Abstract
Matrix metalloproteinase-9 (MMP-9) has been shown to be involved in the development of diabetic nephropathy (DNP). We studied the levels, molecular forms, and degree of activation of urinary MMP-8, -9, -14, trypsin-1 and -2, as well as tumor-associated trypsin inhibitor (TATI) of DNP patients and healthy controls.
Urinary samples were analyzed for MMPs by Western blotting and gelatin zymography and for trypsin-1, -2, and TATI by time-resolved immunofluorometric assays.
Total MMP-8 immunoreactivity, the proportion of active MMP-9, and gelatinolytic activity in urine were significantly higher in DNP patients than in controls. In urine of DNP patients the proportion of active polymorphonuclear neutrophil (PMN)-type (but not fibroblast-type) MMP-8 was increased. MMP-8 and MMP-9 were found to form high molecular weight complexes in DNP urine. Total immunoreactivity of soluble urinary MMP-14 and the levels of trypsin (TRY)-1 and TRY-2, but not of TATI, were also significantly increased in DNP. Zymography, Western blotting, and immunofluorometric analysis of DNP urine showed a significant association especially between activation of MMP-9 as well as PMN-type MMP-8 and TRY-2.
Our findings suggest that a trypsin-MMP cascade is involved in the pathogenesis of DNP, which may offer new possibilities for diagnosis and treatment of DNP with MMP inhibitors.
Introduction
Matrix metalloproteinases (MMPs) have been suggested to play a role in the development of diabetic nephropathy (DNP) Citation1. The genetically distinct but structurally related MMPs are zinc-dependent metalloendopeptidases, which can be classified based on their primary structures and substrate specificities into several groups, such as collagenases (MMP-1, -8, -13), gelatinases (MMP-2, -9), stromelysins (MMP-3, -10, -11, -19), matrilysins (MMP-7, -26), membrane-type MMPs (MT-MMPs) (MMP-14, -15, -16, -17, -24, -25), and other MMPs Citation2. MMPs have traditionally been associated with many physiological and pathological processes, such as inflammation, wound healing, cancer growth and invasiveness, infections, and tissue remodeling Citation2–4. Recently MMPs have been found to regulate extracellular homeostasis by processing many bioactive molecules involved in cell function and signaling and innate immunity Citation2–4. Previously, MMPs have been implicated predominantly in the mediation of tissue destruction in inflammatory and malignant diseases, but recently certain MMPs such as MMP-8 have been found to exhibit defensive anti-inflammatory properties by regulating inflammatory cell activity and apoptosis Citation3, Citation4. Most MMPs are secreted as latent, inactive forms, proMMPs Citation2. Their extracellular or membrane-associated activation involves a complex conformational change, induced by oxidants or proteases such as MMP-14 (MT1-MMP), other MMPs, and serine proteinases such as trypsins Citation5–9. Especially trypsin-2 (TRY-2) can activate proMMP cascades and process directly extracellular matrix (ECM) components including collagens Citation6, Citation9.
Key messages
A trypsin-matrix metalloproteinase (MMP) cascade contributes to the pathogenesis of diabetic nephropathy (DNP).
The finding of the trypsin-MMP cascade in urine may offer new possibilities for diagnosis and treatment of DNP with MMP inhibitors.
Abbreviations
Glomerular matrix proteins constantly undergo metabolic turnover, being degraded primarily by MMPs Citation1, Citation7. Thus, any decrease in turnover would promote excessive matrix accumulation, as seen in diabetic glomerulopathy Citation1, Citation7. The balance between ECM synthesis and degradation is a prerequisite for maintaining the structural and functional integrity of the glomerulus. Changes in this balance may lead to expansion of the glomerular EMC, i.e. renal fibrosis, and decline in renal function Citation1, Citation7, Citation10, Citation11. Expansion of ECM in tubulointerstitium and glomerulus is the typical pathological feature of diabetes Citation1, Citation7, Citation10, Citation11. Renal fibrosis is the main pathological feature of DNP leading to end stage renal disease. Furthermore, collagen IV and fibronectin, which are the major components of EMC, and MMP and TIMP expressions have all been shown to be altered in diabetic and other nephropathies Citation1, Citation7, Citation10, Citation11.
MMP-9 has been shown to be involved in the development of early DNP Citation10. Recently, the concentration of urinary MMP-9 in DNP with macroalbuminuria was shown to be significantly higher than in healthy adults Citation11. Urinary levels of MMP-9 in patients with type II DNP increased in parallel with the clinical stage of the disease Citation11. We have now studied the urinary levels, molecular forms, and degree of activation of MMP-8 and MMP-9 as well as MMP-14 and related these findings to the levels of TRY-1, -2 and tumor-associated trypsin inhibitor (TATI) in urine of adult patients with DNP.
Subjects and methods
Study subjects
Clinical characteristics of the patients (n=10) are shown in . DNP of the studied patients was verified by kidney biopsy taken by clinical indications. The biopsies were analyzed at the Division of Nephrology at Helsinki University Central Hospital by Dr Tom Törnroth, MD, PhD, renal pathologist. The age of the patients varied from 33 to 72 years, and male/female ratio was 9/2. One of the patients had normal serum creatine concentration with heavy proteinuria (dU-prot of 6.1 g), whereas in the rest renal function was moderately or severely decreased. Heavy proteinuria (>3 g/24 h) was observed in eight patients. The study design was approved by the Ethics Committee of Helsinki University Central Hospital, Helsinki, Finland. The control group consisted of ten healthy volunteers. The participants gave informed consent. Urine samples were collected as previously described from ten patients with DNP and from ten healthy voluntary controls Citation12.
Table I. Clinical characteristics of the diabetic nephropathy patients.
Western blotting
The molecular forms and degree of activation of urinary MMPs were analyzed by Western blotting analysis using specific rabbit polyclonal antisera to human MMP-2, -8, -9, and -14 as previously described Citation6, Citation13–15. After sodium dodecyl sulphate-polyacrylamide gel (SDS-PAGE) run under non-reducing conditions, the proteins in the gel were electrotransferred onto a nitrocellulose membrane (Bio-Rad Laboratories, Richmond, California). After blocking with 3% gelatin, the membrane was first reacted with the primary antibody diluted 1:500 and then with an alkaline phosphatase conjugated secondary antibody. Immunoreactive proteins were visualized with nitro blue tetrazolium (Sigma, St. Louis, Missouri) and 5-bromo-4-chloro-3-indolyl-phosphate (Sigma). MMP immunoreactivities were in the linear range of the reaction assessed as previously described Citation15. Quantitation was performed by Bio-Rad Model GS-700 Imaging Densitometer using the Analysis program Citation15. Data are expressed as densitometric units (DU). Human polymorphonuclear neutrophil (PMN) extract Citation16 and rheumatoid synovial culture medium Citation17 were used as positive controls for PMN-type and mesenchymal-type MMP-8 isoforms. Recombinant human MMP-2, -9, and -14 were used as positive controls for MMP-2, -9, and -14 Citation6, Citation18.
Gelatinase assay
Gelatinolytic activity, molecular forms, and the degree of gelatinase A (MMP-2) and B (MMP-9) activation in urine samples were analyzed by zymography using 10% sodium dodecyl sulphate-polyacrylamide gels (SDS-PAGE) containing 1 mg/mL gelatin (Sigma), St. Louis, CA) as a substrate, as described previously Citation6, Citation19. Before electrophoresis, the gels were washed in 50 mM Tris-HCl, 2.5% Tween 80, and 0.02% (w/v) NaN3, pH 7.5 at +37°C for 24 h. The reaction was interrupted by staining the gels with 0.1% Coomassie brilliant blue R250 and destaining Citation6, Citation19. Human recombinant 92-kDa neutrophil MMP-9 and recombinant 72-kDa MMP-2 were used as positive controls Citation6. The gelatinolytic activity was visualized as clear band against a blue background. The band intensities were quantified densitometrically and were in the linear range of the reaction assessed as previously Citation6, Citation19, Citation20. The gelatinolytic activities are expressed as densitometric units, DU Citation20. The gelatinases A (MMP-2) and B (MMP-9) detected by zymography were identified by Western immunoblotting and by specific polyclonal antibodies as described previously Citation6, Citation20.
Time-resolved immunofluorometric assay (IFMA)
The concentrations of TRY-1, TRY-2, and TATI in urine were measured by immunofluorometric assays as previously described Citation21, Citation22. Fluorescence was measured in a 1234 DELFIA research fluorometer (Wallac, Turku, Finland). Detection limits and intra- and interassay coefficients of variations have been described previously Citation23.
Statistics
Data were analyzed by using GraphPad Prism version 4.0 (GraphPad Inc., San Diego, California, USA). Data of two groups were compared by the Mann-Whitney test. The results are presented as median (25%–75% percentiles). Spearman rank correlation was used to analyze correlations between variables. A P value less than 0.05 was considered statistically significant.
Results
Total MMP-8 immunoreactivity in urine of patients with DNP was significantly higher than in healthy controls (3.0 (1.9–7.9) densitometric units (DU) versus 0.01 (0.01–0.02) DU, P < 0.001) (). The proportion of active PMN-type MMP-8 was significantly higher (40 (16–55) DU versus 0.0 (0.0–0.01) DU, P = 0.002) in urine of patients with DNP compared to healthy controls (). The proportion of active fibroblast-derived MMP-8 showed no difference (data not shown). The molecular forms of urinary MMP-8 species in DNP urine are shown in A; both PMN- and fibroblast (Mes)-type MMP-8 isoforms could be detected in partially activated forms together with low levels of low molecular weight (20–30 kDa) fragments (A, lanes 1–3). Total gelatinolytic activity, comprising both MMP-2 and MMP-9, was significantly increased (P<0.01) in DNP as compared to healthy controls (). Among the urinary gelatinases (MMP-2 and MMP-9), MMP-9 was more extensively converted to the active form than MMP-2 (B, lanes 1–3), and the proportion of active MMP-9 in urine was significantly higher (P < 0.05) in urine of patients with DNP compared to healthy controls (). MMP-8 and MMP-9 were found to form high molecular weight complexes in DNP urine but not in urine of healthy controls (). Total immunoreactivity of urinary soluble MMP-14 (MT1-MMP) in DNP was significantly increased (0.34 (0.0–1.0) DU) when compared to healthy controls (0.0 (0.0–0.05) DU, P < 0.05) (C and ). The molecular forms of soluble forms of urinary MMP-14 consisted of 40, 70, and 80 kDa species representing active, pro and complex forms of MMP-14 (C). There was a tendency to elevated TATI levels in urine of DNP patients, but the difference was not statistically significant (). In urine of DNP patients the concentration of TRY-2 was significantly higher (15.9 (1.8–39.6) µg/L) when compared to healthy controls (0 (0–0.09) µg/L, P < 0.001) (A). The concentration of urinary TRY-1 in DNP was also significantly higher (0.5 (0.03–2.7) µg/L) than in healthy controls (0.0 (0.0–0.01) µg/L, P<0.01) (B).
Figure 1. Determination of total MMP-8 immunoreactivity by Western blotting in urine. The concentrations (expressed as densitometric units (DU)) were significantly higher (P < 0.001) in patients with diabetic nephropathy (DNP) than in healthy controls.
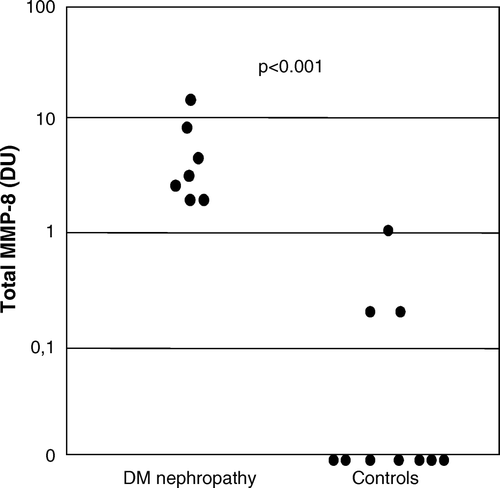
Figure 2. The proportion of active polymorphonuclear neutrophil (PMN) type MMP-8 determined by Western blotting in urine was significantly higher (P = 0.002) in diabetic nephropathy (DNP) patients than in healthy controls.
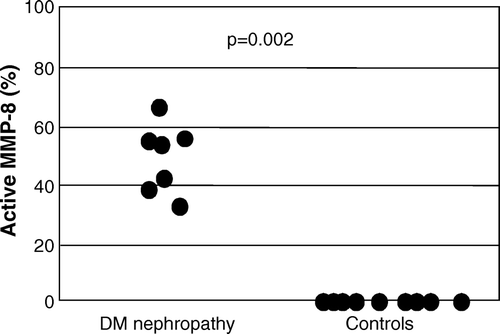
Figure 3. Western blotting of collagenase-2 (MMP-8) (panel A), zymography and Western blot of gelatinase (MMP- 2 and -9) (panel B) and membrane-type I matrix metalloproteinase (MMP-14, panel C) in urine from DNP patients (lanes 1–3, DNP) and healthy controls (lanes 4–6, cont). Also in DNP, but not in urine of healthy control, high molecular weight (>80 kDa) MMP-8 and (>92 kDa) MMP-9 complexes could be detected. Human PMN- and rheumatoid synovial fibroblast-culture media were used as positive control for PMN- and mesenchymal (Mes)-type MMP-8 isoforms and for MMP-9 and -2. Also pure human MMP-2, -9, and -14 were used as positive controls. Mobilities of the molecular weight markers are indicated on the left.
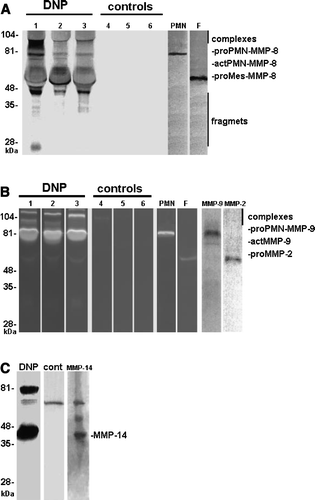
Figure 4. Total immunoreactivity of urinary MMP-14 (MT1-MMP) determined by Western blotting. The concentrations in diabetic nephropathy (DNP) patients were significantly higher (P < 0.05) than in healthy controls.
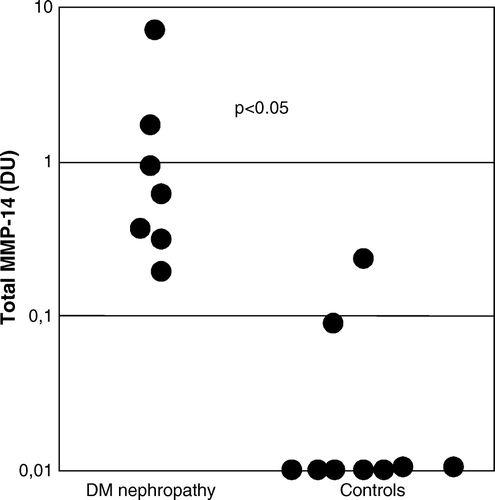
Figure 5. Concentrations of TRY-2 and TRY-1 in urine. In diabetic nephropathy (DNP) patients the concentrations of TRY-2 (panel A) and TRY-1 (panel B) were significantly higher than in healthy controls (P < 0.001 and P<0.01, respectively).
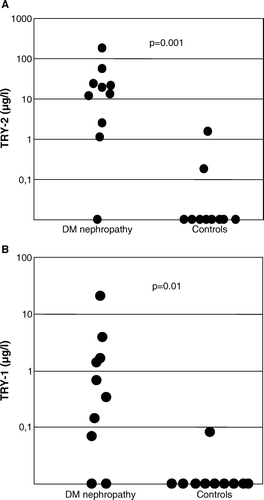
Table II. Gelatinolytic activity, percent active MMP-9 and TATI in urine of patients with diabetic nephropathy, and of healthy controls. Data are presented as median (25%–75% percentiles).
The concentrations of TRY-2, TRY-1, and TATI correlated significantly with active urinary PMN-type MMP-8 in patients with DNP, but not with active fibroblast-type MMP-8 (). TRY-2 and TATI, but not TRY-1, correlated significantly with active MMP-9 in urine of DNP patients (). Correlation analyses (Spearman, two-tailed) showed no correlation between proteinuria and total MMP-8 immunoreactivity (r = 0.12, P > 0.05), percent active MMP-8 (r=0.059, P > 0.05), or percent active MMP-9 (r=0.46, P > 0.05) in urine of patients (n =10) with DNP.
Table III. Correlations between active urinary MMP forms and TRY-2, TRY-1 and TATI in urine of DNP patients (n = 10).
Discussion
We found significantly higher total MMP-8 immunoreactivity in urine of adult patients with DNP than in urine of healthy controls. The proportion of active PMN-type MMP-8, but not active fibroblast-type MMP-8, was also significantly increased. The latent proform of PMN-type MMP-8 was also excreted in urine of DNP patients, but not in urine of healthy controls. This is to the best of our knowledge the first demonstration of MMP-8 and its various molecular isoforms in urine of patients with DNP. The present finding suggests that MMP-8 may play an important role in the pathogenesis of DNP. MMP-8 may mediate tissue destruction by cleaving collagens and proteinase inhibitors (serpins) as well as contribute to an anti-inflammatory protective shield by selectively processing chemokines and cytokines Citation3, Citation4. To further characterize these findings, serum samples as well as samples from DNP patients with different stages and duration of the disease also need to be studied. The present finding of urinary MMP-8 may offer possibilities of treating DNP by modifying chemotherapeutically the secretion and activity of MMP-8.
We also found that urinary total gelatinolytic activity, comprising of both MMP-2 and MMP-9, was significantly increased in DNP when compared to healthy controls. Among urinary gelatinases, MMP-9 was more extensively converted to its active form than MMP-2, and the proportion of active MMP-9 was also more strongly increased. These findings are in accordance with and further extend the recent findings suggesting that MMP-9 is involved in the development of DNP Citation10, Citation11. Ebihara et al. (1998) have shown that MMP-9 is involved in the development of early DNP Citation10. They found that increased plasma MMP-9 levels preceded the occurrence of microalbuminuria. Tashiro et al. (2004) have recently shown that the mean level of urinary MMP-9 in DNP patients with macroalbuminuria was significantly higher than in healthy adults Citation11. In addition urinary MMP-9 levels increased with clinical stage of the disease Citation11. Furthermore, both MMP-8 and MMP-9 were found to form high molecular weight complexes in DNP urine but not in urine of healthy control. These types of complexes have recently been detected in an animal model study of wound healing Citation24. These complexes may regulate MMP-8 and MMP-9 with respect to their stability and turnover, as well as coordinate their substrate targeting Citation24.
This is the first report showing excretion of the soluble form of MMP-14 in the human diabetic urine. The concentration of immunoreactive MMP-14 was significantly higher in urine of patients with impaired renal function due to diabetes mellitus (DM) than in healthy controls. Previously soluble or shedded forms of MMP-14 have been found to be expressed by human mesangial cells Citation25, in culture fluid of renal carcinoma cells Citation26, in inflamed human gingival crevicular fluid Citation27, in human tear fluid Citation8, and in bronchoalveolar lavage fluid from bronchiectasis patients Citation14. The molecular forms of soluble or shedded MMP-14 in DNP urine corresponded to those seen in human inflammatory exudates (i.e. gingival crevicular, tear fluid), and cell culture supernates Citation8, Citation14, Citation15, Citation23–27. The membrane-type matrix metalloproteinases (MT-MMPs) differ from other MMPs in that they have a transmembrane domain that anchors them to cell surface. MT-MMPs have been shown to function as receptors and activators for other proMMPs Citation2, Citation28–30. It is noteworthy that MMP-14 can activate latent proMMP-8 Citation8 and also proMMP-2 Citation29, and that it thus could be part of the MMP-activation cascades involved in the development of DNP. Our results support the findings by McLennan and co-workers Citation31 that MMP-14 plays an important role in DNP.
The concentrations of TRY-1 and TRY-2 in urine of DNP patients were significantly elevated, while those of TATI tended to be elevated without reaching statistical significance. It is noteworthy that TRY-2 is a very efficient activator of proMMP-cascades such as proMMP-8 and -9 in vitro Citation6, Citation9. TRY-2 is a relatively weak activator of proMMP-2 Citation6. High TRY-2 concentrations correlated with activation of MMP-9 and PMN-type, but not fibroblast-type, MMP-8 in DNP urine suggesting that they form serine proteinase-MMP activation cascades in vivo Citation6, Citation9, Citation19, Citation20. TRY-2 can also directly process extracellular matrix components including collagens Citation6, Citation9, and it may thus modify the pathogenesis of DNP both directly and indirectly. It is also noteworthy that in the present study urinary samples were analyzed for MMPs by Western blotting and gelatin zymography Citation6, Citation15, Citation20. Like zymography, Western blotting (electrophoresis under non-reducing conditions) allowed us to separate and identify the different isoforms and molecular forms of the studied MMPs as well as their degree of activation Citation6, Citation15, which is not possible with currently available ELISAs or IFMAs, which detect total immunoreactivity but not individual isoforms or activated and latent species Citation32.
Adler and co-workers recently studied the progression of type II diabetes Citation33 and showed that 10 years following the diagnosis of DM, the prevalence of microalbuminuria was 24.9%, of macroalbuminuria was 5.3%, and elevated plasma creatinine or renal replacement therapy (RRT) was 0.8%. Patients with elevated plasma creatinine or RRT had an annual death rate of 19.2%. MMP-9 has been shown to be involved in the development of early DNP Citation10 increasing in parallel with the clinical stage of the disease Citation11. Studies by Ebihara et al. (1998) Citation10 and Tashiro et al. (2004) Citation11, as well as the results of the present analysis, showing elevated urinary MMP-8, -9 and MMP-14 in DNP, suggest that MMPs may cause tissue destruction that leads to renal damage during the progression of DNP. Connective tissue turnover, formation, and degradation are of critical importance in normal tissue maintenance and remodeling, as well as in the development and progression of various tissue-destructive diseases Citation2, Citation34. We suggest that during the course of DNP synthesis of connective tissue components is induced to compensate for the imbalance of connective tissue turnover due to proteolysis by MMPs, leading to renal fibrosis, which is central to the progression of DNP towards end stage renal failure Citation35–37.
Because of the increased urinary levels of active PMN-derived MMP-8 in DNP, it will be interesting to study, and possibly treat, patients with DNP with synthetic MMP-8 inhibitors. Tetracyclines, especially doxycycline, have been shown to inhibit MMP-8 activity, proMMP-8 activation, and tumor necrosis factor-a-induced expression of MMP-8 by different mechanisms both in vitro and in vivo Citation5, Citation17, Citation38–40. Therapeutically attainable serum concentrations of doxycycline can reduce TRY-2 protein and mRNA expression in vitro Citation19. In addition to MMP-8 inhibition and TRY-2 inhibition and reduction, doxycycline has been also shown to inhibit MMP-9, -13, and MMP-14 Citation5, Citation17–19, Citation40–44. Because of the pro- and anti-inflammatory characteristics of MMP-8 Citation3, Citation4 the less efficient synthetic broad-spectrum MMP inhibitors, such as doxycycline, may be favorable, since doxycycline has been shown in humans to reduce mainly the pathological excess of MMP-8, -9, and -14 activities, thus not causing their complete inhibition, allowing them to carry out their anti-inflammatory protective(s) Citation3, Citation4, Citation40, Citation45, Citation46. It should be kept in mind that tetracyclines, except for doxycycline, should not be used in patients with renal failure Citation47. In this regard, Naini et al. Citation48 medicated with doxycycline the patients with diabetic nephropathy for 2 months with a high degree of compliance and detected no apparent serious side effect. To avoid possible unfavorable antimicrobial side effects it might be useful to use chemically modified non-antimicrobial tetracyclines (CMTs) or low-dose doxycycline (LDD) medication Citation40, Citation48, Citation49. It is noteworthy that Ahuja recently in a case report showed that doxycycline decreased proteinuria in a patient with glomerulonephritis Citation49. The findings of Ahuja Citation49 were further extended and supported by Naini et al. Citation48 who studied the effect of doxycycline therapy on proteinuria in diabetic nephropathy patients. Proteinuria was significantly reduced with doxycycline therapy over a 2-month period of drug administration Citation48. Doxycycline therapy can also reduce salivary and serum levels of MMP-8 Citation38, Citation39.
In summary, the findings of this study, showing elevated levels of MMP-8, -9, and -14 as well as TRY-2 and -1 in urine of patients with DNP, suggest that MMP-8, -9, and -14 as well as TRY-2 and -1 can form a cascade that may play a role in the pathogenesis of DNP. We acknowledge certain limitations of our study. Our study patient number is relatively small. Also our study does not establish tissue origin of studied trypsins and MMPs in DNP urine and relationship between renal urine proteases, renal disease activity, and renoprotective drugs. However, these findings may offer new possibilities for diagnosis and treatment of DNP with broad-spectrum MMP inhibitors like doxycycline. Since our results on elevated urinary trypsin-MMP cascade in DNP are in agreement with other reports Citation50, Citation51 describing increased urinary MMP-2 and -9 in diabetic nephropathy and patients with chronic kidney diseases, further studies concerning the role of MMPs in DNP, with a greater number of patients, with different types of DM, and with different stage of the disease, as well as the role of MMP inhibitors in DNP, are needed. The number of DNP patients is increasing worldwide causing a huge economic burden due the expenses of RRT Citation52, Citation53. Thus, new ways to treat DNP patients are needed, and MMP inhibitors may offer a new possibility in this respect.
Acknowledgements
This study was supported by grants to Anneli Lauhio and to Timo Sorsa from Elsi and Wilhelm Stockmann Stiftelse, the Helsinki University Central Hospital research funds (EVO), Clinical EVO of Infectious Unit and HUS-EVO grants (TYH 5306, TYH 6104, TYH 7114, TYH 2008251, TI 020Y0002 and T1 020 Y 0010). This study was also supported by a grant from the Academy of Finland. We thank Professor Seppo Sarna for statistical advice.
Conflict of interest statement: None declared.
References
- Zaoui P, Cantin J, Alimardani-Bessette M, Monier F, Halimi S, Morel F, et al. Role of metalloproteinases and inhibitors in the occurrence and progression of diabetic renal lesions. Diabetes Metab. 2000; 26(Suppl 4)25–9
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002; 2: 161–74
- Owen CA, Hu Z, Lopez-Otin C, Shapiro SD. Membrane-bound matrix metalloproteinase-8 on activated polymorphonuclear cells is a potent, tissue inhibitor of metalloproteinase-resistant collagenase and serpinase. J Immunol. 2004; 72: 7791–803
- Gueders MM, Balbin M, Rocks N, Foidart JM, Gosset P, Louis R, et al. Matrix metalloproteinase-8 deficiency promotes granulocytic allergen-induced airway inflammation. J Immunol. 2005; 175: 2589–97
- Lauhio A, Sorsa T, Lindy O, Suomalainen K, Saari H, Golub LM, et al. The anticollagenolytic potential of lymecycline in the long-term treatment of reactive arthritis. Arthritis Rheum. 1992; 35: 195–8
- Sorsa T, Salo T, Koivunen E, Tyynelä J, Konttinen Y, Bergmann U, et al. Activation of type IV procollagenases by human tumor-associated trypsin-2. J Biol Chem. 1997; 272: 21067–74
- Marti HP. The role of matrix metalloproteinases in the activation of mesangial cells. Transpl Immunol. 2002; 9: 97–110
- Holopainen JM, Moilanen JA, Sorsa T, Kivela-Rajamaki M, Tervahartiala T, Vesaluoma MH, et al. Activation of matrix metalloproteinas-8 by membrane type 1-MMP and their expression in human tears after photorefractive keratectomy. Invest Ophthalmol Vis Sci. 2003; 44: 2550–6
- Moilanen M, Sorsa T, Stenman M, Nyberg P, Lindy O, Vesterinen J, et al. Tumor-associated trypsinogen-2 (trypsinogen-2) activates procollagenases (MMP-1, -8, -13) and stromelysin-1 (MMP-3) and degrades type I collagen. Biochemistry. 2003; 42: 5414–20
- Ebihara I, Nakamura T, Shimada N, Koide H. Increased plasma metalloproteinase-9 concentrations precede development of microalbuminuria in non-insulin-dependent diabetes mellitus. Am J Kidney Dis. 1998; 32: 544–50
- Tashiro K, Koyanagi I, Ohara I, Ito T, Saitoh A, Horikoshi S, et al. Levels of urinary matrix metalloproteinase-9 (MMP-9) and renal injuries in patients with type 2 diabetic nephropathy. J Clin Lab Anal. 2004; 18: 206–10
- Honkanen E, Teppo AM, Törnroth T, Groop PH, Grönhagen-Riska. Urinary transforming growth factor--1 in membranous glomerulonephritis. Nephrol Dial Transplant. 1997;12:2562–8.
- Lindy O, Konttinen YT, Sorsa T, Ding Y, Santavirta S, Ceponis A, et al. Matrix metalloproteinase 13 (collagenase 3) in human rheumatoid synovium. Arthritis Rheum. 1997; 40: 1391–9
- Maisi P, Prikk K, Sepper R, Pirilä E, Salo T, Hietanen J, et al. Soluble membrane-type 1 matrix metalloproteinase (MT1-MMP) and gelatinase A (MMP-2) in induced sputum and bronchoalveolar lavage fluid of human bronchial asthma and bronchiectasis. APMIS. 2002; 110: 771–82
- Prikk K, Maisi P, Pirilä E, Reintman MA, Salo T, Sorsa T, et al. Airway obstruction correlates with collagenase-2 (MMP-8) expression and activation in bronchial asthma. Lab Invest. 2002; 82: 1535–45
- Sorsa T, Ding Y, Salo T, Lauhio A, Teronen O, Ingman T, et al. Effects of tetracyclines on neutrophil, gingival, and salivary collagenases. A functional and western-blot assessment with special reference to their cellular sources in periodontal diseases. Ann N Y Acad Sci. 1994; 732: 112–31
- Hanemaaijer R, Sorsa T, Konttinen YT, Ding Y, Sutinen M, Visser H, et al. Matrix metalloproteinase-8 is expressed in rheumatoid synovial fibroblasts and endothelial cells. Regulation by tumor necrosis factor-alpha and doxycycline. J Biol Chem. 1997; 272: 31504–9
- Lee HM, Golub LM, Cao J, Teronen O, Laitinen M, Salo T, et al. CMT-3, a non-antimicrobial tetracycline (TC), inhibits MT1-MMP activity: relevance to cancer. Curr Med Chem. 2001; 8: 257–60
- Lukkonen A, Sorsa T, Salo T, Tervahartiala T, Koivunen E, Golub L, et al. Down-regulation of trypsinogen-2 expression by chemically modified tetracyclines: association with reduced cancer cell migration. Int J Cancer. 2000; 86: 577–81
- Paju A, Sorsa T, Tervahartiala T, Koivunen E, Haglund C, Leminen A, et al. The levels of trypsinogen isoenzymes in ovarian tumour cyst fluids are associated with promatrix metalloproteinase-9 but not promatrix metalloproteinase-2 activation. Br J Cancer. 2001; 84: 1363–71
- Itkonen O, Koivunen E, Hurme M, Alfthan H, Schroder T, Stenman UH. Time-resolved immunofluorometric assays for trypsinogen-1 and 2 in serum reveal preferential elevation of trypsinogen-2 in pancreatitis. J Lab Clin Med. 1990; 115: 712–8
- Osman S, Turpeinen U, Itkonen O, Stenman UH. Activation of a time-resolved immunofluorometric assay for tumor-associated trypsin inhibitor (TATI) using the streptavidin-biotin system. J Immunol Methods. 1993; 161: 97–106
- Hedstrom J, Leinonen J, Sainio V, Stenman UH. Time-resolved immunofluorometric assay of trypsin-2 complexed with alpha 1-antitrypsin in serum. Clin Chem. 1994; 40: 1761–5
- Gutierrez-Fernnandez A, Inada M, Balbin M, Fueyo A, Ana S, Pitiot AS, et al. Increased inflammation delays wound healing in mice deficient collagenase-2 (MMP-8). FASEB J. 2007; 21: 2580–91
- Kazes I, Delarue F, Hagege JN, Bouzhir-Sima L, Rondeau E, Sraer JD, et al. Soluble latent membrane-type 1 matrix metalloproteinase secreted by human mesangial cells is activated by urokinase. Kidney Int. 1998; 54: 1976–84
- Zhang Y, Wu XH, Cao GH, Li S. Relationship between expression of matrix metalloproteinase-9 (MMP-9) and angiogenesis in renal cell carcinoma. Ai Zheng. 2004; 23: 326–9
- Tervahartiala T, Pirilä E, Ceponis A, Bouzhir-Sima L, Rondeau E, Sraer JD, et al. The in vivo expression of the collagenolytic matrix metalloproteinases (MMP-2, -8, -13, and -14) and matrilysin (MMP-7) in adult and localized juvenile periodontitis. J Dent Res. 2000; 79: 1969–77
- Overall CM, Lopez-Otin C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer. 2002; 2: 657–72
- Strongin AY, Collier I, Bannikov G, Marmer BL, Grant GA, Goldberg GI. Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. J Biol Chem. 1995; 270: 5331–8
- Knäuper V, Smith B, Lopez-Otin C, Murphy G. Activation of progelatinase B (proMMP-9) by active collagenase-3 (MMP-13). Eur J Biochem. 1997; 248: 369–73
- McLennan SV, Martell SY, Yue DK. High glucose concentration inhibits the expression of membrane type metalloproteinase by mesangial cells: possible role in mesangium accumulation. Diabetologia. 2000; 43: 642–8
- Kolb SA, Lahrtz F, Paul R, Leppert D, Nadal D, Pfister HW, et al. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in viral meningitis: upregulation of MMP-9 and TIMP-1 in cerebrospinal fluid. J Neuroimmunol. 1998; 84: 143–50
- Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR, et al. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int. 2003; 63: 225–32
- Overall CM. Regulation of tissue inhibitor of matrix metalloproteinase expression. Ann NY Acad Sci. 1994; 732: 51–64
- Chow FY, Nikolic-Paterson DJ, Atkins RC, Tesch GH. Macrophages in streptozotocin-induced diabetic nephropathy: potential role in renal fibrosis. Nephrol Dial Transplant. 2004; 19: 2987–96
- Locatelli F, Pozzoni P, Vecchio LD. Renal replacement therapy in patients with diabetes and end-stage renal failure. J Am Soc Nephrol. 2004; 15: 25–9
- Wolf G, Chen S, Ziyadeh FN. From the periphery of the glomerular capillary wall toward the center of disease. Diabetes. 2005; 54: 1626–34
- Lauhio A, Konttinen YT, Tscesche H, Nordström D, Salo T, Lähdevirta J, et al. Reduction of matrix metalloproteinases 8-neutrophil collagenase levels during long-term doxycycline treatment of reactive arthritis. Antimicrob Agents Chemother. 1994; 38: 400–2
- Lauhio A, Salo T, Ding Y, Konttinen YT, Nordström D, Tschesche H, et al. In vivo inhibition of human neutrophil collagenase (MMP-8) activity during long-term combination therapy of doxycycline and non-steroidal anti-inflammatory drugs (NSAID) in acute reactive arthritis. Clin Exp Immunol. 1994; 98: 21–8
- Golub LM, Lee HM, Ryan ME, Giannobile WV, Payne J, Sorsa T. Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Adv Dent Res. 1998; 12: 12–26
- Suomalainen K, Sorsa T, Golub LM, Ramamurthy N, Lee HM, Uitto VJ, et al. Specificity of the anticollagenase action of tetracyclines: relevance to their anti-inflammatory potential. Antimicrob Agents Chemother. 1992; 36: 227–9
- Lauhio A, Salo T, Tjäderhane L, Lähdevirta J, Golub LM, Sorsa T. Tetracyclines in treatment of rheumatoid arthritis. Lancet. 1995; 346: 645–6
- Greenwald RA, Golub LM, Ramamurthy NS, Chowdhury M, Moak SA, Sorsa T. In vitro sensitivity of the three mammalian collagenases to tetracycline inhibition: relationship to bone and cartilage degradation. Bone. 1998; 22: 33–8
- Sorsa T, Ramamurthy NS, Vernillo AT, Zhang X, Konttinen YT, Rifkin BR, et al. Functional sites of chemically modified tetracyclines: inhibition of the oxidative activation of human neutrophil and chicken osteoclast pro-matrix metalloproteinases. J Rheumatol. 1998; 25: 975–8
- Emingil G, Atilla G, Sorsa T, Savolainen P, Baylas H. Effectiveness of adjunctive low-dose doxycycline therapy on clinical parameters and gingival crevicular fluid laminin-5-gamma2 chain levels in chronic periodontitis. J Periodontol. 2004; 75: 1387–96
- Sorsa T, Tjäderhane L, Salo T. Matrix metalloproteinases (MMPs) in oral diseases. Oral Dis. 2004; 10: 311–8
- Meyer B, Meyer B, Salvatore M. Tetracyclines and chloramphenicol. Principles and practice of infectious diseases6th ed, D Mandell, G Bennett, R Douglas, JE Bennett, R Dolin. Elsevier, Churchill Livingstone, Philadelphia 2005; 356–72
- Naini EA, Harandi AA, Moghtaderi J, Bastani B, Amiran A. Doxycycline: a pilot study to reduce diabetic proteinuria. Am J Nephropathol. 2007; 27: 269–73
- Ahuja TS. Doxicycline decreases proteinuria in glomerulonephritis. Am J Kidney Dis. 2003; 42: 376–80
- Diamant M, Hanemaaijer R, Verheijen JH, Smith JW, Lemkes H. Elevated matrix metalloproteinase-2 and -9 in urine, but not in serum, are markers of type I diabetic nephropathy. Diabet Med. 2001; 18: 423–4
- Chang HR, Yang SF, Li ML, Lin CC, Hsieh YS, Lian JD. Relationships between circulating matrix metalloproteinases -2 and -9 and renal function in patients with chronic kidney diseases. Clin Chim Acta. 2006; 366: 243–8
- El Nahas MA, Bello AK. Chronic kidney disease: the global challenge. Lancet. 2005; 365: 331–40
- Van Dijk PC, Jager KJ, Stengel B, Grönhagen-Riska C, Feest TG, Briggs JD. Renal replacement therapy for diabetic end-stage renal disease: data from 10 registries in Europe (1991–2000). Kidney Int. 2005; 67: 1489–99
