Abstract
Background. Abnormal angiogenesis is a pathophysiological component of cardiovascular disease (CVD), where circulating biomarkers of angiogenesis are associated with increased CVD risk in hypertension. We hypothesized that raised levels of angiopoietin (Ang)‐1 and ‐2 would predict events in patients with hypertension treated for CVD.
Methods. We measured angiopoietin levels by enzyme‐linked immunosorbent assay (ELISA) in 251 hypertensive participants (85% male; mean age 63.5 years; 192 free of previous CVD events). Plasma angiopoietin levels were related to the subsequent CVD events over a mean follow‐up period of 57.1 (SD 11) months.
Results. There were 11 cases of myocardial infarction (MI) and 18 cases of stroke during follow‐up. Ang‐2 was a significant predictor of MI, stroke, and composite CVD events, with the greatest event‐free survival amongst those in the lower tertile (all P<0.05). Ang‐1 was not predictive of CVD outcomes. Of CVD risk factors at recruitment (blood pressure, body mass index, plasma glucose, serum and high‐density lipoprotein (HDL)‐cholesterol), Ang‐2 was the only discriminator of incident MI (area under curve (AUC) = 73%, P = 0.013), where a value >4.3 ng/mL optimized specificity and sensitivity. On Cox regression analysis (CVD treatments and risk factors), raised Ang‐2 was an independent predictor of MI, P<0.05, but not stroke or composite outcomes.
Conclusions. Among patients with hypertension, raised levels of Ang‐2 were predictive of MI, and further study is warranted to evaluate the use of this biomarker in CVD management, risk stratification, and prevention.
Introduction
Cardiovascular disease (CVD) is the leading cause of mortality in developed countries and is rapidly achieving epidemic proportions in the developing world Citation1. As such, CVD continues to be a public health priority in both primary and secondary health care. While clinical assessment (focused history, physical examination, and imaging) is the corner‐stone of CVD management, such evaluations have limitations Citation2, Citation3. Moreover, despite advances in the understanding of CVD and its treatment, the precise mechanisms by which its determinants (such as hypertension, diabetes mellitus, or dyslipidaemia) act are not fully understood. For example, even with effective management of blood pressure, antihypertensive medication does not reduce CVD risk back to the levels found in the normotensive population Citation4, Citation5, and our predictions of macrovascular events in diabetes remain underestimated Citation6. Hence, in recent years there has been a surge in the evaluation of the prognostic value of circulating biological markers or ‘biomarkers’—measurable and quantifiable biological parameters that can aid a clinician in CVD disease management, risk stratification, and prevention Citation7.
The process of angiogenesis—the stimulation of the endothelium to form new vessels—appears to be an integral component in the pathophysiology of CVD Citation8–10, with evidence to support the view that atherosclerosis presents a localized area that is rich with inflammatory infiltrates that can activate angiogenesis Citation11. Levels of circulating angiogenic growth factors from the angiopoietin (Ang) family are specific for the endothelium, have been shown to be raised and associated with CVD risk in patients with hypertension Citation12–14, diabetes mellitus Citation15, heart failure Citation16, and acute coronary syndromes Citation17. Physiologically, Ang promote endothelial cell proliferation; specifically, Ang‐1 accelerates the maturation of the blood vessel, while Ang‐2, a natural antagonist of Ang‐1 for a common receptor (endothelial cell‐specific tyrosine kinase receptor Tie‐2), destabilizes the vessel and degrades the basal lamina Citation10, Citation18.
We hypothesized that plasma levels of Ang‐1, Ang‐2, and the soluble Tie‐2 receptor could have prognostic implications amongst patients recognized to be at an increased CVD risk. To test this hypothesis, we analysed the validity and association of these angiogenic biomarkers with the prediction of subsequent myocardial infarction (MI) stroke, vascular events, and death in 251 participants of the Anglo‐Scandinavian Cardiac Outcomes Trial (ASCOT) study, who were attending the Birmingham study centre.
Key messages
Abnormal angiogenesis is a pathophysiological component of cardiovascular disease (CVD).
Circulating biomarkers of angiogenesis are associated with increased CVD risk in hypertension.
In this study, among patients with hypertension, we found that raised levels of angiopoietin‐2 were independently predictive of myocardial infarction.
Further study is warranted to evaluate the use of angiopoietin‐2 as a biomarker in CVD management, risk stratification, and prevention.
Methods
All patients were participants in ASCOT at our centre (City Hospital, Birmingham, UK). Patients were aged 40–80 years with hypertension, either treated (systolic blood pressure (BP) >140 mmHg and/or diastolic BP >90 mmHg) or newly diagnosed (systolic BP >160 mmHg and/or diastolic BP >100 mmHg) subjects. Details of ASCOT are published elsewhere Citation19. Briefly, the inclusion criteria required hypertensive patients with three or more risk factors to be included, that is: 1) left ventricular hypertrophy, 2) other electrocardiogram (ECG) abnormalities (left ventricle strain pattern, abnormal Q waves, left bundle branch block, ST‐T changes compatible with ischaemic heart disease), 3) history of diabetes mellitus (DM) according to World Health Organization criteria, 4) past medical history of a cerebrovascular event, including transient ischaemic attack, 5) male gender, 6) age >55 years, 7) microalbuminuria/proteinuria, 8) positive smoking history, 9) raised plasma total cholesterol/high‐density lipoprotein (HDL) cholesterol ratio, 10) family history of cardiovascular disease, and 11) peripheral vascular disease. Patients were excluded if they had a history of malignant or secondary hypertension, congestive cardiac failure, fasting serum triglycerides >4.5 mmol/L, major concomitant, non‐cardiovascular disease or were taking warfarin. All patients were receiving antihypertensive therapy at the time of recruitment.
Volunteers were interviewed by a specialist nurse, who took a detailed medical history, and an ECG was performed. Blood pressure was measured after the patient was seated in a quiet room for 10 minutes, using the OMRON 705‐CP (Omron Healthcare Europe, Mannheim, Germany), as per the ASCOT protocol; a minimum of three readings were performed and the average of the last two readings was used. All patients were optimally treated for hypertension and cardiovascular risk (diabetes where appropriate) by the study physician and given life‐style advice regarding diet, smoking cessation (strongly encouraged), alcohol and exercise in both verbal and written forms by a trained specialist nurse. Every participant provided written or witnessed consent to be involved within this biomarker substudy of ASCOT, which had local research ethics committee approval.
Laboratory
Blood was drawn after an 8‐hour fasting period with minimal trauma from the antecubital vein into citrated blood bottles. Blood was centrifuged at 3000 rpm for 20 minutes within 30 minutes of collection, and the citrated plasma separated and stored at −80°C prior to analysis. Ang‐1, Ang‐2, and Tie‐2 were measured in plasma by enzyme‐linked immunosorbent assay using commercially available antibodies (R&D systems, Abingdon, U.K.), by methods previously described Citation14, Citation16, where inter‐ and intra‐assay variation for all assays were 10% and 5% respectively.
Data analysis
The study was of a prospective design, with no provision for disease stratification (e.g. known diabetes). On case note analysis, details of the following end‐points (and dates of occurrence) were included: 1) angina, 2) myocardial infarction, 3) non‐haemorrhagic stroke, 4) coronary intervention, 5) peripheral vascular disease, and 6) death. A composite vascular end‐point that included all of these listed was also generated. Data were analysed in SPSS v14 (SPSS Inc., Chicago, IL) using standard and non‐parametric tests as appropriate. Angiogenic biomarkers were of non‐parametric distribution, as determined by normality plots (Kolmorov‐Smirnov test). Differences in cardiovascular risk between base‐line and follow‐up (continuous variables) were determined using the paired t‐test (parametric data) and a non‐parametric equivalent (Wilcoxon) as appropriate. For non‐parametric data, central tendencies were reported as medians and variation by interquartile range (IQR). Similarly, for normally distributed variables, the mean and standard deviation (SD) are reported. The Spearman rank correlation method was used to determine statistical correlations between angiogenic factors with cardiovascular risk. Multivariate logistic regression was used to determine the contribution of various risk factors to the onset of diabetes, using a stepwise approach.
Kaplan‐Meier curves were used to estimate time‐to‐event models for MI, stroke, and composite vascular events (MI (myocardial infarction), angina, peripheral vascular disease, stroke/TIA (transient ischaemic attack), revascularization, or death). Receiver operator characteristic (ROC) curves were used to evaluate the performance of angiogenic indices with conventional CVD risk factors, depicted by the mean area under the curve (AUC) with 95% confidence intervals (CI). Angiogenic indices and conventional CVD risk factors and medication were entered into a Cox regression model to calculate the odds ratio (95% CI) from independent predictors of vascular events. A P‐value<0.05 was considered as statistically significant.
Results
In total, 251 patients with hypertension were recruited for this substudy. Comprehensive assessments of cardiovascular risk factors and vascular outcomes were available on 243 individuals. The mean follow‐up period in these patients was 57.1 (SD 11) months. Base‐line demographic data amongst these patients at base‐line are shown in , and data in relation to changes in cardiovascular risk factors between base‐line and follow‐up are shown in .
Table I. Base‐line characteristics and plasma indices of angiogenesis in 251 patients with hypertension enrolled in ASCOT. Values are n (%), mean (SD), or *median (IQR = interquartile range).
Table II. Base‐line and 6‐year follow‐up of hypertensive patients: indices of risk management. Data are given as mean (SD).
In total, 192 hypertensive patients had no history of overt cardiovascular events. All patients were optimally managed for cardiovascular risk during their 5‐year participation, where the 95% confidence intervals of the change in blood pressure were at least 13% lower, and serum cholesterol 21% lower on follow‐up (all P<0.001). Levels of serum triglycerides and HDL cholesterol also improved significantly (P<0.001), but levels of fasting plasma glucose and body mass index (BMI) were no different. The percentage of patients with diabetes increased from 25% to 29.5% (11 new cases) during this period. Measures of renal function all significantly deteriorated during the follow‐up period, and the percentage of patients with a glomerular filtration rate (GFR) <60 increased from 21.9% to 39.8% (45 new cases).
Relationship to changes in cardiovascular risk and diabetes
On bivariate analysis, changes in cardiovascular risk factors from were unrelated to base‐line indices of angiogenesis, except Ang‐1, which was positively associated with the change in fasting plasma glucose (r = 0.16, P = 0.04). On logistic regression analysis, the onset of diabetes in non‐diabetic patients was independently associated with base‐line serum triglycerides (P = 0.031), and non‐significantly with Ang‐1 (P = 0.05), in a model that included age, gender, body mass index, systolic blood pressure, and serum lipids.
Survival analysis and receiver operating characteristics (ROC)
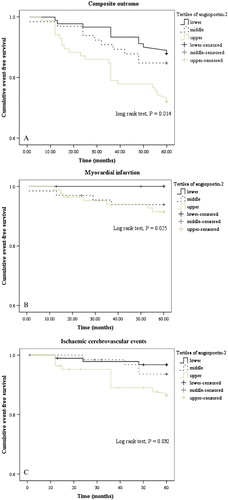
Of the 251 patients, 45 (18%) developed events: there were 11 cases of MI (9.5 per 1000 patient years), 18 cases of stroke/TIA (15.7 per 1000 patient years), and a total of 11 deaths (9.3 per 1000 patient years). On univariate survival analysis, Ang‐2 was a significant predictor of MI (log rank test: P = 0.025), stroke/TIA (P = 0.032), and composite CVD events (P = 0.014), with the greatest survival amongst those in the lowest tertile (see ). On similar analysis, Ang‐1 and levels of the Tie‐2 receptor were not predictive of cardiovascular outcomes.
Figure 1 Kaplan‐Meier cumulative event‐free survival charts between tertiles of plasma angiopoietin‐2 levels, in relation to cardiovascular outcomes. A: Composite cardiovascular outcomes; B: myocardial infarction; C: ischaemic cerebrovascular events.
ROC analysis of cardiovascular risk factors and angiogenic indices at base‐line amongst the 192 patients without a previous history of CVD events as discriminators of incident MI (8 cases), stroke/TIA (13 cases), and composite vascular outcomes (34 cases) are shown in . Base‐line Ang‐2 was the only variable with the ability to discriminate between those with and without new MI (P = 0.028), and BMI was the only discriminator of incident stroke (P = 0.011). With respect to incident MI, the threshold with the greatest arithmetic sum of sensitivity and specificity for Ang‐2 was 4.3 ng/mL.
Table III. Area under the curve (AUC) analysis of angiopoietin‐2 and cardiovascular risk factors with incident vascular outcomes in 192 hypertension patients free of cardiovascular events at base‐line.
On multivariate survival analysis, diabetes, cholesterol‐to‐HDL ratio, and the presence of Ang‐2 >4.3 ng/mL (but not plasma levels) were all independent predictors of MI (P<0.05), and details of the output from the Cox regression model are shown in . On similar analysis, Ang‐2 did not feature in models of stroke/TIA or composite vascular outcomes. For stroke, BMI (P = 0.009) and smoking history (P = 0.037) were negatively‐, and statin therapy (P = 0.016), angiotensin‐converting enzyme (ACE) inhibitor therapy (P = 0.018), and beta‐blocker therapy (P = 0.035) positively associated with event‐free survival. With respect to composite vascular outcomes, diabetes mellitus (P = 0.003), age (P = 0.029), and smoking history (P = 0.04) were negatively associated with event‐free survival.
Table IV. Cox regression analysis of cardiovascular risk factors and raised plasma angiopoietin‐2 (>4.3 pg/mL) at base‐line with incident MI in hypertension patients.
Discussion
We have shown for the first time that plasma levels of Ang‐2, but not Ang‐1 or the soluble Tie‐2 receptor, were consistently associated with CVD outcomes during a 5‐year follow‐up period amongst 251 patients with hypertension in the ASCOT study. As a biomarker, the measurement of Ang‐2 has reasonable specificity and sensitivity at least in relation to MI, and the implication is that raised levels were an independent predictor of event‐free survival in patients who were otherwise optimally managed for CVD.
We have previously shown that Ang‐2 is raised amongst those at an increased risk of MI Citation11, Citation12, Citation14, and our data here support its potential as an independent marker of subclinical atherosclerosis. With respect to the utility of this biomarker, levels of Ang‐2 appear highest following acute events Citation16, Citation17, and may interpret the extent of damage to the myocardium and the risk of future recurrences of ischaemic events. Amongst asymptomatic individuals it is associated with low‐level inflammation Citation11 can be used in a screening capacity—reflecting the severity of underlying coronary disease. In relation to a therapeutic response, we have shown that Ang‐2 remains unchanged amongst patients with overt CVD intensively managed for risk factors over 1 year Citation15.
Diabetes is well known to increase the risk of coronary heart disease (CHD) Citation20, but the insulin‐glucose axis (which defines this disorder), is not a pre‐requisite for the increased CVD risk. Rather, a collective of CHD risk factors, which are concurrently manifest with this diabetes are used in risk estimation Citation21. In the present study, known diabetes was common amongst patients who developed subsequent MI (7 out of 11 cases) and stroke (5 out of 18 cases), but levels of Ang‐2 were unrelated the presence or onset of diabetes amongst hypertensive patients. Serum triglycerides were predictive of diabetes, but were unrelated to CVD outcomes in this analysis. Hence, while Ang‐2 has been reported to be raised in diabetes Citation22, it may be specific for macrovascular risk and complications in these patients. On separate analysis of patients (excluding those who presented with diabetes during the study), there were no differences in the independent association of Ang‐2 with MI (data not shown).
Of note, levels of Ang‐2 were associated with stroke outcomes in this study, and the threshold for events appeared somewhat lower than that for MI. However, Ang‐2 was not an independent predictor for stroke or composite outcomes. As this study was a pragmatic approach to determine the prognostic utility of Ang‐2 measurement, the discrepancies in its association with particular cardiovascular events would suggest that differences in the pathogenesis of stroke and coronary heart disease have implications on circulating levels of this marker. It is likely that the localized area of inflammation associated with atherosclerosis within the coronary circulation has a greater impact on venous Ang‐2 than that associated with the circulation of the brain. Moreover, Ang‐2 levels that increase with small vessel disease are marginalized by other physiological factors.
Ang‐2 and Ang‐1 are ligands with opposing effects on the Tie‐2 receptor Citation23, where the former is a functional antagonist of signal transduction between the latter. Given their ‘interplay’, one may have expected circulating concentrations of Tie‐2 and Ang‐1 to have shared the prognostic utility of Ang‐2 levels. However, there are distinct biological functions of Ang‐2 that may explain the finding that Ang‐1 and the Tie‐2 receptor were unrelated to CVD outcomes in this study. For example, Ang‐2 acts in an autocrine manner—a stored, rapidly available molecule in endothelial cells, which suggests that its biological function is complex, extending its potential beyond the paracrine Ang‐1/Tie‐2‐angiogenesis system Citation24. Moreover, Ang‐2 within endothelial cells is stored within Weibel‐Palade bodies, co‐localizing with inflammatory and pro‐coagulatory factors, such as von Willebrand factor and P‐selectin Citation24. Interestingly, current evidence suggests that circulating levels of these biomarkers have a utility in cardiovascular risk assessment Citation25, Citation26 and here we propose a vascular haemostatic role for Ang‐2.
The limitations of this study are the relatively small size of the cohort and number of events, which are likely to have confounded our assessment of the specificity and sensitivity of the ROC analysis of MI. A larger study specifically stratified for existing overt CVD and gender are needed. CVD progresses with the manifestation of risk factors such as hypertension, which contribute to the development of subclinical atherosclerosis, culminating in events such as MI and stroke that portend premature mortality. In addition, this was an ancillary study to the main ASCOT trial, where the investigations and treatment regimes allowed were very much protocol‐driven and secondary to the main ASCOT trial. The patients in the present analysis were consecutive patients over the initial period of the trial recruitment at the Birmingham study site, and demographic and clinical differences from the main trial exist. All patients also completed a ‘package of care’ of general cardiovascular risk management—which includes education, life‐style advice, dietary advice, and statin use—reflecting contemporary management of hypertension, and it would be impossible to dissect out the individual contributions of each component.
Conclusion
While Ang‐2 levels are already known to identify CVD trait and state Citation10–17, we now report that raised Ang‐2 has prognostic implications amongst patients managed for CVD—an additional tool for risk stratification. Our data presented here add to the limited longitudinal information of the Ang/Tie‐2 system in CVD, but are consistent with those published from oncology studies, where Ang‐2 is a marker of a poor prognosis in breast cancer Citation27 and non‐small cell lung cancer Citation28.
Acknowledgements
We acknowledge the support of the Sandwell and West Birmingham Hospitals NHS Trust Research and Development programme for the Haemostasis Thrombosis and Vascular Biology Unit. We thank Professor DG Beevers, Dr C Spencer, Dr S Nadar and Dr D Felmeden for help with data collection. Other ASCOT Investigators are listed in reference Citation19.
References
- Yusuf S., Reddy S., Ounpuu S., Anand S. Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation 2001; 104: 2746–53
- Kita Chun A., McGee S. R. Bedside diagnosis of coronary artery disease: a systematic review. Am J Med 2004; 117: 334–43
- Pope J. H., Aufderheide T. P., Ruthazer R., Woolard R. H., Feldman J. A., Beshansky J. R., et al. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med 2000; 342: 1163–70
- MacMahon S., Peto R., Cutler J., Collins R., Sorlie P., Neaton J., et al. Blood pressure, stroke, and coronary heart disease. Part 1, Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet 1990; 335: 765–74
- Collins R., Peto R., MacMahon S., Hebert P., Fiebach N. H., Eberlein K. A., et al. Blood pressure, stroke, and coronary heart disease. Part 2, Short‐term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet 1990; 335: 827–38
- McEwan P., Williams J. E., Griffiths J. D., Bagust A., Peters J. R., Hopkinson P., et al. Evaluating the performance of the Framingham risk equations in a population with diabetes. Diabet Med 2004; 21: 318–23
- Vasan R. S. Biomarkers of Cardiovascular Disease: molecular basis and practical considerations. Circulation 2006; 11: 2335–62
- Moulton K. S. Plaque angiogenesis and atherosclerosis. Curr Atheroscler Rep 2001; 3: 225–33
- Le Noble F. A. C., Stassen F. R. M., Hacking W. J. G., Struijker Boudier H. A. J. Angiogenesis and hypertension. J Hypertens 1998; 16: 1563–72
- Felmeden D. C., Blann A. D., Lip G. Y. H. Angiogenesis: basic pathophysiology and implications for disease. Eur Heart J 2003; 24: 586–603
- Patel J. V., Lim H. S., Nadar S., Tayebjee M., Hughes E. A., Lip G. Y. Abnormal soluble CD40 ligand and C‐reactive protein concentrations in hypertension: relationship to indices of angiogenesis. J Hypertens 2006; 24: 117–21
- Felmeden D. C., Spencer C. G., Belgore F. M., Blann A. D., Beevers D. G., Lip G. Y. Endothelial damage and angiogenesis in hypertensive patients: relationship to cardiovascular risk factors and risk factor management. Am J Hypertens 2003; 16: 11–20
- Felmeden D. C., Spencer C. G. C., Chung N., Belgore F. M., Blann A. D., Beevers D. G., et al. Relation of thrombogenesis in systemic hypertension to angiogenesis and endothelial damage/dysfunction (a substudy of the Anglo‐Scandinavian Cardiac Outcomes Trial [ASCOT]). Am J Cardiol 2003; 92: 400–5
- Nadar S. K., Blann A., Beevers D. G., Lip G. Y. Abnormal angiopoietins 1&2, angiopoietin receptor Tie‐2 and vascular endothelial growth factor levels in hypertension: relationship to target organ damage [a sub‐study of the Anglo‐Scandinavian Cardiac Outcomes Trial (ASCOT)]. J Intern Med 2005; 258: 336–43
- Lim H. S., Blann A. D., Chong A. Y., Freestone B., Lip G. Y. Plasma vascular endothelial growth factor, angiopoietin‐1, and angiopoietin‐2 in diabetes: implications for cardiovascular risk and effects of multifactorial intervention. Diabetes Care 2004; 27: 2918–24
- Chong A. Y., Caine G. J., Freestone B., Blann A. D., Lip G. Y. Plasma angiopoietin‐1, angiopoietin‐2, and angiopoietin receptor tie‐2 levels in congestive heart failure. J Am Coll Cardiol 2004; 43: 423–8
- Lee K. W., Lip G. Y., Blann A. D. Plasma angiopoietin‐1, angiopoietin‐2, angiopoietin receptor tie‐2, and vascular endothelial growth factor levels in acute coronary syndromes. Circulation 2004; 110: 2355–60
- Stetler‐Stevenson W. G. Matrix metalloproteinases in angiogenesis: moving target for therapeutic intervention. J Clin Invest 1999; 103: 1237–41
- Sever P. S., Dahlof B., Poulter N. R., Wedel H., Beevers G., Caulfield M., et al. ASCOT Steering Committee, Anglo‐Scandinavian Cardiac Outcomes Trial. Anglo‐Scandinavian Cardiac Outcomes Trial: a brief history, rationale and outline protocol. J Hum Hypertens 2001; 15(Suppl 1)S11–2
- Kannel W. B., McGee D. L. Diabetes and cardiovascular disease: the Framingham study. JAMA 1979; 241: 2035–8
- Hanley A. J., Wagenknecht L. E., D'Agostino R. B., Zinman B., Haffner S. M. Identification of subjects with insulin resistance and beta‐cell dysfunction using alternative definitions of the metabolic syndrome. Diabetes 2003; 52: 2740–7
- Lip P. L., Chatterjee S., Caine G. J., Hope‐Ross M., Gibson J., Blann A. D., et al. Plasma vascular endothelial growth factor, angiopoietin‐2, and soluble angiopoietin receptor tie‐2 in diabetic retinopathy: effects of laser photocoagulation and angiotensin receptor blockade. Br J Ophthalmol 2004; 88: 1543–6
- Jones N., Iljin K., Dumont D. J., Alitalo K. Tie receptors: new modulators of angiogenic and lymphangiogenic responses. Nat Rev Mol Cell Biol 2001; 2: 257–67
- Fiedler U., Scharpfenecker M., Koidl S., Hegen A., Grunow V., Schmidt J. M., et al. The Tie‐2 ligand angiopoietin‐2 is stored in and rapidly released upon stimulation from endothelial cell Weibel‐Palade bodies. Blood 2004; 103: 4150–6
- Lim H. S., Lip G. Y., Blann A. D. Angiopoietin‐1 and angiopoietin‐2 in diabetes mellitus: relationship to VEGF, glycaemic control, endothelial damage/dysfunction and atherosclerosis. Atherosclerosis 2005; 180: 113–8
- Varughese G. I., Patel J. V., Tomson J., Blann A. D., Hughes E. A., Lip G. Y. Prognostic value of plasma soluble P‐selectin and von Willebrand factor as indices of platelet activation and endothelial damage/dysfunction in high‐risk patients with hypertension: a sub‐study of the Anglo‐Scandinavian Cardiac Outcomes Trial. J Intern Med 2007; 261: 384–91
- Sfiligoi C., De Luca A., Cascone I., Sorbello V., Fuso L., Ponzone R., et al. Angiopoietin‐2 expression in breast cancer correlates with lymph node invasion and short survival. Int J Cancer 2003; 103: 466–74
- Tanaka F., Ishikawa S., Yanagihara K., Miyahara R., Kawano Y., Li M., et al. Expression of angiopoietins and its clinical significance in non‐small cell lung cancer. Cancer Res 2002; 62: 7124–9