistical significance.
Results
Serological screening
The frequency of celiac disease‐associated autoantibodies was significantly lower among children in Russian Karelia compared to Finland; 0.6% of the children (12/1988; CI 0.3%–1.1%) in Russian Karelia tested positive for tTGA compared to 1.4% (52/3654; CI 1.1%–1.9%) in the Finnish cohort (P = 0.005). The median tTGA titer in the tTGA‐positive children was 10.0 U/mL (range 5–56) in Russian Karelia compared to 70.3 U/mL (range 5.7–1048) in Finland (P<0.0001). In Russian Karelia, the frequency of tTGA was 0.4% (CI 0.05%–1.5%; antibody range 5–9.7 U/mL) among children of Finnish and/or‐Karelian ancestry compared to 0.7% (CI 0.3%–1.1%; antibody range 5.6–56 U/mL) in other ethnic groups (P = 0.735).
Nine (75%) of the 12 tTGA‐positive Russian Karelia subjects were also positive for EMA. The three tTGA‐positive but EMA‐negative children had very low tTGA levels (). In the Finnish cohort 50 (96%) of the 52 tTGA‐positive subjects tested positive for EMA, and the two remaining EMA‐negative subjects had very low tTGA levels (5.7 and 7.0 U/mL).
Table I. Characteristics of the 12 Russian‐Karelian children who tested positive for tTGA in the primary screening; 8 of these children underwent endoscopy and small‐bowel biopsy to confirm the diagnosis of celiac disease (cases 1–8), while 4 children did not undergo endoscopy (cases 9–12).
IgA‐ and IgG‐class gliadin antibodies were analyzed in a subgroup of 265 Karelian children of Finnish/Karelian ancestry and in 265 Finnish children. IgG‐class gliadin antibodies were detected in 10.2% of the Karelian (CI 6.8%–14.5%) and in 28.3% of the Finnish children (CI 23.0%–34.1%; P<0.0001). IgA‐class gliadin antibodies were observed in 12.1% (CI 8.4%–16.6%) and 16.2% (CI 12.0%–21.2%) of the children, respectively (P = 0.212). There were no significant differences in the levels of IgA‐class and IgG‐class gliadin antibodies between the Karelian and Finnish children testing positive for these antibodies (data not shown).
Biopsy‐proven celiac disease
All Karelian subjects who were positive for tTGA (ten girls and two boys) were invited to small‐bowel biopsy to confirm the diagnosis of celiac disease. Eight of the invited children agreed to undergo biopsy, and representative biopsy samples were obtained from all of them (the cryostat section of one child was damaged during transportation to the laboratory). The diagnosis of celiac disease was confirmed in four children (50%), as the biopsy indicated clear villous atrophy and crypt hyperplasia (). Only one of the biopsied children had previously been diagnosed with celiac disease (case 3, ). That boy was diagnosed at the age of 2 years but had not adhered to a gluten‐free diet. In three of the celiac cases, marked intraepithelial infiltrations of CD3+, α/β+, and γ/δ+ T‐cell receptor‐bearing lymphocytes and mucosal tissue transglutaminase IgA deposits were also observed. Three of the four biopsy‐proven celiac patients reported recurrent abdominal pain and intermittent diarrhea or constipation, two suffered from nausea and vomiting, and one from tiredness. One of them had anemia, one suffered from fainting fits, and one had another autoimmune disease (vitiligo). Thus, the prevalence of biopsy‐proven celiac disease can be estimated to be 1:496 in Russian Karelia, which is only about one‐fifth of the prevalence in the Finnish cohort based on the same screening and diagnostic criteria (1:107) (). Only one (25%) in four patients confirmed to have celiac disease on biopsy was of Finnish/Karelian ancestry while three (75%) children were of mixed ethnic background.
Normal small‐bowel mucosal morphology
Four children with initially low tTGA levels, showed normal small‐bowel mucosal villous morphology (). After the initial screening tTGA had turned negative at the time of the endoscopy in three of these four cases (cases 6, 7, and 8; ). Despite the normal mucosal villous structure, three children had increased density of γ/δ+ intraepithelial lymphocytes (cases 5, 6, and 7; ) and, in addition, one showed high density CD3+ cells together with clear mucosal tissue transglutaminase‐specific IgA‐deposits (case 5; ). All these findings indicated ongoing mucosal inflammation.
HLA typing
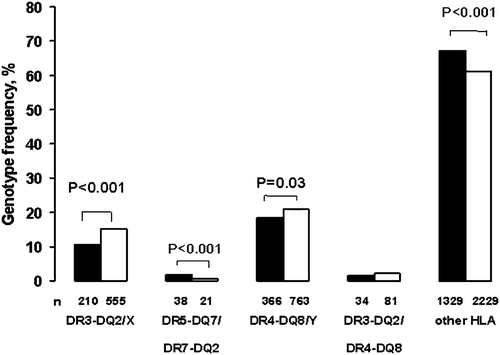
The frequency of the celiac disease‐associated HLA‐DR3‐DQ2 (DQA1*05‐DQB1*02) haplotype was lower among the schoolchildren in Russian Karelia (10.6%; CI 9.3%–12.0%) than among the Finnish schoolchildren (15.2%; CI 14.1%–16.5%; P<0.001), while the frequency of the HLA‐ DR5‐DQ7/DR7‐DQ2 risk genotype was significantly higher among the Karelian children (1.9%; CI 1.4%–2.6% versus 0.6%; CI 0.4%–0.9%; P<0.001) (). The HLA‐DR3‐DQ2 haplotype was associated with tTGA positivity in both populations, but the Finnish children who carried this high‐risk haplotype were more frequently tTGA‐positive than the corresponding Karelian children (7.4%; CI 5.4%–9.9% versus 2.9%; CI 1.1%–6.1%; P = 0.03). Three of the children carrying the HLA‐DR5‐DQ7/DR7‐DQ2 genotype were tTGA‐positive, and they were all Karelian children. Gliadin antibodies were not associated with the HLA‐DR3‐DQ2 haplotype.
Figure 2 Frequency of different human leukocyte antigen(HLA) genotypes in schoolchildren in Russian Karelia (black bars) and in Finland (white bars). Altogether 1977 Russian‐Karelian and 3649 Finnish children were HLA‐typed (X = other than DQ7 or DQ8; Y = other than DQ2; n = absolute number of children carrying different HLA genotypes).
All four Karelian patients confirmed to have celiac disease on biopsy carried HLA genotypes conferring increased disease susceptibility (). One of the four tTGA‐positive children having normal small‐bowel mucosal architecture carried the HLA‐DQB1*02 allele which encodes the beta‐chain of the disease‐associated DQ2 molecule, being present together with DQA1*0201 in the DR7‐DQ2 haplotype. The antibody and HLA status of the four Russian‐Karelian children who were tTGA‐positive but did not undergo endoscopy are shown in .
Discussion
The present results indicate a steep gradient in the prevalence of celiac disease between Russian Karelia and Finland. The frequency of biopsy‐proven celiac disease was 1:496 in Karelia compared to 1:107 in Finland using identical criteria. This difference cannot be due to methodological differences, as the diagnosis was based on identical study design and methodology. The laboratory analyses were carried out in a single laboratory with extensive experience of this kind of studies. The study was based on tTGA screening in an unselected background population (schoolchildren) followed by confirmation of the diagnosis by small‐bowel biopsy in antibody‐positive cases thus excluding the possibility of any selection bias. Furthermore, the gradient remained conspicuous when it was estimated in a different manner: extrapolation from the biopsy findings in the antibody‐positive children in 50% of whom the diagnosis was confirmed in Karelia would give an estimated prevalence of 1 in 331 (6 out of 1988 Karelian children). Extrapolation from the biopsy findings in antibody‐positive Finnish children in 79% of whom the diagnosis was confirmed (34 biopsies in 43 Finnish subjects) would give an estimated prevalence of 1 in 89 (41 out of 3654 Finnish schoolchildren). Accordingly, there is still a 3.7‐fold difference in the prevalence of biopsy‐proven celiac disease between these populations based on extrapolated data. This does not differ from the 4.6‐fold difference based on unextrapolated data.
This study is the first extensive epidemiological survey of gluten intolerance among children in Russian Karelia. The prevalence of celiac disease in Karelian children (1:496) was found to be close to that previously reported from Croatia (1:500), whereas the prevalence has been observed to be substantially higher in most European populations (1:85 to 1:200) Citation1, Citation33. Studies based on serological population screening suggest that celiac disease is quite common in almost all populations with a gluten‐rich diet Citation33, Citation34. Such epidemiological comparisons have often been difficult to interpret due to the fact that celiac disease often presents without clear clinical symptoms, and latent, atypical forms of the disease remain undiagnosed in many patients Citation10, Citation35–40.
Most likely, the etiology of celiac disease is multifactorial. Breast‐feeding duration, and the amount of gluten consumed and the age of introducing gluten into the diet of infants are of importance Citation41, Citation42.
The important virtue of the present study is that it provides a unique opportunity to evaluate the reasons underlying the marked difference in celiac disease between these two populations. This is based on the fact that the border separating the two countries is one of the sharpest welfare gradients in the world. In 2001 the gross national product in Russian Karelia was USD (United States Dollar) 1660 compared to USD 25,130 in Finland (In 2004 the corresponding figures were USD 3410 and USD 32,790). This has a marked influence on the life‐style and socioeconomic circumstances of these two populations and is reflected in a much higher frequency of microbial infections (such as hepatitis A virus, Helicobacter pylori, and Toxoplasma gondii) in Russian Karelia than in Finland Citation24, Citation25. Furthermore, these populations have partly the same ancestry, which makes it possible to assess the contribution of both environmental and genetic factors to the observed difference in disease prevalence.
Possible explanations include differences in dietary gluten exposure, genetic disease susceptibility, and/or other factors modifying disease risk. Cereals are an important constituent of the diet in both populations. There is no evidence that exposure to gluten differs markedly between these two countries, even though there is no detailed data available on the age at introduction and amount of gluten introduced in children in Russian Karelia. The only available information is on the age of introducing cereals in infants in both populations. Thus, the Diabetes Prediction and Prevention (DIPP) Nutrition Study in Finland has showed that the median age of introduction to oats is 5 months, whereas it is 6 months for wheat, barley, and rye Citation43. In the Russian Federation the age at first exposure to cereal is 4–5 months Citation44, Citation45. According to the Food and Agriculture Organization Statistics the annual consumption of grain and grain products per person is 106 kg in Finland compared to 155 kg in Russia Citation23. Accordingly, it seems that different exposure to gluten is hardly the reason for the conspicuous difference in the disease prevalence.
Lack of breast‐feeding has been reported to be associated with type 1 diabetes, celiac disease, and a number of other chronic childhood disorders. The conspicuous difference in socioeconomic situation between Finland and Russian Karelia does have a profound effect on the living conditions in the two countries affecting early feeding. Thus, the duration of breast‐feeding in Finland is two times longer than that in Russia. According to the WHO Global Data Bank on Breastfeeding and Complementary Feeding, infants in Finland are breast‐fed for 6–10 months, while infants in the Russian Federation are breast‐fed for 3–5 months (years of survey 1983–1997, in Finland; 1992–1996, in the Russian Federation). These data are in contrast to the observations that an increased duration of breast‐feeding may decrease the incidence of celiac disease. Taken together, these findings indicate that other factors may also contribute to the considerable difference seen in the incidence of celiac disease between Russian Karelia and Finland. The search for such factors should be intensified. Differences in the use of antibiotics in children are a factor that can potentially contribute to the observed difference in the incidence of celiac disease. There are, however, no comparable data available in the two countries on the consumption of antibiotics in children.
A difference similar to that seen for tTGA was observed in the frequency of IgG‐class gliadin antibodies between the two populations. It is possible that the more common immunization to dietary gliadin in the Finnish population is a key event inducing the disease process in individuals with genetic predisposition to celiac disease. This is also supported by the fact that tTGA were more frequent in the Finnish than in the Karelian children who carried the major genetic risk marker for the disease (the HLA‐DR3‐DQ2 haplotype). This suggests that some still undefined environmental factor(s) may trigger the process in genetically susceptible Finnish children or protect genetically susceptible children in Russian Karelia.
The previously described major susceptibility haplotype DR3‐DQ2 was associated with biopsy‐proven disease both in Russian Karelia and in Finland. This haplotype also showed an association with tTGA‐positivity in the background population. It was slightly more frequent in the Finnish population compared to the Karelian children (15.2% versus 10.6%), but it is very unlikely that such a small difference could explain the marked difference in disease prevalence. In contrast, it seems that the genetically susceptible children are more prone to the disease in Finland, reflected by the more frequent occurrence of tTGA in genetically susceptible Finnish children compared to genetically predisposed Karelian children. Our data on the other risk‐associated genotype Citation46, i.e. HLA‐DR5‐DQ7/DR7‐DQ2 which also encodes the risk‐associated DQ2 molecule, show in contrast that this genotype is more common in Russian Karelia than in Finland (1.9% versus 0.6%). This genotype is common among patients with celiac disease in southern Europe but rare in northern Europe Citation47.
In conclusion, the present study suggests that some nongenetic factors must contribute to the low disease incidence in Russian Karelia. In the present study it is not possible to identify the operative environmental factors, but one may speculate that they may be linked to the markedly different socioeconomic conditions resulting, for example, in differences in gut microbial flora, the frequency of intestinal and parasitic infections and in a variety of dietary factors other than gluten. We have previously reported that there is a lower incidence rate of other immune‐mediated diseases, i.e. type 1 diabetes and atopic sensitization, among children under the age of 15 years in Russian Karelia when compared to Finland Citation24, Citation48. These findings in combination with the present observation imply that there must be substantial differences in the programming of the immune system in young children related to the contrasting standard of living in these two adjacent countries. Studies are in progress to find out whether this phenomenon could be explained by the hygiene hypothesis and the role of early microbial exposures in the maturation of regulatory elements of the immune system.
This study offers a unique opportunity to increase our knowledge of celiac disease by future research. Most important is the identification of environmental factors affecting the prevalence of celiac disease in different populations. Collection of accurate data on quality and quantity of gluten intake in early childhood and weaning practices provides the opportunity to determine the effects of these factors on the age at disease presentation and underlying mechanisms of maturation of the immune system in young children. Large prospective screening programs could facilitate early diagnosis of celiac disease and minimize the risk for onset of other autoimmune disorders.
Acknowledgements
This study was supported by the EU as a part of the INCO‐Copernicus Programme (EPIVIR study, contract number IC15‐CT98‐0316) and by grants from the Päivikki and Sakari Sohlberg Foundation, the Tampere Tuberculosis Foundation, the Academy of Finland, and the University of Tampere. These funders had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript. We thank Eveliina Jalonen, Mervi Kekäläinen, Terttu Lauren, and Ritva Suominen for their skilful technical assistance. We express our gratitude to Dr Elena Kozubova, Dr Oleg Leksunov, and Dr Vladimir Petrov from the Committee on Public Health, Ecology and Social Protection, Petrozavodsk City Administration, for the valuable help and support. We would like to thank Virginia Mattila and Sisko Tauriainen for their suggestions and input in the preparation of this manuscript. We express our gratitude to all children and parents who participated in the study. The authors have no conflict of interest to declare. The principal investigator (HH) had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The EPIVIR study group includes: H. Hyöty (coordinator), M. Knip, H. Viskari, University of Tampere, Finland; J. Ilonen, University of Turku, Finland; A. Reunanen, National Public Health Institute, Helsinki, Finland; R. Uibo (scientific coordinator), L. Salur, University of Tartu, Estonia; J. Ludvigsson, University of Linköping, Sweden; D. Marciulionyte, Kaunas University of Medicine, Lithuania; R. Hermann, G. Soltesz, University of Pécs, Hungary; M. Füchtenbusch, A. Ziegler, Munich, Germany; A. Kondrashova, A. Romanov, University of Petrozavodsk, Russia.
References
- Accomando S., Cataldo F. The global village of celiac disease. Dig Liver Dis 2004; 36: 492–8
- Hoffenberg E. J., MacKenzie T., Barriga K. J., Eisenbarth G. S., Bao F., Haas J. E., et al. A prospective study of the incidence of childhood celiac disease. J Pediatr 2003; 143: 308–14
- Rewers M. Epidemiology of celiac disease: what are the prevalence, incidence, and progression of celiac disease?. Gastroenterology 2005; 128: S47–51
- Dube C., Rostom A., Sy R., Cranney A., Saloojee N., Garritty C., et al. The prevalence of celiac disease in average‐risk and at‐risk Western European populations: a systematic review. Gastroenterology 2005; 128: S57–67
- Greco L. Epidemiology of coeliac disease. Coeliac disease, M. Mäki, P. Collin, J. K. Visakorpi. Coeliac Disease Study Group, Tampere 1997; 9–14
- Maki M., Mustalahti K., Kokkonen J., Kulmala P., Haapalahti M., Karttunen T., et al. Prevalence of Celiac disease among children in Finland. N Engl J Med 2003; 348: 2517–24
- Lowichik A., Book L. Pediatric celiac disease: clinicopathologic and genetic aspects. Pediatr Dev Pathol 2003; 6: 470–83
- Castano L., Blarduni E., Ortiz L., Nunez J., Bilbao J. R., Rica I., et al. Prospective population screening for celiac disease: high prevalence in the first 3 years of life. J Pediatr Gastroenterol Nutr 2004; 39: 80–4
- Ress K., Harro M., Maaroos H. I., Harro J., Uibo R., Uibo O. High prevalence of coeliac disease: need for increasing awareness among physicians. Dig Liver Dis 2007; 39: 136–9
- Maki M., Collin P. Coeliac disease. Lancet 1997; 349: 1755–9
- Collin P., Reunala T., Mäki M. New diagnostic strategy for coeliac disease. Coeliac disease, M. Mäki, P. Collin, J. K. Visakorpi. Coeliac Disease Study Group, Tampere 1997; 47–51
- Treem W. R. Emerging concepts in celiac disease. Curr Opin Pediatr 2004; 16: 552–9
- Fasano A., Catassi C. Current approaches to diagnosis and treatment of celiac disease: an evolving spectrum. Gastroenterology 2001; 120: 636–51
- Reif S., Lerner A. Tissue transglutaminase—the key player in celiac disease: a review. Autoimmun Rev 2004; 3: 40–5
- Ciccocioppo R., Di Sabatino A., Ara C., Biagi F., Perilli M., Amicosante G., et al. Gliadin and tissue transglutaminase complexes in normal and coeliac duodenal mucosa. Clin Exp Immunol 2003; 134: 516–24
- Papadopoulos G. K., Wijmenga C., Koning F. Interplay between genetics and the environment in the development of celiac disease: perspectives for a healthy life. J Clin Invest 2001; 108: 1261–6
- Dewar D., Pereira S. P., Ciclitira P. J. The pathogenesis of coeliac disease. Int J Biochem Cell Biol 2004; 36: 17–24
- Sollid L. M. Coeliac disease: dissecting a complex inflammatory disorder. Nat Rev Immunol 2002; 2: 647–55
- Liu J., Juo S. H., Holopainen P., Terwilliger J., Tong X., Grunn A., et al. Genomewide linkage analysis of celiac disease in Finnish families. Am J Hum Genet 2002; 70: 51–9
- Sollid L. M. Molecular basis of celiac disease. Annu Rev Immunol 2000; 18: 53–81
- Greco L., Romino R., Coto I., Di Cosmo N., Percopo S., Maglio M., et al. The first large population based twin study of coeliac disease. Gut 2002; 50: 624–8
- Hernell O., Ivarsson A., Persson L. A. Coeliac disease: effect of early feeding on the incidence of the disease. Early Hum Dev 2001; 65 Suppl: S153–60
- Food and Agriculture Organization Statistics Division Food Balance Sheet. 2002, http://faostat.fao.org/site/354/default.aspx
- Seiskari T., Kondrashova A., Viskari H., Kaila M., Haapala A. M., Aittoniemi J., et al. Allergic sensitization and microbial load—a comparison between Finland and Russian Karelia. Clin Exp Immunol 2007; 148: 47–52
- von Hertzen, Laatikainen T., Pitkänen T., Vlasoff T., Mäkelä M. J., Vartiainen E., et al. Microbial content of drinking water in Finnish and Russian Karelia—implications for atopy prevalence. Allergy 2007; 63: 288–92
- The WHO Database on Breastfeeding and Complementary Feeding, web. address: http://www.who.int/research/iycf/bfcf/bfcf.asp
- Sulkanen S., Halttunen T., Marttinen A., Leivo E. L., Laurila K., Maki M. Autoantibodies in celiac disease: importance of fibroblasts. J Pediatr Gastroenterol Nutr 1998; 27: 206–13
- Sulkanen S., Halttunen T., Laurila K., Kolho K. L., Korponay‐Szabo I. R., Sarnesto A., et al. Tissue transglutaminase autoantibody enzyme‐linked immunosorbent assay in detecting celiac disease. Gastroenterology 1998; 115: 1322–8
- Jarvinen T. T., Kaukinen K., Laurila K., Kyronpalo S., Rasmussen M., Maki M., et al. Intraepithelial lymphocytes in celiac disease. Am J Gastroenterol 2003; 98: 1332–7
- Korponay‐Szabo I. R., Halttunen T., Szalai Z., Laurila K., Kiraly R., Kovacs J. B., et al. In vivo targeting of intestinal and extraintestinal transglutaminase 2 by coeliac autoantibodies. Gut 2004; 53: 641–8
- Kaukinen K., Peraaho M., Collin P., Partanen J., Woolley N., Kaartinen T., et al. Small‐bowel mucosal transglutaminase 2‐specific IgA deposits in coeliac disease without villous atrophy: a prospective and randomized clinical study. Scand J Gastroenterol 2005; 40: 564–72
- Nejentsev S., Sjöroos M., Soukka T., Knip M., Simell O., Lövgren T., et al. Population‐based genetic screening for the estimation of Type 1 diabetes mellitus risk in Finland: selective genotyping of markers in the HLA‐DQB1, HLA‐DQA1 and HLA‐DRB1 loci. Diabet Med 1999; 16: 985–92
- Henker J., Losel A., Conrad K., Hirsch T., Leupold W. [Prevalence of asymptommatic coeliac disease in children and adults in the Dresden region of Germany]. Dtsch Med Wochenschr 2002; 127: 1511–5
- Rostami K., Malekzadeh R., Shahbazkhani B., Akbari M. R., Catassi C. Coeliac disease in Middle Eastern countries: a challenge for the evolutionary history of this complex disorder?. Dig Liver Dis 2004; 36: 694–7
- Green P. H., Jabri B. Coeliac disease. Lancet 2003; 362: 383–91
- Collin P., Kaukinen K., Valimaki M., Salmi J. Endocrinological disorders and celiac disease. Endocr Rev 2002; 23: 464–83
- Ascher H. Paediatric aspects of coeliac disease: old challenges and new ones. Dig Liver Dis 2002; 34: 216–24
- Catassi C., Fasano A. New developments in childhood celiac disease. Curr Gastroenterol Rep 2002; 4: 238–43
- Freeman H., Lemoyne M., Pare P. Coeliac disease. Best Pract Res Clin Gastroenterol 2002; 16: 37–49
- Kennedy N. P., Feighery C. Clinical features of coeliac disease today. Biomed Pharmacother 2000; 54: 373–80
- Hernell O., Ivarsson A., Persson L. A. Coeliac disease: effect of early feeding on the incidence of the disease. Early Hum Dev 2001; 65 Suppl: S153–60
- Schack‐Nielsen L., Michaelsen K. F. Breast feeding and future health. Curr Opin Clin Nutr Metab Care 2006; 9: 289–96
- Virtanen S., Kenward M. G., Erkkola M., Kautiainen S., Kronberg‐Kippilä C., Hakulinen T., et al. Age at introduction of new foods and advanced beta cell autoimmunity in young children with HLA‐conferred susceptibility to type 1 diabetes. Diabetologia 2006; 49: 1512–21
- Feeding practices to children during their first year of life. Methodical Recommendation of the Ministry of Health of the USSR. Ministry of Health, Moscow 1982, [in Russian]
- Contemporary principles and methods of feeding practices to children during their first year of life. Methodical Recommendation of the Ministry of Health of the Russian Federation N225. Ministry of Health, Moscow 1999, [in Russian]
- Fernandez‐Arquero M., Figueredo M. A., Maluenda C., de la Concha E. G. HLA‐linked genes acting as additive susceptibility factors in celiac disease. Hum Immunol 1995; 42: 295–300
- Margaritte‐Jeannin P., Babron M. C., Bourgey M., Louka A. S., Clot F., Percopo S., et al. HLA‐DQ relative risks for coeliac disease in European populations: a study of the European Genetics Cluster on Coeliac Disease. Tissue Antigens 2004; 63: 562–7
- Kondrashova A., Reunanen A., Romanov A., Karvonen A., Viskari H., Vesikari T., et al. A six‐fold gradient in the incidence of type 1 diabetes at the eastern border of Finland. Ann Med 2005; 37: 67–72