Abstract
A significant proportion of depressed patients eventually present with treatment‐resistant/refractory major depression (TRD), a debilitating condition that imposes significant health, social, and economic burdens. Recently, a growing level of consensus has been reached on the general meaning of TRD, according to which, depression is considered resistant when at least two trials with antidepressants from different pharmacologic classes (adequate in terms of dose, duration, outcome, and compliance) failed to achieve clinical remission. Regarding the management of TRD, a two‐step approach is suggested, involving first the evaluation of factors that may contribute to treatment nonresponse (such as comorbid medical and psychiatric conditions), and second, the use of the four classical strategies for enhancing antidepressant efficacy (namely optimization, augmentation, combination, and switching). Finally, future research on TRD should include studies addressing, among other issues, the validity of the proposed definitional criteria, the evaluation of reliable predictors of treatment outcome, and the development of novel therapeutic strategies.
Introduction
In its broadest sense, treatment‐resistant/refractory depression (TRD) is found among a significant number of patients in therapy Citation1–3. Indeed, about one‐third of patients treated for major depressive disorder (MDD) do not respond satisfactorily to the first antidepressant Citation3, Citation4, and up to 15% of cases will be significantly depressed in spite of multiple pharmacological and psychotherapeutic approaches Citation5, Citation6.
However, systematic research on TRD has been hampered to a significant extent owing to the inconsistent ways in which it has been characterized and defined in the specialized literature Citation7–9. Indeed, one can find more than 15 disparate definitions of TRD while searching the specialized literature Citation10, Citation11. Furthermore, our current knowledge on TRD is based mainly on naturalistic follow‐up studies of the outcome of depressive disorders in general Citation2, Citation12. Thus, it is possible that the true proportion of poorly responsive patients has been overestimated, as an unknown number of apparently resistant patients have been included who may have been inadequately treated, poorly compliant, or affected by other conditions that prolong chronic depression Citation2, Citation10. Finally, although available data suggest that resistant patients may share a number of characteristics (e.g. clinical, neurobiological, environmental), the relative heterogeneity of TRD speaks against a classification of this condition as a discrete subtype of depression Citation3, Citation13.
Key messages
Broadly speaking, treatment‐resistant depression (TRD) still characterizes a significant number of patients in therapy.
Major depression is usually considered resistant when at least two appropriate trials of antidepressants from different pharmacological classes failed to produce clinical remission.
The somatic management of TRD should be done in a systematic way, through the use of clinical strategies that include optimization, augmentation, combination, and/or switching.
Defining resistance to treatment in unipolar depression
TRD is broadly defined as the occurrence of an insufficient clinical response following adequate antidepressant therapy (in terms of dose, duration, and compliance) among patients suffering from MDD Citation4, Citation7, Citation14. How many adequately delivered trials are required, and when these trials are to be delivered (e.g. only in the current episode) to declare treatment resistance remain debatable Citation5, Citation14. Nevertheless, an emerging consensus in the specialized literature is that clinically significant treatment resistance is present if a current episode of depression has not adequately benefited from at least two adequate trials of different classes of antidepressants Citation6–8, Citation14, Citation15. Furthermore, TRD is usually best understood not as an all‐or‐none phenomenon, but as occurring along a continuum ranging from partial response to complete treatment resistance Citation3, Citation10, Citation12.
Factors associated with treatment resistance in depression
A number of clinical, sociodemographic, and biological variables have been studied in relationship to response/resistance to antidepressant therapy Citation11. However, this literature yields largely unreliable results, mainly because of methodological variability and the heterogeneity of depression itself Citation7. Nevertheless, although pointing to the need for further studies, we summarize below some of the factors most commonly associated with TRD.
Age at onset
With age at onset, both ends of the spectrum have been described as risk factors for TRD. More specifically, onset of depression in patients over 60 years has been associated with features that may lead to resistance, including a greater likelihood of psychotic symptoms, the presence of brain changes (e.g. vascular), and more comorbid medical conditions Citation16.
Family history of affective disorders
A positive family history of depression is sometimes referred as a predictive variable for treatment resistance Citation15. In particular, there are studies showing that a positive family history is associated with early onset of depression and with chronicity, both of which have been linked to TRD Citation1. Moreover, from a clinical perspective, a family history of resistance may suggest a worse prognosis for the individual patient Citation11.
Chronicity of depressive episodes
Index depressive episodes lasting 2 years or more, or double depression (i.e. major depression superimposed upon dysthymic disorder) have been associated with poorer outcome in some but not all studies Citation1, Citation16. Additionally, delay in initiating treatments is also a main predictor of chronicity and nonresponse Citation4, Citation12, Citation17.
Depression subtypes
The recognition of depression subtypes is an important element in the evaluation of TRD because they may respond in somewhat different ways to the available therapies Citation6, Citation13. Important differential diagnoses that may influence treatment response in TRD are the melancholic, psychotic, atypical, seasonal, and bipolar types of depression Citation4, Citation16.
Briefly, melancholic depressions appear to show a greater degree of response to tricyclic antidepressants (TCAs) and to electroconvulsive therapy (ECT) Citation1, Citation5, Citation7, psychotic depressions usually respond better to the addition of an antipsychotic agent to the antidepressant regimen Citation4, Citation18, atypical depressions have been shown to respond better to classical monoamine oxidase inhibitors (MAOIs) than to TCAs or placebo Citation13, Citation17, seasonal depressions appear to have a good response to phototherapy Citation12, Citation16, and bipolar depressions are usually responsive to mood stabilizers (e.g. lithium) combined, if necessary, with antidepressants and/or an antipsychotics Citation2, Citation4. For more detailed information on depression subtypes and their response to treatment see Ayuso‐Gutierrez Citation19.
Psychiatric comorbidity
Depressed patients with comorbid panic attacks or obsessive‐compulsive disorder may present poorer outcomes and often more resistance to treatment Citation4, Citation16, Citation17. In addition, subjects with significant anxiety may be more susceptible to the antidepressants' side effects Citation4. Covert alcohol and substance abuse/dependence may also contribute to the emergence of TRD Citation1, Citation17, and, accordingly, full abstinence should be recommended, as even occasional use may have important clinical effects Citation2, Citation16. Finally, comorbid personality disorders have been associated with poor response to antidepressants in some but not all studies Citation6, Citation7, Citation13, with a negative impact being more clearly demonstrated for cluster C diagnoses (i.e. those disorders mostly characterized by ‘anxious‐fearful’ attributes) Citation3.
Comorbid medical illnesses and depression‐related medications
Organic factors may contribute to affective illness in as much as 50% of patients Citation5. Therefore, in patients with suspected TRD it is critical to rule out the presence of underlying medical disorders, especially from an endocrinologic origin (e.g. hypothyroidism, Cushing's syndrome) Citation1, Citation2, Citation13. Other conditions that should be potentially investigated include neurological disorders (both cortical and subcortical), pancreatic carcinoma, connective tissue disorders, vitamin deficiencies, and certain viral infections Citation4. Additionally, several medications (including immunosuppressants, steroids, and sedatives) may precipitate or contribute to chronic depression and adversely affect the outcome of antidepressant treatment Citation17.
Finally, a diagnosis of secondary depression (e.g. due to a general medical condition) is usually associated with a significant likelihood of chronicity despite adequate interventions Citation12, Citation13, and, therefore, aggressive antidepressant therapy may need to accompany adequate medical management Citation4.
Evaluating the adequacy of antidepressant treatments
There is strong evidence that up to 60% of depressed patients initially classified as suffering from TRD fall into the category of ‘pseudo‐TRD’, as on systematic clinical data gathering it is shown that no objectively adequate treatment course was previously administered Citation4, Citation5, Citation7, Citation10. Additionally, community‐based surveys have revealed that less than 50% of patients actually receive either an adequate dosage or duration of antidepressant treatment Citation5, Citation6, and this figure does not take into account the high noncompliance rate for taking medications as prescribed Citation10.
Therefore, a fundamental task of the clinician is to review the adequacy of treatments received to date Citation7. In particular, at each level of treatment resistance, it is required that an adequate trial of antidepressant be given Citation2. This raises the critical question of how one defines an adequate trial of treatment, and on what basis one may determine when a true level of resistance has been attained.
Adequacy in terms of dosage
Underdosing of antidepressants has historically been known to be one of the main causes of nonresponse to treatment Citation12. This is especially problematic in the light of recent studies showing a ‘therapeutic decrement’, whereby patients who have not responded to one antidepressant have a 20% lower likelihood of responding to the next antidepressant Citation3.
Therefore, current consensus is that the maximum tolerated antidepressant dose should be used, according to specific dosage recommendations Citation6, Citation10. Additionally, it is important to evaluate the concomitant use of metabolic inducers that may be associated with a relative reduction in antidepressant blood levels Citation7, and the possibility that some patients could be rapid/fast metabolizers, and show poorer response to standard doses of antidepressants. In both cases, dosage adjustments may produce a significant treatment improvement Citation20.
For some antidepressants (e.g. imipramine and nortriptyline), drug efficacy may be correlated with the presence of adequate antidepressant levels as measured in the plasma Citation8, Citation20. An useful guide is that nonresponse in the absence of side effects should raise the possibility of a less than adequate antidepressant dosage, and, accordingly, a dose increase could be an effective measure in these cases Citation4, Citation8.
Another important cause of underdosing may be medication intolerance Citation4, as, in clinical practice, patients experiencing difficulty tolerating standard doses of medication may often be wrongly left on subtherapeutic doses. Importantly, many of the side effects eventually abate, and, if not, they may become better tolerated, especially after the onset of improvement Citation11.
Adequacy in terms of duration
Pressures of the clinical setting commonly lead practitioners to change pharmacologic strategies too early. However, shifting regimens and incorrectly drawing the conclusion that a given medication is ineffective may also be considered as a cause of nonresponse and/or ‘pseudo‐TRD’ Citation4, Citation5.
Although most definitions of adequate treatment length are derived from the 6‐week industry‐sponsored drug trials, the optimal duration of antidepressant drug treatment necessary for TRD patients may be considerably longer. Accordingly, it has been suggested that prolonged trials of treatment, lasting more than 10 weeks, may lead to a therapeutic response in certain resistant cases Citation2, Citation11, Citation14. Furthermore, in elderly depressed patients, 12 weeks or more may be necessary for a satisfactory clinical improvement Citation16, Citation17. However, there is currently a lack of compelling evidence to support the advantage of prolonged trials over 6–8 weeks compared to other treatment strategies Citation6, Citation10, Citation14. Of interest, it has been shown that minimal improvement after 4 weeks of antidepressant use predicted poorer outcome at 8 weeks Citation7.
Adequacy in terms of clinical outcome
Most experts nowadays agree that what constitutes inadequate response to treatment of depression is the failure to achieve remission and not the failure to observe response Citation15, Citation21. Briefly, remission refers to a state where the patient is free of depressive symptoms Citation11 (e.g. a score of 7 or less on the 17‐item Hamilton Depression Rating Scale Citation22), while response refers to an improvement of at least 50% from baseline levels. The clinical implication is that only remitted patients improve fully, not only in terms of symptoms, but also in terms of premorbid levels of social and interpersonal functioning Citation23. Therefore, not achieving remission despite adequate treatment usually results in the presence of residual depressive symptoms (e.g. insomnia, fatigue, psychic and somatic anxiety), which have been consistently shown to be associated with poorer outcomes, increased risk of relapse, and impaired social functioning Citation5, Citation16, Citation21, Citation23.
Adequacy in terms of patient compliance
Another important step toward the assessment of resistance in depression concerns the level of drug treatment adherence Citation1, Citation4, as it has been estimated that nonadherence may account for as many as 20% of cases considered to be resistant Citation13. However, the actual prevalence of significant noncompliance may be grossly underestimated and difficult to assess, as direct questioning, blood level monitoring, and pill counting may not accurately reflect adherence in many patient populations Citation4. Thus, a collateral history from past records or the patient's companion may be useful to assist in the evaluation of adherence Citation16.
Managing TRD: current perspectives
The traditional literature on TRD has several limitations that create a profound gap between what is needed and what is available for an evidence‐based practice. Some of these limitations include: 1) the use of response (i.e. partial remission) instead of complete remission as the main outcome of studies; 2) the use of exclusion criteria that reduce the external validity of results (e.g. noninclusion of patients with suicide risk and/or comorbidity with other axis I psychiatric disorders such as anxiety disorders and substance abuse/dependence); 3) the availability of few randomized controlled clinical trials (RCTs); 4) the lack of placebo arms in many of the existent RCTs; 5) the short‐term follow‐up employed (i.e. usually 8 weeks or less); and 6) the presence of possible conflicts of interest (as the majority of current studies are funded by the pharmaceutical industry) Citation10, Citation12, Citation14.
Despite the lack of sound scientific data to support clinical decision, there is a growing consensus that patients who do no present full remission after a first antidepressant trial should be systematically approached and treated Citation20. Classically, four strategies have been employed to enhance treatment efficacy, namely 1) optimization of dose or treatment duration, 2) augmentation with a primarily nonantidepressant drug, 3) combination of antidepressants, and 4) switching to another antidepressant Citation15, Citation24, Citation25.
Optimization
Dose optimization is the simplest and fastest way to deal with insufficient clinical response. However, finding the best antidepressant dose for an individual patient is not always easy, as there is a wide interindividual variation in pharmacokinetics and pharmacodynamics Citation25. Providing enough time for a dose to achieve its maximum effect (i.e. optimizing the duration of treatment) is also a basic strategy that is often neglected Citation20. Importantly, it is well known that some patients will only respond later in the course of a medication trial (i.e. beyond 4 or 6 weeks) Citation26. Nevertheless, in clinical settings there is usually a strong pressure for switching drugs prematurely rather than waiting for a response to eventuate, leading to frequent medication changes prior to completion of an adequate trial Citation20.
Augmentation
Augmentation is the addition of another (nonantidepressant) medication to the current pharmacological regimen with the purpose of enhancing the effects of the antidepressant drug (see Table ) Citation15. Generally, this strategy is used for those patients who initially presented partial response to treatment, as it keeps the improvement already achieved and rapidly adds on additional positive effects Citation27, Citation28.
Table I. Most commonly used augmentation strategies.a
Below we briefly discuss the most commonly used augmentation strategies:
Lithium. Adding lithium to TCAs, monoamine oxidase inhibitors (MAOIs), or selective serotonin reuptake inhibitors (SSRIs) is the most studied augmentation strategy. The response rate of the addition of lithium to a tricyclic is around 50%–60% Citation29. However, the role of lithium augmentation for newer antidepressants (SSRIs or serotonin‐norepinephrine reuptake inhibitors (SNRIs)) remains to be established Citation26.
Thyroid hormone. Triiodothyronine (T3) augmentation is associated with response rates ranging between 25% and 60% Citation30. Although most studies on T3 augmentation to date have involved tricyclics, a recent study in SSRI nonresponders has shown that T3 is probably a better option when compared to lithium considering its side effect profile Citation31.
Buspirone. Positive data on the efficacy of buspirone augmentation is based solely on open studies, as controlled investigations reported mixed results Citation28. In a recent report from the STAR*D study (see below), the augmentation of an SSRI with buspirone was as effective as the combination of an SSRI plus bupropion Citation32.
Pindolol. Although pindolol has been used especially in Europe to accelerate the response to SSRIs, two small open studies did not show benefits in antidepressant‐resistant patients Citation33, Citation34.
Psychostimulants. Several uncontrolled studies in monotherapy nonresponders have shown that when a stimulant was added to the medication regimen (mostly tricyclics) response rates were up to 78% Citation29. Moreover, the combination of psychostimulants with MAOIs seems to be especially effective Citation35. Nonetheless, despite the absence of controlled studies, some experts have described dramatic benefits of stimulants in selected depressed patients Citation36.
Antipsychotics. This class of medication has been combined with tricyclics since the mid‐1970s when investigators observed that patients with delusional depression responded poorly to antidepressant monotherapy Citation37, but had a 92% response rate to a combination of perphenazine and imipramine Citation38. More recently, Ostroff and Nelson Citation39 have suggested that risperidone might be useful as an augmenting agent in patients who have failed SSRIs. Shelton and colleagues Citation40, in a double‐blind study of nonresponders to fluoxetine monotherapy, found greater improvement in those who received fluoxetine plus olanzapine.
Combination
Combining two antidepressants is a frequently used strategy. The rationale behind it is that two drugs with different mechanisms of action may have complementary or synergistic effects when compared to each drug used alone Citation5, Citation24. Traditionally, combination therapy has been used when antidepressant monotherapy fails (see Table ). Nevertheless, there is some evidence from controlled studies suggesting that combination started at the beginning of antidepressant therapy may increase the rates of remission when compared to the usual sequential approach Citation26.
Table II. Most commonly used combination strategies.a
Below we briefly discuss the most commonly used combination strategies:
Tricyclic + MAOI. Open studies and anecdotal reports of this combination are encouraging Citation29, Citation41, Citation42, although to date no controlled studies confirm these findings. Clinicians tend to avoid this cocktail due to the risk of a hypertensive crisis. Thus, although this combination seems to be generally safe if low doses of both drugs are started simultaneously (or if a low dose of a MAOI is added to an ongoing tricyclic trial), clinicians should monitor carefully patients started on it.
SSRI + noradrenergic tricyclic (e.g. nortriptyline). This strategy consists of adding a SSRI to a noradrenergic tricyclic or vice versa Citation28. A prospective study comparing the combination of desipramine and fluoxetine with either drug alone found that the combination was associated with significantly higher remission. Although this study did not specifically address resistant patients, those who failed to respond to a single drug (50% of the sample) showed the same trend of favorable response to the combination Citation28.
Bupropion + SSRI. This is a popular combination, although supported only by uncontrolled studies. The rationale for this combination is the presumed complementary effect of noradrenergic/dopaminergic stimulation by bupropion added to serotoninergic stimulation by the SSRI. The presence of continued anergic symptoms after an adequate trial of an SSRI is thought to predict a response to this strategy Citation43.
∝2‐Antagonist + SSRI. Controlled studies of yohimbine Citation44, mianserin Citation45, and mirtazapine Citation46 have shown beneficial effects from the addition of an ∝2‐antagonist to an SSRI in depressed patients not responding to SSRI monotherapy.
Antidepressants + electroconvulsive therapy (ECT). Clinical experience and the preliminary results from the Consortium for Research in ECT Citation47 suggest that the benefits of ECT are superior to those of drug‐switching or augmentation strategies Citation48. Indeed, a recent comprehensive meta‐analytic study showed that ECT is more effective than drug therapy in depression Citation49. Furthermore, in a short‐term study, Folkerts and colleagues Citation50 found that ECT was markedly superior to paroxetine in patients with resistant depression.
Antidepressants + novel somatic treatments. Repetitive transcranial magnetic stimulation (rTMS), vagus nerve stimulation (VNS), and deep brain stimulation (DBS) are new nonpharmacologic therapies for TRD. Of these modalities, only VNS has been approved by the US Food and Drug Administration (FDA) for the long‐term management of TRD based on open trials Citation51–53 and on a RCT Citation54, although the high costs associated with VNS limit somewhat its clinical utility. Regarding rTMS, study results have been mixed Citation55, although a recent meta‐analysis has shown it to have a clinically significant effect in depression Citation56. Finally, DBS is an experimental approach still in its infancy, and further studies are needed to assess its clinical usefulness for TRD Citation57.
Switching
Generally there are two main reasons for switching an antidepressant: the lack of a satisfactory response after an adequate trial or the presence of significant and intolerable side effects Citation28. Again, there is no consistent data to determine what is the ‘best switch’ (see Table ) Citation26. Nonetheless, although it is commonly held that the switch of antidepressants within the same class (especially in the case of tricyclics) is less effective than the switch of antidepressants to a different class, switching between SSRIs (e.g. fluoxetine, paroxetine, citalopram) appears sometimes to be associated with a favorable clinical response Citation7.
Table III. Most commonly used switching strategies.a
Below we briefly discuss the most commonly used switching strategies:
SSRI to TCA. This switching is based on the idea that a dual action serotoninergic/noradrenergic antidepressant reaches a wider spectrum of neuroreceptors and may improve efficacy. One major limitation of this strategy is the likelihood of increasing side effects. For example, Peselow and collaborators Citation58 showed that 73% of 15 patients responded to imipramine after failing paroxetine. Additionally, Thase and colleagues Citation59 found that 44% of the 117 patients with chronic depression who failed sertraline responded to imipramine. Finally, Nierenberg and collaborators Citation60 showed that of depressed subjects who presented no improvement after monotherapy with various drugs (including SSRIs) approximately 40% responded and 12% remitted to a trial of 6 weeks with nortriptyline.
SSRI to SNRI. The rationale of this strategy is the same as the previous one (see above). Poirier and Boyer Citation61, in a double‐blind randomized study, compared venlafaxine and paroxetine in 122 patients who had failed two antidepressant trials. The venlafaxine and paroxetine groups had 52% and 33% response rates, and 42% and 20% remission rates, respectively. In a multicenter open study, Kaplan Citation62 found that 58% of 152 patients who failed at least one prior antidepressant (not specifically an SSRI) had at least 50% improvement when switched to venlafaxine.
SSRI to bupropion. Although this switching is commonly used there is almost no literature to support it. Clinicians dealing with patients presenting anergic depression and psychomotor retardation frequently use bupropion after a failure of an SSRI based on the idea that noradrenergic/dopaminergic stimulation is effective for these symptoms Citation43. McGrath et al. Citation63, in a small open study (n = 18), showed that 28% of those who failed fluoxetine 40 mg/day showed a 50% improvement in response to bupropion.
SSRI to SSRI. As mentioned above, when switching antidepressants, the usual clinical recommendation is to switch to another class of antidepressant. Nevertheless, there is evidence showing that a switch from one SSRI to another could be useful. In these studies, 42%–63% of patients have presented a response after being switched to another SSRI. Joffe and colleagues Citation64 published the only report that enrolled patients who were resistant to prior SSRI treatment. In this open study, 51% of 55 patients who were resistant to a first SSRI trial had a marked or complete response after 5 weeks on a second SSRI. Recently, as part of the STAR*D study (see below), 727 patients without remission after citalopram were randomized to three alternatives: bupropion, sertraline, or venlafaxine. All treatment options did not differ significantly with respect to outcomes, tolerability, or adverse effects. This result thus questions the usual clinical practice of changing the group of antidepressant when there is a previous failure with an agent from the same family Citation65.
SSRI to mirtazapine. Fava and collaborators Citation66 found that 48% of 69 patients resistant to various SSRIs responded to a switch to mirtazapine.
Switching to MAOIs. The literature concerning switching to MAOIs is relatively old, as their side effects profile have decreased their use. Nevertheless, several case reports and open studies as well as one double‐blind study indicate that MAOIs are effective in about 50% of patients who are resistant to tricyclics Citation67. Until recently, there were no studies using MAOIs in SSRI‐resistant patients. In one of the reports from the STAR*D project (see below), a group of highly depressed patients who had not achieved remission in three previous trials were randomly assigned to receive tranylcypromine or venlafaxine plus mirtazapine Citation68. Briefly, there was no significant statistical difference between the two treatments. However, tranylcypromine was less well tolerated.
Clinical decision‐making based on ‘traditional’ studies: some limitations
The approach based solely on the results provided by ‘traditional’ trials, although useful, does not answer the crucial question for the clinician treating subjects with TRD: what is the next best option for my patient?
The main reason for this is that ‘traditional’ studies are generally limited to case reports, open trials or small comparisons with placebo. Moreover, few of these studies have compared different active strategies, and very few have examined predictors of response to different treatment strategies. In addition, in most studies, the main inclusion criterion is the failure to respond to only one antidepressant trial and, accordingly, samples are composed by patients with milder expressions of ‘difficult‐to‐treat depression’ that differ somewhat from daily practice. Finally, each study has its own inclusion and exclusion criteria, and thus it is sometimes difficult to compare their results.
New perspectives in TRD: the STAR*D project
Recently, an important study in the field of TRD, namely the Sequenced Treatment Alternatives to Relieve Depression (STAR*D), has been completed. The STAR*D was a 7‐year, multimillion dollar project funded by the National Institute of Mental Health (NIMH) which is by far the most complex and largest prospective study examining the next ‘best step’ for patients who do not benefit from initial and subsequent antidepressant treatment Citation69. Overall, more than 4,000 outpatients, aged 18–75 years, have been enrolled in this four‐step treatment alternatives study Citation70.
Below we summarize the main findings of the STAR*D project Citation31, Citation68, Citation70–72:
Level 1. A total of 3,671 patients started with citalopram as the first line of treatment. The remission rate (i.e. a score ⩽7) according to the 17‐item Hamilton Depression Rating Scale (HAM‐D17) was 27.5%. Therefore, these results closely resemble those of the traditional 8‐week efficacy clinical trials.
Level 2. Patients without remission in Level 1 were randomized to receive augmentation with either bupropion (n = 279) or buspirone (n = 286), and presented similar levels of remission (29.7% and 30.1%, respectively) according to the HAM‐D17. Both strategies were well tolerated. In the other arm of Level 2 (i.e. switching) patients who did not remit with citalopram were randomized to three alternatives: bupropion (n = 239), sertraline (n = 238) or venlafaxine (n = 250). Remission rates (as assessed by the HAM‐D17) were 21.3% for sustained‐release bupropion, 17.6% for sertraline, and 24.8% for extended‐release venlafaxine. Treatment options did not differ significantly with respect to outcomes, tolerability or adverse effects. These results, in summary, question the usual practice of changing the class of antidepressants when there is a previous failure with a molecule from the same family (in this case citalopram for sertraline).
Level 3. A total of 142 depressed patients who had not achieved remission with a second‐line switch or augmentation trial (Levels 1 and 2) were randomly assigned to augmentation with lithium (n = 63) or liothyronine (T3) (n = 70). Remission rates were 15.9% for lithium and 24.7% for T3 according to the HAM‐D17, a difference that was not statistically significant. Nonetheless, lithium was more frequently associated with side effects. The main conclusion was that T3 is probably a better option than lithium when one considers effectiveness and side effect profile. In the other arm of Level 3 (i.e. switching), depressed patients were randomly assigned to receive mirtazapine (n = 114) or nortriptyline (n = 121). Remission rates for mirtazapine were 12.3%, and for nortriptyline were 19.8% according to the HAM‐D17, a statistically nonsignificant difference. Thus, this result suggests that switching antidepressants as a third step of treatment is associated with only a modest chance of remission.
Level 4. A total of 109 patients with major depression who had not achieved remission in the three previous levels were randomly assigned to receive tranylcypromine (n = 58) or venlafaxine plus mirtazapine (n = 51). Remission rates for tranylcypromine were 6.9%, and for venlafaxine plus mirtazapine were 13.7% according to the HAM‐D17. Although there was no significant difference in terms of efficacy between these two treatments, tranylcypromine was less well tolerated. Accordingly, highly resistant depressed patients have only modest clinical benefits after four medication switches, and the use of other treatment alternatives (such as electroconvulsive therapy) should be considered for this population.
In conclusion, some findings from the STAR*D project confirm previous notions stemming from the traditional literature on the field. For example, approximately one‐third of patients achieved remission with the first antidepressant trial. Moreover, overall remission rates according to the 16‐item Quick Inventory for Depressive Symptomatology—Self‐Report were 36.8% for step 1, 30.6% for step 2, 13.7% for step 3, and 13% for step 4. Thus, the decrease in remission rates after the second step supports the idea that TRD is best defined by two prior treatment failures Citation73. Also, the STAR*D has shown that relapse rates were higher in patients with response compared to those with remission, therefore supporting the notion that remission should be the main outcome of an antidepressant treatment. STAR*D data also showed that remission is associated with better prognosis even if it is reached after several treatments Citation70. Finally, the STAR*D project reported original data on relapse rates after each treatment step. Briefly, rates were lower in the initial steps for those with remission (step 1 = 33.5% of relapse; step 4 = 50% of relapse), and also for those with partial response (step 1 = 58.6% of relapse; step 4 = 83.3% of relapse) Citation70.
One major limitation of the STAR*D project was the counterpart of its major methodological advance: the randomization procedure through which subjects were able to choose the treatments to which they would like to be randomized. Importantly, severity of symptoms at entry was associated with choice (e.g. patients who chose cognitive psychotherapy or augmentation strategies had milder depressions when compared to those who chose switching to a new medication). The second main limitation was the lack of a placebo (control) arm, as it was not possible to determine whether nonspecific factors accounted for differences found among therapeutic strategies.
For more information on the STAR*D project see especially Rush et al. Citation70, Nierenberg et al. Citation31, McGrath et al. Citation68, Fava et al. Citation72, and Thase et al. Citation74.
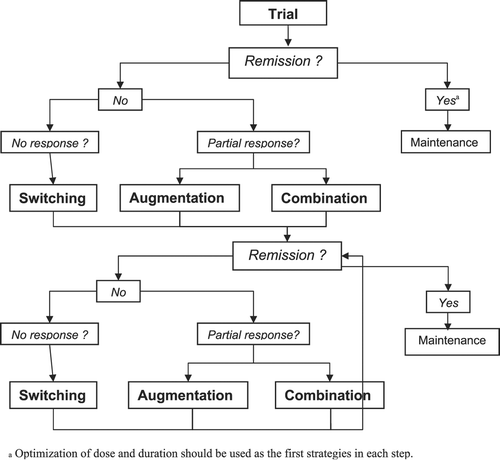
A simplified algorithm for managing TRD
Owing to the current state of knowledge in the field of TRD, it seems more plausible to propose an algorithm of treatment based on the use of particular strategies rather than on specific drugs. Thus, our proposed algorithm (Figure ) was developed based on the Canadian Psychiatric Association's Clinical Guidelines for the Treatment of Depressive Disorders Citation75, but we considered (complete) remission (instead of merely response to treatment) as the gold‐standard outcome of any antidepressant treatment.
Figure 1 Simplified algorithm for the management of TRD(partially based on Kennedy et al. Citation75). TRD = Treatment resistant/refractory major depression.
Conclusions
Despite the numerous options available for the treatment of depression, many patients do not achieve a satisfactory improvement with adequate doses of antidepressants given for sufficient duration, and are eventually classified as presenting with TRD. In general, these patients require major involvement of health care services, and this often leads to lengthy hospitalizations and high human, family, and societal costs.
However, in spite of its significant impact, there is still some debate regarding what is the most suitable definition of TRD, and this is probably one of the main problems affecting its management. Therefore, it is of major concern to reach an agreed‐upon operational definition of TRD. Such a foundation is essential for the interpretation of research findings and their translation to clinical application, as this process is only possible through comparison of results obtained with acceptable consistency and validity.
Regarding the somatic treatment of resistant/refractory depression, the literature has been sparse and based mainly on open trials and anecdotal reports, with few double‐blind randomized trials. Accordingly, clinicians continue to make treatment decisions based on little and inconclusive systematic evidence. The recently published STAR*D project has answered many important questions, but has also left many of them awaiting further clarification.
Undoubtedly, a better understanding of TRD and the many facets of its etiology, as well as the availability of new and effective therapies, will hopefully decrease its morbidity and mortality, and minimize the confusion and ‘therapeutic nihilism’ for both clinicians and patients.
References
- Fava M., Davidson K. G. Definition and epidemiology of treatment‐resistant depression. Psychiatr Clin North Am 1996; 19: 179–200
- O'Reardon J. P., Amsterdam J. D. Overview of treatment‐resistant depression and its management. Treatment‐Resistant Mood Disorders, J. D Amsterdam, M Hornig, A. A Nierenberg. Cambridge University Press, New York 2001; 30–45
- O'Reardon J. P., Brunswick D. J., Amsterdam J. D. Treatment‐resistant depression in the age of serotonin: evolving strategies. Curr Opin Psychiatry 2000; 13: 93–8
- Berman R. M., Narasimhan M., Charney D. S. Treatment‐refractory depression: definitions and characteristics. Depress Anxiety 1997; 5: 154–64
- O'Reardon J. P., Amsterdam J. D. Treatment‐resistant depression: progress and limitations. Psychiatr Ann 1998; 28: 633–40
- Bird D., Haddad P. M., Dursun S. M. An overview of the definition and management of treatment‐resistant depression. Klin Psikofarmakol Bul 2002; 12: 92–101
- Fava M. Diagnosis and definition of treatment‐resistant depression. Biol Psychiatry 2003; 53: 649–59
- Ananth J. Treatment‐resistant depression. Psychother Psychosom 1998; 67: 61–70
- Berlim M. T., Turecki G. What is the meaning of treatment resistant/refractory major depression (TRD)? A systematic review of current randomized trials. Eur Neuropsychopharmacol 2007; 17: 696–707
- Souery D., Lipp O., Massat I., Mendlewicz J. The characterization and definition of treatment‐resistant mood disorders. Treatment‐Resistant Mood Disorders, J. D Amsterdam, M Hornig, A. A Nierenberg. Cambridge University Press, New York 2001; 3–29
- Berlim M. T., Turecki G. Definition, assessment and staging of treatment resistant/refractory major depression: a review of current concepts and methods. Can J Psychiatry 2006; 51: 875–82
- Souery D., Amsterdam J., de Montigny C., Lecrubier Y., Montgomery S., Lipp O., et al. Treatment resistant depression: methodological overview and operational criteria. Eur Neuropsychopharmacol 1999; 9: 83–91
- Fagiolini A., Kupfer D. J. Is treatment‐resistant depression a unique subtype of depression?. Biol Psychiatry 2003; 53: 640–8
- Rush A. J., Thase M. E., Dube S. Research issues in the study of difficult‐to‐treat depression. Biol Psychiatry 2003; 53: 743–53
- Keller M. B. Issues in treatment‐resistant depression. J Clin Psychiatry 2005; 66: 5–12
- Kornstein S. G., Schneider R. K. Clinical features of treatment‐resistant depression. J Clin Psychiatry 2001; 62(Suppl 16)18–25
- Sackeim H. A. The definition and meaning of treatment‐resistant depression. J Clin Psychiatry 2001; 62(Suppl 16)10–7
- Rothschild A. J. Challenges in the treatment of depression with psychotic features. Biol Psychiatry 2003; 53: 680–90
- Ayuso‐Gutierrez J. L. Depressive subtypes and efficacy of antidepressive pharmacotherapy. World J Biol Psychiatry 2005; 6(Suppl 2)31–7
- Fleck M. P., Horwath E. Pharmacologic management of difficult‐to‐treat depression in clinical practice. Psychiatr Serv 2005; 56: 1005–11
- Rush A. J., Kraemer H. C., Sackeim H. A., Fava M., Trivedi M. H., Frank E., et al. Report by the ACNP Task Force on response and remission in major depressive disorder. Neuropsychopharmacology 2006; 31: 1841–53
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23: 56–62
- Nierenberg A. A., DeCecco L. M. Definitions of antidepressant treatment response, remission, nonresponse, partial response, and other relevant outcomes: a focus on treatment‐resistant depression. J Clin Psychiatry 2001; 62(Suppl 16)5–9
- Pridmore S., Turnier‐Shea Y. Medication options in the treatment of treatment‐resistant depression. Aust N Z J Psychiatry 2004; 38: 219–25
- Souery D., Papakostas G. I., Trivedi M. H. Treatment‐resistant depression. J Clin Psychiatry 2006; 67(Suppl 6)16–22
- Fava M., Rush A. J. Current status of augmentation and combination treatments for major depressive disorder: a literature review and a proposal for a novel approach to improve practice. Psychother Psychosom 2006; 75: 139–53
- Nelson J. C. Overcoming treatment resistance in depression. J Clin Psychiatry 1998; 59(Suppl 16)13–9, discussion 40–2
- Nelson J. C. Managing treatment‐resistant major depression. J Clin Psychiatry 2003; 64(Suppl 1)5–12
- Nierenberg A. A., Katz J., Fava M. A critical overview of the pharmacologic management of treatment‐resistant depression. Psychiatr Clin North Am 2007; 30: 13–29
- Thase M., Rush A. J. Treatment‐resistant depression. Psychopharmacology: The Fourth Generation of Progress, 4th ed, F. E Bloom, D. J Kupfer. Raven Press, New York 1995
- Nierenberg A. A., Fava M., Trivedi M. H., Wisniewski S. R., Thase M. E., McGrath P. J., et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry 2006; 163: 1519–30, quiz 1665
- Trivedi M. H., Fava M., Wisniewski S. R., Thase M. E., Quitkin F., Warden D., et al. Medication augmentation after the failure of SSRIs for depression. N Engl J Med 2006; 354: 1243–52
- Moreno F. A., Gelenberg A. J., Bachar K., Delgado P. L. Pindolol augmentation of treatment‐resistant depressed patients. J Clin Psychiatry 1997; 58: 437–9
- Perez V., Soler J., Puigdemont D., Alvarez E., Artigas F. A double‐blind, randomized, placebo‐controlled trial of pindolol augmentation in depressive patients resistant to serotonin reuptake inhibitors. Grup de Recerca en Trastorns Afectius. Arch Gen Psychiatry 1999; 56: 375–9
- Fawcett J., Kravitz H. M., Zajecka J. M., Schaff M. R. CNS stimulant potentiation of monoamine oxidase inhibitors in treatment‐refractory depression. J Clin Psychopharmacol 1991; 11: 127–32
- Price L., Carpenter L., Rasmussen S. Drug combining strategies. Treatment‐Resistant Mood Disorders, J Amsterdam, M Hornig, A Nierenberg. Cambridge University Press, Cambridge 2001
- Glassman A. H., Kantor S. J., Shostak M. Depression, Delusions, and Drug Response. Am J Psychiatry 1975; 132: 716–9
- Nelson J. C., Bowers M. B Jr. Delusional unipolar depression: description and drug response. Arch Gen Psychiatry 1978; 35: 1321–8
- Ostroff R. B., Nelson J. C. Risperidone augmentation of selective serotonin reuptake inhibitors in major depression. J Clin Psychiatry 1999; 60: 256–9
- Shelton R. C., Tollefson G. D., Tohen M., Stahl S., Gannon K. S., Jacobs T. G., et al. A novel augmentation strategy for treating resistant major depression. Am J Psychiatry 2001; 158: 131–4
- Razani J., White K. L., White J., Simpson G., Sloane R. B., Rebal R., et al. The safety and efficacy of combined amitriptyline and tranylcypromine antidepressant treatment. A controlled trial. Arch Gen Psychiatry 1983; 40: 657–61
- White K., Simpson G. Combined MAOI‐tricyclic antidepressant treatment: a reevaluation. J Clin Psychopharmacol 1981; 1: 264–82
- Shelton R. C., Tomarken A. J. Can recovery from depression be achieved?. Psychiatr Serv 2001; 52: 1469–78
- Cappiello A., McDougle C. J., Malison R. T., Heninger G. R., Price L. H. Yohimbine augmentation of fluvoxamine in refractory depression: a single‐blind study. Biol Psychiatry 1995; 38: 765–7
- Dam J., Ryde L., Svejso J., Lauge N., Lauritsen B., Bech P. Morning fluoxetine plus evening mianserin versus morning fluoxetine plus evening placebo in the acute treatment of major depression. Pharmacopsychiatry 1998; 31: 48–54
- Carpenter L. L., Yasmin S., Price L. H. A double‐blind, placebo‐controlled study of antidepressant augmentation with mirtazapine. Biol Psychiatry 2002; 51: 183–8
- Petrides G., Fink M., Husain M. M., Knapp R. G., Rush A. J., Mueller M., et al. ECT remission rates in psychotic versus nonpsychotic depressed patients: a report from CORE. J ECT 2001; 17: 244–53
- Pearlman C. Electroconvulsive therapy in clinical psychopharmacology. J Clin Psychopharmacol 2002; 22: 345–6
- UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta‐analysis. Lancet 2003; 361: 799–808
- Folkerts H. W., Michael N., Tolle R., Schonauer K., Mucke S., Schulze‐Monking H. Electroconvulsive therapy vs. paroxetine in treatment‐resistant depression—a randomized study. Acta Psychiatr Scand 1997; 96: 334–42
- Rush A. J., Sackeim H. A., Marangell L. B., George M. S., Brannan S. K., Davis S. M., et al. Effects of 12 months of vagus nerve stimulation in treatment‐resistant depression: a naturalistic study. Biol Psychiatry 2005; 58: 355–63
- Sackeim H. A., Rush A. J., George M. S., Marangell L. B., Husain M. M., Nahas Z., et al. Vagus nerve stimulation (VNS) for treatment‐resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacology 2001; 25: 713–28
- Rush A. J., George M. S., Sackeim H. A., Marangell L. B., Husain M. M., Giller C., et al. Vagus nerve stimulation (VNS) for treatment‐resistant depressions: a multicenter study. Biol Psychiatry 2000; 47: 276–86
- Rush A. J., Marangell L. B., Sackeim H. A., George M. S., Brannan S. K., Davis S. M., et al. Vagus nerve stimulation for treatment‐resistant depression: a randomized, controlled acute phase trial. Biol Psychiatry 2005; 58: 347–54
- Loo C. K., Mitchell P. B. A review of the efficacy of transcranial magnetic stimulation (TMS) treatment for depression, and current and future strategies to optimize efficacy. J Affect Disord 2005; 88: 255–67
- Herrmann L. L., Ebmeier K. P. Factors modifying the efficacy of transcranial magnetic stimulation in the treatment of depression: a review. J Clin Psychiatry 2006; 67: 1870–6
- Mayberg H. S., Lozano A. M., Voon V., McNeely H. E., Seminowicz D., Hamani C., et al. Deep brain stimulation for treatment‐resistant depression. Neuron 2005; 45: 651–60
- Peselow E. D., Filippi A. M., Goodnick P., Barouche F., Fieve R. R. The short‐ and long‐term efficacy of paroxetine HCl: A. Data from a 6‐week double‐blind parallel design trial vs. imipramine and placebo. Psychopharmacol Bull 1989; 25: 267–71
- Thase M. E., Rush A. J., Howland R. H., Kornstein S. G., Kocsis J. H., Gelenberg A. J., et al. Double‐blind switch study of imipramine or sertraline treatment of antidepressant‐resistant chronic depression. Arch Gen Psychiatry 2002; 59: 233–9
- Nierenberg A. A., Papakostas G. I., Petersen T., Kelly K. E., Iacoviello B. M., Worthington J. J., et al. Nortriptyline for treatment‐resistant depression. J Clin Psychiatry 2003; 64: 35–9
- Poirier M. F., Boyer P. Venlafaxine and paroxetine in treatment‐resistant depression. Double‐blind, randomised comparison. Br J Psychiatry 1999; 175: 12–6
- Kaplan E. M. Efficacy of venlafaxine in patients with major depressive disorder who have unsustained or no response to selective serotonin reuptake inhibitors: an open‐label, uncontrolled study. Clin Ther 2002; 24: 1194–200
- McGrath P., Fava M., Stewart J. Bupropion for SSRI‐resistant depression. 2000, In: 39th Annual Meeting of the American College of Neuropsychopharmacology; 2000; San Juan, Puerto Rico
- Joffe R. T., Levitt A. J., Sokolov S. T., Young L. T. Response to an open trial of a second SSRI in major depression. J Clin Psychiatry 1996; 57: 114–5
- Rush A. J., Trivedi M. H., Wisniewski S. R., Stewart J. W., Nierenberg A. A., Thase M. E., et al. Bupropion‐SR, sertraline, or venlafaxine‐XR after failure of SSRIs for depression. N Engl J Med 2006; 354: 1231–42
- Fava M., Dunner D. L., Greist J. H., Preskorn S. H., Trivedi M. H., Zajecka J., et al. Efficacy and safety of mirtazapine in major depressive disorder patients after SSRI treatment failure: an open‐label trial. J Clin Psychiatry 2001; 62: 413–20
- Nolen W., Hoencamp E., Haffmans P. Classical and selective monoamine oxidase inhibitors in refractory major depression. Refractory Depression—Current Strategies and Future Directions, W Nolen, J Zohar, S Roose. John Wiley & Sons, New York 1994
- McGrath P. J., Stewart J. W., Fava M., Trivedi M. H., Wisniewski S. R., Nierenberg A. A., et al. Tranylcypromine versus venlafaxine plus mirtazapine following three failed antidepressant medication trials for depression: a STAR*D report. Am J Psychiatry 2006; 163: 1531–41, quiz 1666
- Rush A. J., Fava M., Wisniewski S. R., Lavori P. W., Trivedi M. H., Sackeim H. A., et al. Sequenced treatment alternatives to relieve depression (STAR*D): rationale and design. Control Clin Trials 2004; 25: 119–42
- Rush A. J., Trivedi M. H., Wisniewski S. R., Nierenberg A. A., Stewart J. W., Warden D., et al. Acute and Longer‐Term Outcomes in Depressed Outpatients Requiring One or Several Treatment Steps: A STAR*D Report. Am J Psychiatry 2006; 163: 1905–17
- Trivedi M. H., Rush A. J., Wisniewski S. R., Nierenberg A. A., Warden D., Ritz L., et al. Evaluation of outcomes with citalopram for depression using measurement‐based care in STAR*D: implications for clinical practice. Am J Psychiatry 2006; 163: 28–40
- Fava M., Rush A. J., Wisniewski S. R., Nierenberg A. A., Alpert J. E., McGrath P. J., et al. A comparison of mirtazapine and nortriptyline following two consecutive failed medication treatments for depressed outpatients: a STAR*D report. Am J Psychiatry 2006; 163: 1161–72
- Nelson J. C. The STAR*D Study: A Four‐Course Meal That Leaves Us Wanting More. Am J Psychiatry 2006; 163: 1864–6
- Thase M. E., Friedman E. S., Biggs M. M., Wisniewski S. R., Trivedi M. H., Luther J. F., et al. Cognitive therapy versus medication in augmentation and switch strategies as second‐step treatments: a STAR*D report. Am J Psychiatry 2007; 164: 739–52
- Kennedy S. H., Lam R. W., Cohen N. L., Ravindran A. V. Clinical guidelines for the treatment of depressive disorders. IV. Medications and other biological treatments. Can J Psychiatry 2001; 46(Suppl 1)38S–58S