Abstract
Cells with stem cell-like attributes, such as self-renewal and pluripotency, have been isolated from hematological malignancies and from several solid tumor types. Tumor-initiating cells, also referred to as cancer stem cells, are thought to be responsible for the initiation and growth of tumors. Like their normal counterparts, putative cancer stem cells show remarkable resistance to radiation and chemotherapy. Their capacity for surviving apparently curative treatment can result in tumor relapse. Novel approaches that target tumor-initiating cells in addition to differentiated malignant cells, which constitute the bulk of the tumor, are required for improved survival of patients with metastatic tumors. Oncolytic viruses enter cells through infection and may therefore be resistant to defense mechanisms exhibited by cancer stem cells. Oncolytic adenoviruses can be engineered to attack tumor stem cells, recognized by linage-specific cell surface markers, dysfunctional stem cell-signaling pathways, or upregulated oncogenic genes. Normal stem cells may possess innate resistance to adenoviruses, as most humans have sustained numerous infections with various wild-type serotypes. This review focuses on current literature in support of cancer stem cells and discusses the possibility of using oncolytic virotherapy for killing these tumor-initiating cells.
Introduction
Cancer is a globally encountered disease with somber statistics. It is estimated that approximately one in two men and one in three women will during their lifetime develop some type of cancer. At present time, this corresponds to about 10 million newly diagnosed cancer patients per year worldwide. Available means of treatment, i.e. surgery, chemotherapy, ionizing radiation, hormonal therapies, small molecular inhibitors, and monoclonal antibodies, benefit many patients. Still, individuals with solid tumors metastatic to distant organs have poor prospects of being completely cured. With few exceptions, relapses always occur after seemingly effective treatment, and regrettably refractory cell clones typically display increased resistance to treatment modalities.
A cancerous cell can be characterized by unlimited proliferative capability, resistance to apoptotic mechanisms, and an invasive phenotype Citation1. These traits are acquired through genetic and epigenetic changes, which transform a normal cell into a malignant cell. Rapid development in cancer research during the last quarter of a century has provided us with a good insight into the mechanisms and mutations that are present in cancerous cells. Yet, the origin of cancer, i.e. the identity of the tumor-initiating cells, is still a subject of debate. An increasing amount of data suggests that cancer originates from tumor cells with stem cell-like characteristics (the cancer stem cell hypothesis). A stem cell origin may explain the morphologic and functional heterogeneity detected in most tumors. However, alternative theories that may explain some of the intriguing features of refractory tumors have been presented. One of these, the mutator phenotype hypothesis, postulates that random mutations throughout the genome of a malignant cell contribute to the heterogeneity of the tumor and will induce for instance rapid resistance to radiation and chemotherapy Citation2, Citation3. It has also been proposed that the increase in mobility and invasiveness of cancerous cells can be ascribed to epithelial-mesenchymal transition (EMT), a process that usually occurs during development Citation4, Citation5. At the moment, there is no evidence indicating that one of these theories must exclude the others.
Abbreviations
The cancer stem cell hypothesis postulates that tumors arise from specific tumor-initiating cells, also known as cancer stem cells, which possess unique stem cell characteristics that render them resistant to conventional cancer modalities. Presently used means of cancer treatment would thus leave the cancer stem cells unharmed and allow them to induce regrowth of the tumor after seemingly effective treatment. Radiation therapy and most of the currently available chemotherapeutic agents target mainly replicating cells, whereas non-cycling, quiescent cancer stem cells are expected to sustain much less damage Citation6, Citation7. Indeed, adult stem cells demonstrate a remarkable resistance to radiation and chemotherapy, a trait shared also by proposed tumor-initiating cells Citation8. In part, this is due to the high level of anti-apoptotic proteins expressed by stem cells, which reduce the tendency for apoptosis following radiotherapy and chemotherapy Citation9, Citation10. Due to their quiescent stem cell nature, putative cancer stem cells also remain unaffected by hormonal therapy and small molecular inhibitors that halt the cycling of tumor cells. In addition, adult stem cells express high levels of multidrug transporters, which exclude cytotoxic drugs from the cell Citation11, Citation12. Cancer stem cells are thus expected to expel antitumor drugs efficiently Citation13. New approaches are obviously needed that target specifically tumor-inducing cancer stem cells in order to minimize tumor relapses. Oncolytic virotherapy represent a promising approach to target and kill the putative cancer stem cells, while leaving normal somatic stem cells unharmed. In the following sections we will review the current literature in support of tissue-specific cancer stem cells and discuss the potential of using oncolytic viruses for their elimination.
Key messages
Accumulating evidence indicates that cancer is initiated and maintained by a small population of malignant cells with stem cell-like properties, including self-renewal and pluripotency.
Tumor-initiating cells, also referred to as cancer stem cells, are strikingly resistant to radiation and chemotherapy, which render them unharmed by most currently used modes of cancer treatment. Following seemingly successful cancer therapy, quiescent cancer stem cells may reactivate and repopulate the tumor.
Oncolytic adenoviruses are not susceptible to cancer stem cell resistance mechanisms and constitute a promising approach for elimination of tumor-initiating cells. Oncolytic adenoviruses can be modified to replicate specifically in tumor cells while leaving normal cells unharmed. Experimental data implies that oncolytic adenoviruses have the potential to kill tumor-initiating cells, in addition to the bulk of cancerous cells that make up the tumor.
Adult stem cells and the stem cell niche
The ability of adult tissue to regenerate itself after injury or disease, or to replace worn out cells, is maintained by tissue-specific stem cells. Stem cells are found at specific locations in the tissues, so-called stem cell niches, where they lie quiescent surrounded by mature cells Citation14. The stem cell niche constitutes a unique microenvironment that bears great importance in maintaining stem cell homeostasis by regulating stem cell proliferation Citation15–17. Activation of the stem cell is tightly regulated by different signaling pathways such as Wnt, Sonic hedgehog (Shh), Notch, Octamer-4 (Oct-4), bone morphogenetic protein (BMP), and Janus family kinase (JFK), which are normally involved in embryonic development Citation18–20. Dysfunction of these signaling pathways has been detected in several types of cancer Citation21, Citation22.
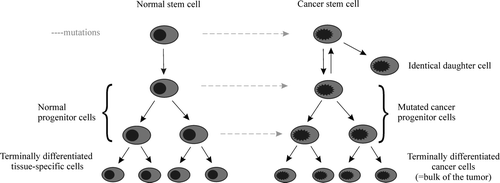
A stem cell is characterized by its ability to self-renew and to produce most of the mature cell types of the specific tissue (unlimited potency). Upon activation, the stem cell undergoes asymmetric cell division producing one identical daughter cell with self-renewal characteristics and one early transient-amplifying cell (early TA), which possesses high proliferative capability. Through a series of rapid cell divisions, the early TA cell gives rise to late TA cells followed by tissue-specific progenitor cells and finally to the bulk of differentiated cells that make up the organ or tissue. The slow cycling of the stem cell protects it from the genetically dangerous task of multiple cell divisions and promotes long-time survival. Instead, the TA cells are faced with the risky endeavor of multiple cell replications. The lifetime of the TA cell is limited, and it is repeatedly replaced by a new expendable TA cell.
Asymmetric cell division does not increase the number of stem cells. The expansion of the stem cell population that is observed during development or in response to injury is instead achieved through symmetric cell divisions Citation23, which yield two identical, un-differentiated daughter cells.
The cancer stem cell hypothesis
The idea that tumors arise from cells with stem cell-like features is far from new. Already some 100 years ago, the German pathologist Julius Cohnheim came up with the revolutionary hypothesis that tumors are derived from what he called ‘embryonal cell rests’, i.e. cells with stem cell-like properties Citation24. However, it was not until the 1960s that the idea got more concrete support when Till and McCulloch through their ground-breaking work on stem cells proposed that tissue-specific stem cells can induce cancer Citation25. Work by Pierce in the beginning of the 1970s on mouse teratocarcinomas led him to speculate that tumors contain a small number of malignant stem cells that maintain stem cell function and give rise to progenitor cells with various functions and degree of differentiation Citation26.
The current definition of a tumor-initiating cancer stem cell is a cell that bears the ability to undergo asymmetric cell division, i.e. self-renew, and the ability to produce the heterogeneous mixture of cells that comprise a tumor Citation27 (). The tumor-initiating cell may originate from tissue or organ-specific stem cells or the immediate progeny, which have dysfunctions in the normally tight regulation of self-renewal and differentiation Citation21, Citation22. Alternatively, cancer stem cells may arise from progenitor cells that attain the ability of self-perpetuation through alterations in major signaling pathways like the Wnt pathway Citation28. Only a minute fraction of the total number or malignant cells that make up a tumor is expected to be cancer stem cells.
Figure 1. The cancer stem cell hypothesis ascribes tumor initiation and growth to a subset of cancer stem cells, with features that resemble normal stem cells. Cancer stem cells are thought to originate from tissue or organ-specific stem cells or the immediate progeny through mutations that alter their normally tight regulation of self-renewal. Activation of quiescent cancer stem cells induces asymmetric cell division, which yields one identical daughter cell with stem cell-like characteristics and one early transiently amplifying cell, which in turn are responsible for the bulk of dividing cells that produce late transiently amplifying cells, tissue-specific progenitor cells, and finally the differentiated cells of the tissue. The cancer stem cell and its identical daughter cell are resistant to currently used means of cancer treatment. The bulk of tumor cells is not immortal and is hence sensitive to chemotherapy and radiation.
The first solid evidence in favor of cancer stem cells came from studies of the hematopoietic system. The laboratory of John Dick eloquently showed that only a specific cell population, bearing CD34+CD38− cell surface markers, were able to induce acute myelogenous leukemia (AML) in non-obese diabetic/severe combined immunodeficient (NOD/SCID) mice, whereas a 1000-fold higher number of other cells not featuring these cellular markers were non-tumorigenic Citation29, Citation30. The CD34+CD38− cell population, which constituted less than 0.01% of all leukemia cells, displayed clonogenic features, since the cell population could be passed from animal to animal while retaining the same AML phenotype Citation31, Citation32.
Approximately 10 years after the initial reports on tumor-initiating cells, Al-Hajj et al. were able to isolate putative cancer stem cells from solid tumors Citation33. The proposed breast cancer stem cells displayed stem cell features such as an extended pluripotency and were found in the CD44+CD24−/low population isolated from breast cancer patients Citation33. The cells expressing the CD44+CD24−/low profile together with epithelial specific antigen (ESA) on the cell surface were highly tumorigenic. Only 200 of these cells were enough to induce new tumors in NOD/SCID mice, whereas up to 20,000 cells lacking the CD44+CD24−/low phenotype were not tumorigenic Citation33. In addition, CD44+CD24−/low cells were able to form clonogenic tumors through multiple in vivo passages in immunodeficient mice. Normal mammary stem/progenitor cells grow in vitro as non-adherent spherical cell clusters, so-called mammospheres, and the same behavior was detected in cultures of the tumor-initiating CD44+CD24−/low cells Citation34. The CD44+CD24−/low cell population constituted approximately 10% of the total malignant cell population Citation33. The number is higher than could be expected based on xenograft studies. It is therefore crucial to remember that not all of the cells expressing this marker profile will necessarily be true cancer stem cells. Instead the true stem cell population can be found among the cells expressing these specific markers. Recently, it was suggested that an increased activity of aldehyde dehydrogenase can be used as a marker for the stem cell phenotype Citation35. The breast tumor specimens that engrafted in mice were obtained mainly from metastatic pleural effusion Citation33, which suggests a pleural effusion enrichment in tumor-initiating CD44+CD24−/low cells.
With the improvement in methods of stem cell isolation and identification, putative cancer stem cells have been reported from several organs and tissues. Human brain tumors were shown to harbor a tumor-initiating cell population that expresses the characteristic neural stem cell markers CD133 and nestin Citation36, Citation37. As few as 100 CD133+ cells were capable of inducing brain tumors in NOD/SCID mice upon injection into the forebrain, whereas 100,000 CD133− cells did not cause tumor induction Citation36, Citation37. Thus far, the presence of tumor-initiating, clonogenic cells has been shown in prostate cancer Citation38, ovarian cancer Citation39, liver cancer Citation40, melanoma Citation41, sarcoma Citation42, myeloma Citation43, colorectal cancer Citation44, Citation45, pancreatic cancer Citation46, and head and neck cancer Citation47. Interestingly, many of the surface markers on the putative tissue-specific tumor-initiating cells are common to several types of cancer stem cells, including CD44, CD133, α6, and β1 integrin.
Cancer stem cells display several stem cell features in addition to the ability to perpetuate themselves and the unlimited differentiation capability. These include active expression of telomerase, inactivation of apoptotic pathways, and increase in the activity of membrane transporters, as well as the ability to migrate and thus metastasize Citation48. Development of metastatic breast cancer may include dissemination of tumor cells to the bone marrow, an event associated with a poor outcome. A recent study indicates that cancer stem cells are highly represented among the early disseminating tumor cells Citation49. The inactivation of apoptotic pathways is due to a high expression of anti-apoptotic proteins, through which the cancer stem cells escape apoptosis initiated by radiation and chemotherapy Citation9, Citation10. Moreover, cancer stem cells are thought to express adenosine 5′-triphosphate (ATP)-binding cassette (ABC) drug transporters such as the multidrug resistance protein (mdr) Citation50, which actively pump cytotoxic drugs out of the cell. Normally, lipophilic fluorescent dyes like Hoechst 33342 and rhodamine 123 accumulate in the cytoplasm of the cells. However, the membrane transporters of stem cells efficiently exclude such dyes Citation13. The cancer stem cell population can hence be isolated as the ‘side population’ that is not stained by the dye. The resistance of cancer stem cells towards current cancer modalities can lead to an enrichment of tumor-initiating cells compared to other malignant cells upon chemotherapy. For example, neoadjuvant chemotherapy of breast cancer patients was recently shown to noticeably enrich the tumor cell population for cells expressing the CD44+CD24−/low phenotype Citation51. The resistance of cancer stem cells towards commonly used means of cancer therapy represents a great obstacle in the treatment of tumors. The risk for regrowth of the tumor is evident, even after apparently successful treatments, if cancer stem cells are left unharmed. New strategies are needed for targeting tumor-initiating cells, in addition to the bulk of malignant cells that make up a tumor. A promising approach is the use of oncolytic viruses for targeted elimination of cancer stem cells. In the following sections, we will discuss the possibility of adenoviral-based virotherapy for targeting cancer cells and cancer stem cells in particular.
Oncolytic adenoviruses for treatment of cancer
The structural and biological properties of adenoviruses have been studied since their discovery in the early 1950s. Several inherent characteristics have been found that make adenoviruses suitable for virotherapy. The members of the family of Adenoviridae normally cause mild infection of the upper respiratory tract or the eyes, or gastroenteritis. Still, adenoviruses can infect a great variety of dividing and non-dividing cells. An adenoviral vector is unlikely to cause unwanted mutations of host DNA, since adenoviral DNA is not integrated into the host genome. In addition, the structure of the virus is stable, and it can be produced at high titers according to good manufacturing practices. Moreover, the safety record of adenoviral-based gene therapy for cancer is very good, with more than 15,000 patients treated thus far without reported mortality due to treatment Citation52.
The human adenovirus is composed of an up to 38 kb linear DNA genome encircled by a non-enveloped icosahedral capsid Citation53. The virus capsid consists of three major and several minor proteins Citation54. The most abundant protein in the capsid is hexon, of which there are 720 copies per virion. Five penton monomers make up a penton base fundament that is found at all of the 12 icosahedral vertices. Each penton base anchors a trimeric fiber polymer, at the end of which a globular knob is located. The fiber knob is thought to constitute the major attachment and recognition point of the adenovirus to host cell receptors, although recently the fiber shaft has been proposed to play an important role also.
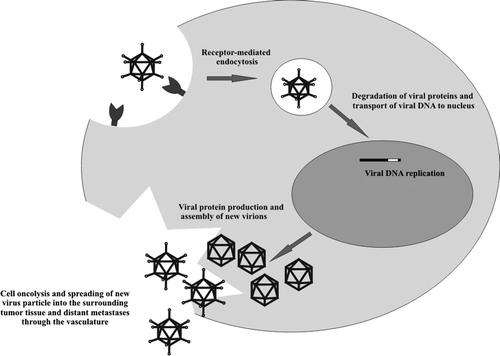
The primary cellular receptor for most adenovirus serotypes, including the commonly used serotype 5 (Ad5), is the Coxsackie-adenovirus receptor (CAR) Citation55, Citation56. Initial high-affinity binding of the fiber knob to CAR is followed by virus internalization into the cell through formation of a clathrin-coated vesicle (). The internalization is triggered by interaction between an arginine-glycine-aspartate (RGD) motif in the penton base and cellular αvβ integrins Citation57. In the cytoplasm, the clathrin-coated vesicle carrying the adenovirus is fused with endosomes, where the adenovirus is digested followed by endosomal lysis and liberation of the adenoviral DNA. Thereafter, the viral genome is transported to the nucleus and expression of viral DNA is initiated. Viral gene expression leads to the formation of numerous new virus particles, which eventually will burst and kill the cell. A wealth of newly synthesized adenoviruses is released into the surrounding tissues and vasculature where they are free to infect new cells.
Figure 2. A simplified view of the events that follow upon infection of a malignant cell by an oncolytic adenovirus (Ad). Recognition of the Ad by its primary receptor on the cell surface leads to the internalization of the virus via clathrin-coated pits. The virus is transported to endosomes, where the viral proteins are degraded and the viral DNA is released. Viral DNA is thereafter transported to the nucleus were the host cell DNA replication machinery is utilized for the production of viral DNA. Viral capsid proteins are produced in the cytosol, and a multitude of new virions are assembled from the viral protein and DNA, ultimately leading the oncolysis of the cell.
An oncolytic adenovirus is genetically altered to replicate preferentially in tumor cells. For this purpose, different types of modification strategies can be exploited. One is a so-called loss-of-function modification, where the adenoviral genome is mutated so that the virus can only replicate in cells that compensate for the feature lost by the mutation. Typically, part of the immediate-early (E1A) or early (E1B) adenoviral genes is deleted. As a consequence, the adenovirus is unable to express viral proteins that are needed for binding and inhibition of host cellular proteins that impair viral DNA replication. However, the modified adenovirus will be able to replicate in malignant cells where cell cycle pathway defects have made the binding of those viral proteins unnecessary.
Most, if not all, human cancer types are thought to be deficient in the p16/retinoblastoma tumor suppressor/cell cycle regulation (Rb) pathway, which has a central role in controlling the cell cycle Citation58. The Δ24 generation of oncolytic adenoviruses takes specifically advantage of this deficiency. Ad5-Δ24 (also known as dl922–947) has a 24-bp deletion in the constant region 2 (CR2) of E1A and is therefore unable to bind to the Rb protein and induce an S-phase-like state in normal cells Citation59, Citation60. Ad5-Δ24 will thus replicate preferentially in tumor cells where the p16/Rb-pathway is abnormal Citation59, Citation60. An optional approach for targeting oncolytic adenovirus to tumor cells is insertion of a tumor-specific promoter (TSP) into the adenoviral genome. This can restrict viral replication to malignant cells expressing transcription factors that activate the inserted promoter (reviewed in Citation61). Usually, the TSP is placed to regulate the expression of the E1A gene, but other early genes can also be subjected to control by a TSP. By combining these two types of transcriptional control, i.e. an oncolytic adenovirus with a loss-of-function mutation and TSP, further specificity can be achieved Citation62.
The oncolytic potency of a replicating adenovirus is largely determined by the capacity of the virus to enter target cells Citation63–65. The primary cellular receptor for most adenovirus serotypes, CAR, is abundantly expressed on nearly all normal epithelial cells, but is unfortunately poorly expressed, lacking, or expressed in the wrong cellular compartment in many cancer cells Citation66–77. Inadequate expression of CAR on tumor cells can be circumvented by alterations of the fiber knob, which allow the adenovirus to enter through non-CAR-dependent mechanisms.
In Ad5/3 chimeric vectors, the knob part of the Ad5 fiber has been exchanged by the knob from serotype 3. Ad3 belongs to a different subgroup of Adenoviridae and uses different receptors for cellular entry Citation67, Citation68, Citation78, Citation79. The altered tropism of Ad5/3 allows CAR-independent transduction and results in increased infection of cells expressing low amounts of CAR. Other modifications of the adenoviral capsid that improve infectivity include insertion of an integrin-binding arginine-glycine-aspartate (RGD) motif in the HI-loop of the fiber knob (Ad5-Δ24RGD) Citation69, Citation80 or insertion of seven positively charged lysine residues, preceded by a glycine-serine linker sequence, into the C-terminus of the fiber of Ad5 (Ad5.pK7-Δ24) Citation81. Additional methods for enhanced adenoviral tropism have recently been reviewed Citation69, Citation71, Citation72. Recent advances in cancer biology and virology have facilitated the development of more sophisticated oncolytic adenoviruses that can have several different mechanisms for targeting the virus to specific cancer cells combined with capsid modification for enhanced target infection.
Virotherapy targeting cancer stem cells
Cancer stem cells (in parallel to stem cells in general) are remarkably resistant to most commonly used means of cancer treatment, but they form a small minority of the cells present in the tumor. If, however, the differentiated majority of malignant cells in a tumor respond to treatment, this can result in a decrease in tumor size. Currently, most cancer drugs are approved based on response rates, instead of e.g. patient survival, and therefore the approval process may have in fact selected for agents that are effective against the wrong tumor cell population. At the moment, almost no patient diagnosed with metastasis to distant organs will benefit from available treatment strategies. The presence of therapy-resistant cancer stem cells is thought to be the reason for tumor recurrence after apparently successful treatment.
Virotherapy may, however, have the potential to target also cancer stem cells. Viruses enter cells through infection and may therefore be unaffected by the defense mechanisms that cancer stem cells exhibit. Years of work on different mouse models have shown that oncolytic adenoviruses can efficiently target and kill cancer cells in vivo. Of key importance for efficient oncolysis is the capacity for entering the target cell. It has been reported that CAR is poorly expressed on mesenchymal stem cells, suggesting that lack of CAR may constitute a problem for infectivity of also other types of stem cells Citation82, Citation83 (unpublished observations, G. Bauerschmitz, University of Helsinki, 2007). Interestingly, capsid-modified adenoviral vectors Ad5/3-Δ24, Ad5-Δ24RGD-4C, and Ad5.pK7-Δ24 are able to overcome CAR deficiency for effective gene delivery to mesenchymal stem cells Citation83.
The identification and isolation of putative cancer stem cells has provided the opportunity to test the potential of virotherapy for targeting these cells. In recent work by Eriksson et al., the ability of capsid-modified oncolytic adenoviruses to kill putative breast cancer stem cells was studied Citation84. Breast cancer stem cells are believed to reside in the CD44+/CD24−/low cell population Citation33, Citation34. Previously, injection of CD44+/CD24−/low cells into the mammary fat pads of NOD/SCID mice had been shown to induce rapid tumor formation Citation33, Citation84. Infection of the CD44+/CD24−/low cells with Ad5/3-Δ24 prior to injection, however, prevented tumor occurrence in mice during a follow-up time of 120 days Citation84. In addition, when established CD44+/CD24−/low-derived tumors were treated with Ad5/3-Δ24 or Ad5.pK7-Δ24, tumor growth was abrogated Citation84. The Ad5/3-Δ24 construct utilizes the serotype 3 receptor, abundantly expressed on many tumor types, for cellular entry, and has in addition a 24-bp deletion in the E1A gene Citation65, Citation81. The Ad5.pK7-Δ24 construct utilizes heparin sulfate proteoglycans for internalization into the cell and harbors the same E1A deletion. The oncolytic potential of these two viruses extends beyond cancer stem cell, since Ad5/3-Δ24 and Ad5.pK7-Δ24 have previously been shown to effectively kill differentiated breast cancer cells Citation81. In addition, the biodistribution of Ad5/3-Δ24 and Ad5.pK7-Δ24 in the body resembles that of Ad5, which is an adenovirus that has been safe in more than 15,000 cancer patients. The fact that Δ24 generation viruses are able to replicate in putative breast cancer stem cells suggests that defects in the p16/Rb pathway may arise early in carcinogenesis and are present in cancer stem cells, in addition to other malignant cells as previously suggested Citation58. Possible mechanisms for deregulation of this pathway in cancer stem cells include p16 methylation and overexpression of the polycomb gene Bmi1, which downregulates p16 Citation85, Citation86.
One plausible approach for targeting oncolytic adenovirus towards cancer stem cells is the use of TSPs active in cancer stem cells. Currently, little is known about promoters active in cancer stem cells. However, based on the high resistance of tumor-initiating cells towards chemotherapy and their obvious stem cell-like features, possible candidates can be nominated. These include the promoters for multidrug resistance protein (mdr), telomerase (hTERT), and cyclo-oxygenase 2 (Cox-2) (unpublished observations, G. Bauerschmitz, University of Helsinki, 2007). We have constructed oncolytic adenoviruses featuring the Ad5/3 capsid modification and mdr, hTERT and Cox-2 promoters in control of the E1A gene. The virus constructs harbored, in addition, the 24-bp deletion in the E1A gene for improved selectivity towards cancer cells. The oncolytic potential of Ad5/3-mdr-Δ24 and Ad5/3-hTERT-Δgp (featuring an additional deletion in the E3A gene) was tested in vitro on CD44+/CD24−/low breast cancer stem cells and was found to be equivalent to the efficient Ad5/3-Δ24. Moreover, the size of CD44+/CD24−/low cell-induced tumors was decreased upon intratumoral administration of Ad5/3-mdr-Δ24 and Ad5/3-Cox-2-Δ24 (unpublished observations, G. Bauerschmitz, University of Helsinki, 2007). The results imply that transcriptional targeting of oncolytic adenoviruses with TSPs may be an efficient way to eradicate cancer stem cells and that damage to normal stem cells may be avoided through transcriptional control.
Another possible approach to targeting oncolytic adenovirus towards cancer stem cells is to use single-chain monoclonal antibodies or other bispecific adapter molecules through which the virus can attach to a specific cell surface protein Citation87. Using this strategy, an oncolytic adenovirus with a truncated, soluble form of CAR fused to the epidermal growth factor receptor (EGFR) was constructed, which target the virus to tumor cells that overexpress EGFR Citation64, Citation88. Recently, Carette and co-workers engineered an oncolytic adenovirus that upon replication in cancer cells produces progenitor virions, which are targeted to EGFR Citation89.
The ability of adenoviral vectors to infect putative tumor-initiating cells is not limited only to breast cancer stem cells. Ad serotype 16 and the chimpanzee Ad CV23 have recently been shown to effectively infect low-passage brain tumor cell lines, as well as CD133+ and CD133− tumor cells Citation90. Brain tumor stem cells have been proposed to reside in the cell population bearing the neural stem cell marker CD133+ Citation36, Citation37. Interestingly, putative brain tumor stem cells have recently been reported to be susceptible to Ad5-Δ24RGD Citation91. Hematopoietic and mesenchymal stem cells have, in addition, been found to be efficiently transduced by different types of modified adenoviruses Citation83, Citation92. Therefore, it is important to abrogate replication in normal tissue stem cells by utilizing tight transcriptional control. Moreover, normal tissue stem cells might have protective measures against adenoviruses, since most humans have been infected or even treated with numerous wild-type adenovirus strains without severe toxicity Citation93. Because tumors seem inherently more susceptible to adenoviruses than normal cells Citation93, some of these protective measures may be lost during carcinogenesis, and theoretically this might be an early event present already in cancer stem cells. Studies are needed to investigate these aspects further.
Final remarks
In our opinion, virotherapy with oncolytic adenoviruses has great promise for treating tumors resistant to available treatment modalities. Still, several problems need to be overcome in order to increase treatment efficacy. In vivo, any antitumor approach will encounter obstacles not present in vitro. Stromal cells and matrix, necrotic, hypoxic, and hyperbaric tissue may limit the efficient dissemination of viruses and other agents within tumors. With regard to adenovirus, another problem is bioavailability following systemic administration. Intravenously administered adenovirus tends to accumulate into the liver (at least in mice), and tissue-specific macrophages, such as Kupffer cells, efficiently remove adenovirus particles from the blood Citation74, Citation94, Citation95.
Nevertheless, an urgent need for novel antitumor approaches is evident, since chemotherapy and other conventional therapy forms are not likely to significantly improve patient survival further. Thus, agents that target cancer stem cells may have great potential. Oncolytic adenoviruses have indeed been shown to be efficient not only against differentiated tumor cells, but also against tumor-initiating cells. Moreover, virotherapy can sensitize tumor cells to radiation and chemotherapy, and combination treatments may hence have strong antitumor effects Citation71. In conclusion, oncolytic virotherapy has potential for increasing treatment options for patients with currently incurable cancer.
Acknowledgements
This work was supported by EU FP6 THERADPOX and APOTHERAPY, HUCH Research Funds (EVO), Sigrid Jusélius Foundation, Academy of Finland, Emil Aaltonen Foundation, Biocentrum Helsinki and the Finnish Cancer Society. AH is K Albin Johansson Research Professor of the Finnish Cancer Institute.
References
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000; 100: 57–70
- Loeb LA, Loeb KR, Anderson JP. Multiple mutations and cancer. Proc Natl Acad Sci USA. 2003; 100: 776–81
- Bielas JH, Loeb KR, Rubin BP, True LD, Loeb LA. Human cancers express a mutator phenotype. Proc Natl Acad Sci U S A. 2006; 103: 18238–42
- Fidler IJ. The pathogenesis of cancer metastasis: the'seed and soil' hypothesis revisited. Nat Rev Cancer. 2003; 3: 453–8
- Mani SA, Yang J, Brooks M, Schwaninger G, Zhou A, Miura N, et al. Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis and is associated with aggressive basal-like breast cancers. Proc Natl Acad Sci U S A. 2007; 104: 10069–74
- Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005; 5: 275–84
- Eckfeldt CE, Mendenhall EM, Verfaillie CM. The molecular repertoire of the'almighty' stem cell. Nat Rev Mol Cell Biol. 2005; 6: 726–37
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001; 414: 105–11
- Peters R, Leyvraz S, Perey L. Apoptotic regulation in primitive hematopoietic precursors. Blood. 1998; 92: 2041–52
- Domen J, Gandy KL, Weissman IL. Systemic overexpression of BCL-2 in the hematopoietic system protects transgenic mice from the consequences of lethal irradiation. Blood. 1998; 91: 2272–82
- Chaudhary PM, Roninson IB. Expression and activity of P-glycoprotein, a multidrug efflux pump, in human hematopoietic stem cells. Cell. 1991; 66: 85–94
- Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996; 183: 1797–806
- Hirschmann-Jax C, Foster AE, Wulf GG, Nuchtern JG, Jax TW, Gobel U, et al. A distinct ‘side population’ of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci U S A. 2004; 101: 14228–33
- Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978; 4: 7–25
- Xie T, Spradling AC. A niche maintaining germ line stem cells in the Drosophila ovary. Science. 2000; 290: 328–30
- Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003; 425: 836–41
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, et al. Defining the epithelial stem cell niche in skin. Science. 2004; 303: 359–63
- Dontu G, Jackson KW, McNicholas E, Kawamura MJ, Abdallah WM, Wicha MS. Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res. 2004; 6: 605–15
- Liu S, Dontu G, Wicha MS. Mammary stem cells, self-renewal pathways, and carcinogenesis. Breast Cancer Res. 2005; 7: 86–95
- Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005; 434: 843–50
- Kubo M, Nakamura M, Tasaki A, Yamanaka N, Nakashima H, Nomura M, et al. Hedgehog signaling pathway is a new therapeutic target for patients with breast cancer. Cancer Res. 2004; 64: 6071–4
- Tai MH, Chang CC, Kiupel M, Webster JD, Olson LK, Trosko JE. Oct4 expression in adult human stem cells: evidence in support of the stem cell theory of carcinogenesis. Carcinogenesis. 2005; 26: 495–502
- Morrison SJ, Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature. 2006; 441: 1068–74
- Houghton J, Morozov A, Smirnova I, Wang TC. Stem cells and cancer. Semin Cancer Biol. 2007; 17: 191–203
- Till JE, McCulloch EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961; 14: 213–22
- Pierce GB. Teratocarcinoma: model for a developmental concept of cancer. Curr Top Dev Biol. 1967; 2: 223–46
- Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, et al. Cancer stem cells—perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006; 66: 9339–44
- Jamieson CH, Ailles LE, Dylla SJ, Muijtjens M, Jones C, Zehnder JL, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med. 2004; 351: 657–67
- Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994; 367: 645–8
- Vormoor J, Lapidot T, Pflumio F, Risdon G, Patterson B, Broxmeyer HE, et al. Immature human cord blood progenitors engraft and proliferate to high levels in severe combined immunodeficient mice. Blood. 1994; 83: 2489–97
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997; 3: 730–7
- Dick JE. Normal and leukemic human stem cells assayed in SCID mice. Semin Immunol. 1996; 8: 197–206
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003; 100: 3983–8
- Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, et al. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005; 65: 5506–11
- Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007; 1: 555–67
- Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003; 63: 5821–8
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004; 432: 396–401
- Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005; 65: 10946–51
- Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, Dinulescu DM, Connolly D, Foster R, et al. Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian Inhibiting Substance responsiveness. Proc Natl Acad Sci U S A. 2006; 103: 11154–9
- Ma S, Chan KW, Hu L, Lee TK, Wo JY, Ng IO, et al. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007; 132: 2542–56
- Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S, et al. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005; 65: 9328–37
- Gibbs CP, Kukekov VG, Reith JD, Tchigrinova O, Suslov ON, Scott EW, et al. Stem-like cells in bone sarcomas: implications for tumorigenesis. Neoplasia. 2005; 7: 967–76
- Matsui W, Huff CA, Wang Q, Malehorn MT, Barber J, Tanhehco Y, et al. Characterization of clonogenic multiple myeloma cells. Blood. 2004; 103: 2332–6
- O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007; 445: 106–10
- Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A. 2007; 104: 10158–63
- Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007; 67: 1030–7
- Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007; 104: 973–8
- Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea—a paradigm shift. Cancer Res. 2006; 66: 1883–90
- Balic M, Lin H, Young L, Hawes D, Giuliano A, McNamara G, et al. Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype. Clin Cancer Res. 2006; 12: 5615–21
- Mickley LA, Spengler BA, Knutsen TA, Biedler JL, Fojo T. Gene rearrangement: a novel mechanism for MDR-1 gene activation. J Clin Invest. 1997; 99: 1947–57
- Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong C, et al. Let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007; 131: 1109–23
- Kanerva A, Raki M, Hemminki A. Gene therapy of gynaecological diseases. Expert Opin Biol Ther. 2007; 7: 1347–61
- Russell WC. Update on adenovirus and its vectors. J Gen Virol. 2000; 81: 2573–604
- Fabry CM, Rosa-Calatrava M, Conway JF, Zubieta C, Cusack S, Ruigrok RW, et al. A quasi-atomic model of human adenovirus type 5 capsid. EMBO J. 2005; 24: 1645–54
- Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, et al. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997; 275: 1320–3
- Tomko RP, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci U S A. 1997; 94: 3352–6
- Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993; 73: 309–19
- Sherr CJ. Cancer cell cycles. Science. 1996; 274: 1672–7
- Fueyo J, Gomez-Manzano C, Alemany R, Lee PS, McDonnell TJ, Mitlianga P, et al. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene. 2000; 19: 2–12
- Heise C, Hermiston T, Johnson L, Brooks G, Sampson-Johannes A, Williams A, et al. An adenovirus E1A mutant that demonstrates potent and selective systemic anti-tumoral efficacy. Nat Med. 2000; 6: 1134–9
- Saukkonen K, Hemminki A. Tissue-specific promoters for cancer gene therapy. Expert Opin Biol Ther. 2004; 4: 683–96
- Bauerschmitz GJ, Guse K, Kanerva A, Menzel A, Herrmann I, Desmond RA, et al. Triple-targeted oncolytic adenoviruses featuring the cox2 promoter, E1A transcomplementation, and serotype chimerism for enhanced selectivity for ovarian cancer cells. Mol Ther. 2006; 14: 164–74
- Douglas JT, Kim M, Sumerel LA, Carey DE, Curiel DT. Efficient oncolysis by a replicating adenovirus (ad) in vivo is critically dependent on tumor expression of primary ad receptors. Cancer Res. 2001; 61: 813–7
- Hemminki A, Dmitriev I, Liu B, Desmond RA, Alemany R, Curiel DT. Targeting oncolytic adenoviral agents to the epidermal growth factor pathway with a secretory fusion molecule. Cancer Res. 2001; 61: 6377–81
- Kanerva A, Zinn KR, Chaudhuri TR, Lam JT, Suzuki K, Uil TG, et al. Enhanced therapeutic efficacy for ovarian cancer with a serotype 3 receptor-targeted oncolytic adenovirus. Mol Ther. 2003; 8: 449–58
- Dmitriev I, Krasnykh V, Miller CR, Wang M, Kashentseva E, Mikheeva G, et al. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J Virol. 1998; 72: 9706–13
- Kanerva A, Mikheeva GV, Krasnykh V, Coolidge CJ, Lam JT, Mahasreshti PJ, et al. Targeting adenovirus to the serotype 3 receptor increases gene transfer efficiency to ovarian cancer cells. Clin Cancer Res. 2002; 8: 275–80
- Kanerva A, Wang M, Bauerschmitz GJ, Lam JT, Desmond RA, Bhoola SM, et al. Gene transfer to ovarian cancer versus normal tissues with fiber-modified adenoviruses. Mol Ther. 2002; 5: 695–704
- Bauerschmitz GJ, Lam JT, Kanerva A, Suzuki K, Nettelbeck DM, Dmitriev I, et al. Treatment of ovarian cancer with a tropism modified oncolytic adenovirus. Cancer Res. 2002; 62: 1266–70
- Anders M, Hansen R, Ding RX, Rauen KA, Bissell MJ, Korn WM. Disruption of 3D tissue integrity facilitates adenovirus infection by deregulating the coxsackievirus and adenovirus receptor. Proc Natl Acad Sci U S A. 2003; 100: 1943–8
- Kanerva A, Hemminki A. Adenoviruses for treatment of cancer. Ann Med. 2005; 37: 33–43
- Raki M, Rein DT, Kanerva A, Hemminki A. Gene transfer approaches for gynecological diseases. Mol Ther. 2006; 14: 154–63
- Kangasniemi L, Kiviluoto T, Kanerva A, Raki M, Ranki T, Sarkioja M, et al. Infectivity-enhanced adenoviruses deliver efficacy in clinical samples and orthotopic models of disseminated gastric cancer. Clin Cancer Res. 2006; 12: 3137–44
- Sarkioja M, Kanerva A, Salo J, Kangasniemi L, Eriksson M, Raki M, et al. Noninvasive imaging for evaluation of the systemic delivery of capsid-modified adenoviruses in an orthotopic model of advanced lung cancer. Cancer. 2006; 107: 1578–88
- Rajecki M, Kanerva A, Stenman UH, Tenhunen M, Kangasniemi L, Sarkioja M, et al. Treatment of prostate cancer with Ad5/3Delta24hCG allows non-invasive detection of the magnitude and persistence of virus replication in vivo. Mol Cancer Ther. 2007; 6: 742–51
- Guse K, Dias JD, Bauerschmitz GJ, Hakkarainen T, Aavik E, Ranki T, et al. Luciferase imaging for evaluation of oncolytic adenovirus replication in vivo. Gene Ther. 2007; 14: 902–11
- Guse K, Ranki T, Ala-Opas M, Bono P, Sarkioja M, Rajecki M, et al. Treatment of metastatic renal cancer with capsid-modified oncolytic adenoviruses. Mol Cancer Ther. 2007; 6: 2728–36
- Havenga MJ, Lemckert AA, Ophorst OJ, van Meijer M, Germeraad WT, Grimbergen J, et al. Exploiting the natural diversity in adenovirus tropism for therapy and prevention of disease. J Virol. 2002; 76: 4612–20
- Shayakhmetov DM, Li ZY, Ni S, Lieber A. Targeting of adenovirus vectors to tumor cells does not enable efficient transduction of breast cancer metastases. Cancer Res. 2002; 62: 1063–8
- Suzuki K, Fueyo J, Krasnykh V, Reynolds PN, Curiel DT, Alemany R. A conditionally replicative adenovirus with enhanced infectivity shows improved oncolytic potency. Clin Cancer Res. 2001; 7: 120–6
- Ranki T, Kanerva A, Ristimaki A, Hakkarainen T, Sarkioja M, Kangasniemi L, et al. A heparan sulfate-targeted conditionally replicative adenovirus, Ad5.pk7-Delta24, for the treatment of advanced breast cancer. Gene Ther. 2007; 14: 58–67
- Mizuguchi H, Sasaki T, Kawabata K, Sakurai F, Hayakawa T. Fiber-modified adenovirus vectors mediate efficient gene transfer into undifferentiated and adipogenic-differentiated human mesenchymal stem cells. Biochem Biophys Res Commun. 2005; 332: 1101–6
- Hakkarainen T, Sarkioja M, Lehenkari P, Miettinen S, Ylikomi T, Suuronen R, et al. Human mesenchymal stem cells lack tumor tropism but enhance the antitumor activity of oncolytic adenoviruses in orthotopic lung and breast tumors. Hum Gene Ther. 2007; 18: 627–41
- Eriksson M, Guse K, Bauerschmitz G, Virkkunen P, Tarkkanen M, Tanner M, et al. Oncolytic Adenoviruses Kill Breast Cancer Initiating CD44+CD24−/Low Cells. Mol Ther. 2007; 15: 2088–93
- Merlo A, Herman JG, Mao L, Lee DJ, Gabrielson E, Burger PC, et al. 5′ CpG island methylation is associated with transcriptional silencing of the tumour suppressor p16/CDKN2/MTS1 in human cancers. Nat Med. 1995; 1: 686–92
- Jacobs JJ, Kieboom K, Marino S, DePinho RA, van Lohuizen M. The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature. 1999; 397: 164–8
- Dmitriev I, Kashentseva E, Rogers BE, Krasnykh V, Curiel DT. Ectodomain of coxsackievirus and adenovirus receptor genetically fused to epidermal growth factor mediates adenovirus targeting to epidermal growth factor receptor-positive cells. J Virol. 2000; 74: 6875–84
- Hemminki A, Wang M, Hakkarainen T, Desmond RA, Wahlfors J, Curiel DT. Production of an EGFR targeting molecule from a conditionally replicating adenovirus impairs its oncolytic potential. Cancer Gene Ther. 2003; 10: 583–8
- Carette JE, Graat HC, Schagen FH, Mastenbroek DC, Rots MG, Haisma HJ, et al. A conditionally replicating adenovirus with strict selectivity in killing cells expressing epidermal growth factor receptor. Virology. 2007; 361: 56–67
- Skog J, Edlund K, Bergenheim AT, Wadell G. Adenoviruses 16 and CV23 Efficiently Transduce Human Low-passage Brain Tumor and Cancer Stem Cells. Mol Ther. 2007; 15: 2140–5
- Jiang H, Gomez-Manzano C, Aoki H, Alonso MM, Kondo S, McCormick F, et al. Examination of the therapeutic potential of Delta-24-RGD in brain tumor stem cells: role of autophagic cell death. J Natl Cancer Inst. 2007; 99: 1410–4
- Shayakhmetov DM, Papayannopoulou T, Stamatoyannopoulos G, Lieber A. Efficient gene transfer into human CD34+ cells by a retargeted adenovirus vector. J Virol. 2000; 74: 2567–83
- Smith RR, Huebner RJ, Rowe WP, Schatten WE, Thomas LB. Studies on the use of viruses in the treatment of carcinoma of the cervix. Cancer. 1956; 9: 1211–8
- Alemany R, Suzuki K, Curiel DT. Blood clearance rates of adenovirus type 5 in mice. J Gen Virol. 2000; 81: 2605–9
- Ranki T, Sarkioja M, Hakkarainen T, von Smitten K, Kanerva A, Hemminki A. Systemic efficacy of oncolytic adenoviruses in imagable orthotopic models of hormone refractory metastatic breast cancer. Int J Cancer. 2007; 121: 165–74