Abstract
Background. Obese youths may be susceptible to develop the metabolic syndrome (MS) later in life.
Aim. To study childhood predictors of MS in adulthood.
Method. Prospective cohort study including 2,195 subjects, aged 3–18 years at base-line in 1980, who were re-examined in 1983, 1986, and 2001.
Results. In adults (aged 24–39 years) in 2001, the prevalence of MS (using the International Diabetes Federation criteria) was 19% in men and 12% in women. Multivariable logistic regression model selected obesity, male sex, high triglycerides, high insulin, high C-reactive protein (CRP), and family history of hypertension and type 2 diabetes, as independent predictors of adult MS. Youth obesity (body mass index (BMI)>80th age- and sex-specific percentile) was the strongest risk factor for MS. During the 21-year follow-up, there was an increasing trend in BMI, insulin, systolic blood pressure, and triglycerides, and a decreasing trend in high-density lipoprotein cholesterol in obese subjects who developed MS in adulthood compared to those obese subjects who did not develop MS.
Conclusions. Youth determinants of adult MS included obesity, high triglycerides, high insulin, high CRP, and family history of hypertension and type 2 diabetes. Identifying these risk factors at an early stage could help identifying children and adolescence at greater risk of developing MS later in life.
Introduction
Cardiovascular diseases are the leading causes of mortality in the adult population worldwide. A constellation of cardiovascular risk factors including central obesity, hypertension, dyslipidemia, and impaired glucose metabolism with insulin resistance is commonly called the metabolic syndrome Citation1. The clustering of metabolic risk factors begins already in childhood Citation2–4 and these multiple risk factors tend to persist from childhood into adulthood Citation5. Nevertheless, overall this cluster tracking may not be particularly strong. We have previously shown in the Young Finns cohort that on average only about 25% of children and young adults initially at high risk (with all three risk factors, serum total cholesterol, high-density lipoprotein (HDL) cholesterol, and diastolic blood pressure in the extreme tertile) remained there after 6 years Citation6. In the 3-year follow-up of the Princeton School District Study, approximately half of adolescents with base-line metabolic syndrome lost the diagnosis at follow-up regardless of the definitions used Citation7. These observations indicate that there may be substantial instability in the diagnosis of the metabolic syndrome. Dichotomous approach may also exclude too much clinically important information. Thus, the clinical utility of the diagnosis may be reduced in young people, and alternative approaches are needed in primary prevention to identify young people who are at risk of developing the metabolic syndrome as adults.
Abbreviations
Obesity is strongly associated with the insulin resistance and it increases the risk of cardiovascular disease morbidity in adults Citation8. Previously, the Harvard Alumni Health Study has shown a direct correlation between body weight and mortality Citation9. The prevalence of obesity is increasing worldwide in all age groups, including children and adolescents. According to the recent epidemiological studies, there has been a 3-fold increase in the prevalence of overweight in US children and adolescents during the past two decades Citation10. Ogden et al. Citation11 have shown that 17.1% of 2–19-year-old US children are overweight. Overweight children are at increased risk for adult obesity Citation12, Citation13. We have previously reported that the risk of being obese in adulthood (body mass index (BMI) > 30 kg/m2) was increased by 3-fold in overweight or obese (BMI > 80th percentile) children (ages 3–9 years) and by 4-fold in overweight or obese youths (ages 12–18 years) Citation12. Alarmingly, the incidence of type 2 diabetes and other morbidities reported in obese children has increased markedly in the past 20 years. Obesity is causally related to the metabolic syndrome. Weiss et al. Citation14 have examined the effects of varying degrees of obesity on the prevalence of the metabolic syndrome in a large cohort of children and adolescents. They observed that the prevalence of the metabolic syndrome increased and each element of the syndrome worsened directly with the degree of obesity. However, not all obese youths will go on to develop the metabolic syndrome. The pathophysiological mechanisms that predispose some obese individuals to develop the metabolic syndrome are not fully understood.
Key messages
Childhood determinants of the adult metabolic syndrome included obesity, family history of hypertension, family history of type 2 diabetes, high triglycerides, high insulin, and high C-reactive protein.
Identifying these risk factors in children and adolescents could be helpful in pediatric metabolic risk assessment.
In the present analysis, we used longitudinal data from the Cardiovascular Risk in Young Finns Study to examine the association of childhood and adolescence risk variables and adulthood metabolic syndrome. In addition, to gain insight of the changes in risk profile during maturation in obese individuals who develop the adult metabolic syndrome, we compared serial changes in metabolic variables and life-style from childhood to young adulthood between obese individuals who developed and those who did not develop metabolic syndrome in adulthood.
Materials and methods
Subjects
The Cardiovascular Risk in Young Finns Study is an on-going epidemiological study of cardiovascular disease risk factors and their determinants from childhood to adulthood. The original sample size was 4,320 healthy Caucasian children and adolescents aged 3, 6, 9, 12, 15, and 18 years, and a total of 3,596 (83.2%) subjects participated in the first cross-sectional study in 1980. The follow-up studies were performed for the whole study group in 1983, 1986, and 2001, when 2,991 (83.2%), 2,799 (78.3%), and 2,624 (75.9%) subjects participated. Details of the data collection and the study design have been presented previously Citation15. The analyses were restricted to non-pregnant subjects. In the present analysis, our study population with complete data included 2,195 subjects (1,014 men and 1,181 women) aged 3 to 18 years in 1980, and 24 to 39 years in 2001.
Clinical characteristics
Height and weight were measured, and body mass index (BMI) was calculated. In 1980, 1983, and 1986, biceps, triceps, and subscapular skinfold thicknesses were measured in triplicate to the nearest millimeter from the non-dominating arm using Harpenden skinfolds calipers (Holtain and Bull, British Indicators Ltd, Luton, Beds, UK). Their sum variable was used in statistical analysis. Blood pressure was measured from the brachial artery with a standard mercury sphygmomanometer in 1980 and 1983, and with a random-zero sphygmomanometer in 1986 and 2001. The average of three measurements was used in statistical analysis.
Data on smoking, alcohol consumption, physical activity, family history of coronary heart disease, hypertension, or type 2 diabetes, and socio-economic status were obtained from questionnaires. The data on breast-feeding and birth measurements (height and weight) were obtained from the parents by a questionnaire in 1983 when the subjects were 6 to 21 years old. The information on smoking habits was collected in subjects aged 12 years or older. Subjects smoking on a weekly basis or more often were considered as smokers, and those reporting consuming beer, wine, or liquor once a week or more often were classified as regular users of alcohol. Physical activity measurements were done in 1980 for subjects at 9 years or older. The physical activity index was calculated as previously explained Citation16.
A positive family history was defined as mother and/or father having coronary heart disease, hypertension, or diabetes mellitus. Metabolic syndrome in 2001 was defined using the International Diabetes Federation (IDF) Citation17, the National Institute of Health Adult Treatment Panel III (NCEP) Citation18, and the European Group for the Study of Insulin Resistance (EGIR) Citation19 guide-lines. The data are shown using the IDF criteria, but similar results were seen using any of the criteria. A subject's socio-economic status was determined using information on parental education, occupation, and family's income as previously described Citation20.
Blood samples
For the determination of serum lipid levels, venous blood samples were drawn after an overnight fast. All of the lipid determinations were done using standard methods Citation21, Citation22. In 1980, 1983, and 1986 serum insulin was measured using a modification of the immunoassay method of Herbert et al. Citation23. In 2001, serum insulin was measured by a microparticle enzyme immunoassay kit (Abbott Laboratories, Diagnostic Division, Dainabot), and glucose concentrations were analyzed enzymatically (Olympus Diagnostica GmbH, Hamburg, Germany). Childhood serum samples taken in 1980 were stored at −20°C. In 2005, they were used to determine serum high-sensitive C-reactive protein (CRP) analyzed by an automated analyzer (Olympus AU400) using a turbidimetric immunoassay kit (‘CRP-UL’ assay, Wako Chemicals, Neuss, Germany). During the storage, the samples were not thawed or refrozen. The long-term stability of CRP has been previously reported. We excluded subjects with CRP levels more than 10 mg/L. Details have been presented elsewhere Citation24.
Statistical methods
Data are presented as means±SD for continuous variables and as percentages for categorical variables, unless otherwise stated. Group comparisons were performed using t test for continuous variables and chi-square test for categorical variables. Stepwise logistic regression analysis was done to establish the independent childhood determinants of the adulthood metabolic syndrome. In multivariable model, risk factors were defined as values at or above the age- and sex-specific 80th percentile for BMI, systolic blood pressure, triglycerides, insulin, CRP, and at or below the age- and sex-specific 20th percentile for HDL cholesterol.
The primary aim was to examine study childhood determinants of the adulthood metabolic syndrome. In addition, we also present the childhood and adolescence risk factor data by the presence or absence of adulthood metabolic syndrome and by the number of adulthood metabolic syndrome components.
The longitudinal association of childhood obesity status with risk variables was assessed by repeated measures analysis of variance (adjusted for age, sex, and pubertal status) to study childhood risk factors in obese subjects with and without the metabolic syndrome in adulthood and to compare these groups at different time points. Values for triglycerides, insulin, and CRP were log-transformed before analysis because of skewed distributions.
All statistical analyses were done stratified by two groups according to the base-line age when the risk factor assessments were done—in 3–9- and 12–18-year-olds. This age stratification paralleled pubertal staging, as 85% of 12–18-year-olds at base-line were classified having puberty on-going or completed Citation25.
All tests were performed with Statistical Analysis System (SAS) version 8.1, and statistical significance was inferred at a two-tailed P < 0.05.
Results
In adulthood (year 2001), the prevalence of the metabolic syndrome (using the IDF criteria) was 19% in men and 12% in women. Subjects with the metabolic syndrome had higher waist circumference than those subjects without the syndrome (mean±SD; 100±11 cm versus 81±10 cm, P<0.0001). The base-line characteristics in 1980 of the study subjects, stratified by base-line age group, with and without IDF metabolic syndrome in adulthood are shown in . Subjects who had the metabolic syndrome in adulthood had higher skinfold thickness, BMI, systolic blood pressure, triglycerides, insulin, and lower HDL cholesterol in childhood than subjects without the metabolic syndrome. In males, the adulthood metabolic syndrome was associated with older age and higher CRP levels in youth. The metabolic syndrome in adulthood was generally more prevalent in subjects with positive family history of hypertension or type 2 diabetes.
Table I. Childhood and adolescent characteristics of study subjects with and without the IDF metabolic syndrome diagnosis in adulthood.
The levels of risk variables measured in childhood and adolescence according to the number of metabolic syndrome components measured in adulthood are shown in . Risk factor levels showed significant positive trends in BMI, skinfold thickness, blood pressure, triglycerides, insulin, and CRP, and a negative trend in HDL cholesterol with increasing number of adulthood metabolic syndrome components.
Table II. Adjusted mean levels of youth risk variables by number of IDF metabolic syndrome components present in adulthood.a
In all subjects, significant 21-year tracking correlations were observed between childhood/adolescence and adulthood metabolic risk variables. The highest Spearman's correlation coefficient was seen with HDL cholesterol (r=0.45, P<0.0001), followed by BMI (r=0.41, P<0.0001), systolic blood pressure (r=0.30, P<0.0001), triglycerides (r=0.26, P<0.0001), and glucose (r=0.17, P<0.0001, 15-year correlation).
Youth determinants of the adult metabolic syndrome
Multivariable logistic regression models were constructed to examine the independent contributions of childhood and adolescents risk variables to the development of the adult metabolic syndrome. In children (aged 3–9 years at base-line), childhood obesity, high triglycerides, family history of hypertension, and age were independently associated with the adulthood metabolic syndrome (, Model I). In adolescents (aged 12–18 years at base-line), the independent determinants of the adulthood metabolic syndrome included obesity, male sex, high insulin level, family history of hypertension, high triglycerides, family history of type 2 diabetes, and high CRP level (, Model II). Risk variables that were not associated with the adulthood metabolic syndrome in the multivariable models included elevated blood pressure, low HDL cholesterol, smoking, alcohol use, physical activity index, family history of coronary heart disease, birth size, breast-feeding, and parental socio-economic status. Similar results were seen when the adult metabolic syndrome was defined by using the NCEP or the EGIR criteria (data not shown).
Table III. Childhood (Model I) and adolescent (Model II) characteristics that predict adulthood International Diabetes Federation (IDF) metabolic syndrome (n = 1955).
Characteristics of obese children and adolescents who developed the metabolic syndrome
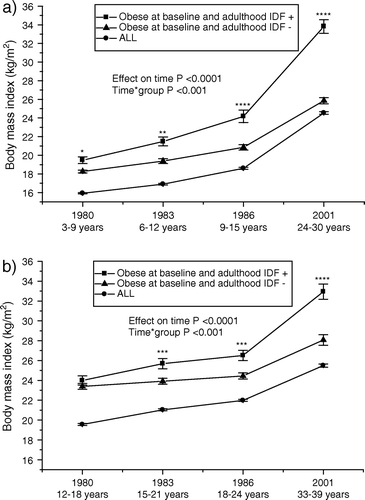
Because not all overweight and obese youths appear to be at equal risk of developing metabolic syndrome, we studied the development of risk factor levels in initially obese subjects with and without the metabolic syndrome in young adulthood. The longitudinal serial changes in study variables in initially obese subjects who developed and did not develop the adult metabolic syndrome are shown in , along with the data in all study subjects. Among 3–9-year-olds at base-line, there were 169 obese children, and 38 (22%) of these subjects were diagnosed with the metabolic syndrome in adulthood. Among 12–18-year-olds at base-line, there were 125 obese adolescents, and 38 (30%) of these subjects were diagnosed with the metabolic syndrome in adulthood. Obese children and adolescents who had the metabolic syndrome diagnosis as adults had significantly higher BMI already during childhood and adolescence than those who did not have the diagnosis as adults. They also increased their BMI more rapidly, especially between 1986 and 2001, i.e. during the transition into adulthood (). Obese subjects at base-line who later developed the metabolic syndrome had higher waist circumference in adulthood (mean±SD, 106±11 cm) than those who did not develop the syndrome (mean±SD, 87±12 cm), P<0.0001.
Figure 1. Serial changes in body mass index (mean±SEM) a) from childhood (3–9 years) and b) from adolescence (12–18 years) to young adulthood in all subjects, and initially obese subjects (obesity status was defined in 1980) with respect to adult metabolic syndrome (using the International Diabetes Federation (IDF) criteria). Statistical comparisons between obese groups. *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001.
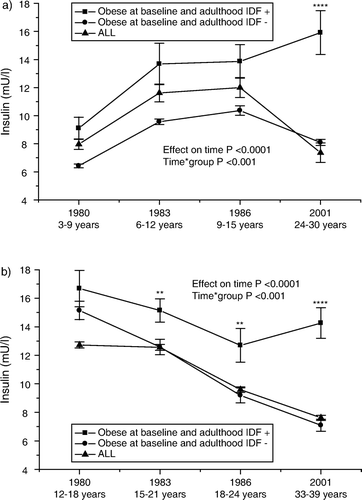
Regarding insulin, the physiological state of insulin resistance during puberty Citation26 is reflected as high insulin levels during adolescence in all subjects as well as in obese subjects (). Normally insulin levels decrease after puberty Citation27 and this normalization is seen in obese youths who do not develop the metabolic syndrome. In contrast, those individuals who develop the metabolic syndrome continue presenting with high insulin levels in adulthood ().
Figure 2. Serial changes in insulin (mean±SEM) a) from childhood (3–9 years) and b) from adolescence (12–18 years) to young adulthood in all subjects, and in initially obese subjects (obesity status was defined in 1980) with respect to adult metabolic syndrome (using the International Diabetes Federation (IDF) criteria). Statistical comparisons between obese groups. * P<0.05; ** P<0.01; *** P<0.001; **** P<0.0001.
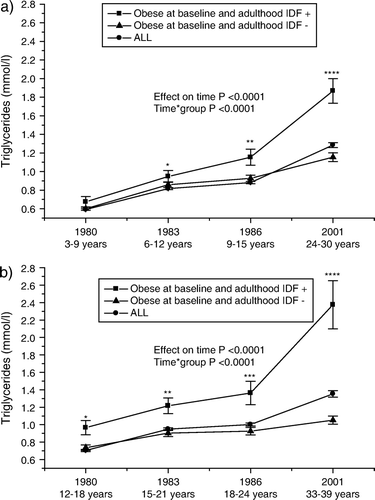
The trends in triglycerides levels resemble those seen in BMI: a rapid increase especially during the transition from adolescence/early adulthood to adulthood ().
Figure 3. Serial changes in triglycerides (mean±SEM) a) from childhood (3–9 years) and b) from adolescence (12–18 years) to young adulthood in all subjects, and in initially obese subjects (obesity status was defined in 1980) with respect to adult metabolic syndrome (using the International Diabetes Federation (IDF) criteria). Statistical comparisons between obese groups. *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001.
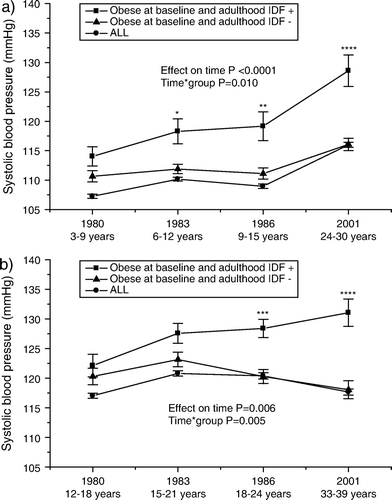
Development of systolic blood pressure levels is shown in . Obese children and adolescents who develop the metabolic syndrome have a steady rise in systolic blood pressure by age. In comparison, obese individuals who do not develop the metabolic syndrome show no change by age in systolic blood pressure. Their blood pressure changes over time resemble the trend seen in all subjects Citation22.
Figure 4. Serial changes in systolic blood pressure (mean±SEM) a) from childhood (3–9 years) and b) from adolescence (12–18 years) to young adulthood in all subjects, and in initially obese subjects (obesity status was defined in 1980) with respect to adult metabolic syndrome (using the International Diabetes Federation (IDF) criteria). Statistical comparisons between obese groups. * P<0.05; **P<0.01; ***P<0.001; ****P<0.0001.
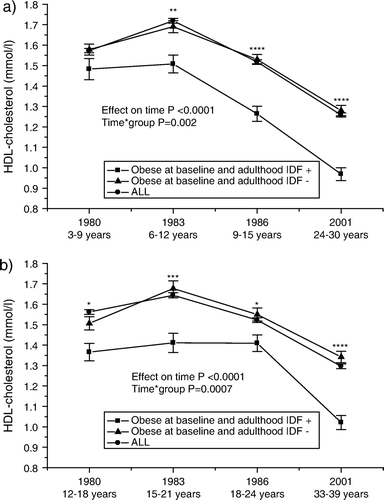
The trends in HDL cholesterol levels are shown in . Obese youths who develop the metabolic syndrome have consistently lower HDL cholesterols compared to those who do not develop the syndrome.
Figure 5. Serial changes in high-density lipoprotein (HDL) cholesterol (mean±SEM) a) from childhood (3–9 years) and b) from adolescence (12–18 years) to young adulthood in all subjects, and in initially obese subjects (obesity status was defined in 1980) with respect to adult metabolic syndrome (using the International Diabetes Federation (IDF) criteria). Statistical comparisons between obese groups. *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001.
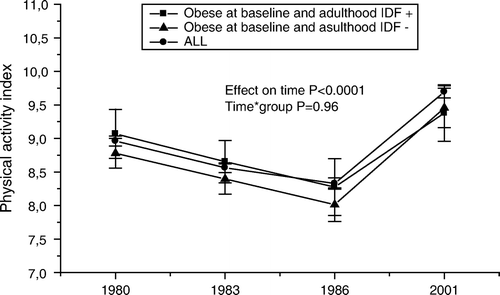
The trends in physical activity levels are shown in . No significant differences were seen in the levels of physical activity indices between obese youth who developed and did not develop the metabolic syndrome.
Figure 6. Serial changes in physical activity (mean±SEM) from adolescence (12–18 years) to young adulthood in all subjects, and in initially obese subjects (obesity status was defined in 1980) with respect to adult metabolic syndrome (using the International Diabetes Federation (IDF) criteria). Statistical comparisons between obese groups. *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001.
The smoking rates were similar between obese subjects who developed and did not develop the metabolic syndrome, and resembled the rates in all subjects (data not shown).
Discussion
In the present study, the independent youth determinants of the adult metabolic syndrome included obesity, male sex, family history of hypertension, family history of type 2 diabetes, high triglycerides, high insulin, and high CRP. Obesity was a strong predicting risk factor for the development of the adult metabolic syndrome. Furthermore, the serial data demonstrated that those obese youths who developed the metabolic syndrome had significantly higher BMI already during adolescence than those who did not develop the syndrome. They also gained weight more rapidly during transition into adulthood. Our findings thus confirm the results of previous studies which have shown that childhood obesity predicts the development of the metabolic syndrome in adulthood Citation28, Citation29.
In addition to obesity, elevated level of triglycerides was another individual component of the metabolic syndrome that independently predicted the risk. The serial trends in triglyceride levels in obese individuals who developed the adult metabolic syndrome demonstrated a constant rise during childhood and adolescence and a rapid increase during the transition into young adulthood. Elevation in triglyceride levels may thus be a specific marker of the early metabolic events related to the pathogenesis of the metabolic syndrome. In adults, the simultaneous occurrence of hypertriglyceridemia and obesity has been suggested as a high-risk phenotype with atherogenic and diabetogenic profiles Citation30.
High insulin level in adolescence was also an independent risk factor for the adult metabolic syndrome. We have previously shown in the Young Finns cohort that high fasting insulin measured in children predicted the clustering of high triglycerides, low HDL cholesterol, and elevated systolic blood pressure in a 6-year follow-up Citation31. The current observation with considerably longer follow-up provides more evidence for the idea that hyperinsulinemia may be causally related with the deterioration of lipid and blood pressure profiles. In the serial data, the deviation in insulin metabolism was clearly seen in obese individuals who developed the adult metabolic syndrome. Normally insulin levels decrease after puberty, but this normalization was not seen in these individuals who continued to have elevated insulin levels through out the follow-up. In line with these observations, Bao et al. Citation32 have previously shown in the longitudinal Bogalusa Heart Study that when insulin concentrations are increased in childhood, they tend to remain elevated in adulthood, and that those adults with consistently elevated insulin levels also tend to have a combination of increased obesity, hypertension, and dyslipidemia. There are several mechanisms that could explain the cause and effect relation between insulin and clustering of metabolic risk factors. Free fatty acid concentrations are suggested to be the link between insulin resistance and dyslipidemia Citation33. In patients with insulin resistance, the suppression of free fatty acid release from adipose tissue is impaired, providing increased production of very-low-density lipoprotein (VLDL) and triglycerides by the liver Citation33. The defect in lipoprotein lipase activity in insulin resistance also contributes to the decrease in plasma HDL cholesterol, and the LDL cholesterol pool may become enriched with small, dense, highly atherogenic LDL particles Citation34. Hyperinsulinemia or insulin resistance may also raise blood pressure by increasing sympathetic nervous system activation Citation35, stimulation of vascular smooth muscle cell growth, promoting the renal reabsorption of sodium, and altering cell electrolyte composition Citation36.
Recently, in the Fels Longitudinal Study of 493 study participants, Sun et al. Citation37 showed a direct relation between childhood blood pressure and the metabolic syndrome in adulthood. The investigators concluded that the effects of childhood BMI on the adulthood metabolic syndrome were mediated by childhood blood pressure. We could not confirm these observations in the present study. Children and adolescents who eventually developed the adult metabolic syndrome had higher blood pressure levels at base-line compared to others. In addition, obese youths who developed the metabolic syndrome had a steady rise in systolic blood pressure by age. However, elevated blood pressure in youth was not an independent predictor of the adult metabolic syndrome in models adjusted for several other metabolic risk factors.
We have previously shown in this cohort that maintaining a high level of physical activity from youth to adulthood is associated with lower risk of abdominal obesity in women Citation38. In the present analysis, however, the level of physical activity was not an independent determinant of the adult metabolic syndrome. We were also unable to demonstrate significant differences in the level of physical activity in serial measurements between obese youths who differed regarding the metabolic syndrome status in adulthood. These findings suggest that the assessment of habitual physical activity level in children and adolescents may not provide useful information about the future risk of the metabolic syndrome.
Increased CRP associates with obesity Citation39 and is an independent predictor of cardiovascular events Citation40. In the present study, an elevated CRP level in adolescence was an independent predictor of the adult metabolic syndrome. Previous studies have suggested that children and adolescents with the metabolic syndrome show evidence of low-grade inflammation. Ford et al. Citation41 analyzed data from the National Health and Nutrition Examination Survey and showed that the concentrations of CRP were higher among 12–17-year-old subjects with the metabolic syndrome than in subjects without the syndrome. In another US study, De Ferranti et al. Citation42 reported in 12–19-year-old adolescents that CRP concentrations increased with the increasing number of metabolic abnormalities and were higher in adolescents with the metabolic syndrome than in those without the syndrome. In the present study, the relation between elevated CRP and adult metabolic syndrome remained independent in a multivariable model after adjustments for other confounding factors such as obesity. Together, these observations point to the possibility that inflammation may have role in the pathogenesis of the metabolic syndrome.
We found that family history for hypertension and type 2 diabetes were independent predictors of the adult metabolic syndrome. A recent study by Ventura et al. Citation43 demonstrated that girls at the age of 13 classified as having higher risk for the metabolic syndrome and its components were more likely to have a family history of obesity, type 2 diabetes and gestational diabetes. There are various potential mechanisms explaining the relations between the metabolic syndrome and positive family history. There may be some underlying genetic variants, which predispose some vulnerable individuals to the development of the metabolic syndrome Citation44, Citation45. Genes may also modify the impact of obesity and other conventional risk factors. Further genetic studies are needed to clarify the involvement of genetic variants in the metabolic syndrome. The practical implication is that the assessment of family risk of hypertension and type 2 diabetes may be helpful in the pediatric risk assessment for the propensity to develop metabolic syndrome.
A potential limitation of the study is the non-participation in the follow-up studies. The participation rate of the latest follow-up study in 2001 was approximately 65%. We have previously reported that the base-line characteristics are similar between study subjects and those lost to follow-up Citation22. Therefore, the present study cohort seems to be representative of the original study population. We measured only fasting insulin level that is a crude measure of insulin resistance. We did not measure waist circumference in 1980 and 1986 and blood glucose in 1980. Information on family history of coronary heart disease, type 2 diabetes, and hypertension were collected in 2001, when participants were 24–39 years of age. Obviously, the rates for positive family histories would have been lower if collected in 1980.
In adults, the metabolic syndrome predicts the development of type 2 diabetes and cardiovascular diseases Citation46, Citation47. Among young adults, individuals with the metabolic syndrome have evidence of subclinical atherosclerosis indicated by increased carotid intima-media thickness Citation48. We have previously shown that exposure to metabolic risk factors in adolescence may contribute to the development of structural and functional vascular markers of subclinical atherosclerosis Citation25, Citation49, Citation50. Data are also emerging linking childhood exposure to metabolic risk factors to cardiovascular end-points decades later Citation51; Morrison et al. Citation51 recently reported from the longitudinal Princeton LRC School Study that those children with the cluster of factors defined as pediatric metabolic syndrome were significantly more likely to have cardiovascular disease decades later as adults. These observations thus emphasize the importance of early identification of children and adolescents at increased risk of the metabolic syndrome. In the present study, youth determinants of adult metabolic syndrome included obesity, family history of hypertension, family history of type 2 diabetes, high triglycerides, high insulin, and high CRP. Identifying these risk factors in children and adolescents could be helpful in pediatric metabolic risk assessment.
Acknowledgements
This study was financially supported by the Academy of Finland (grant no. 77841 and 210283), the Juho Vainio Foundation, the Finnish Foundation of Cardiovascular Research, the Finnish Cultural Foundation, Special Federal Grants for the Turku University Hospital, the Turku University Foundation, the Research Foundation of Orion Corporation, the Research Foundation of AstraZeneca, the Yrjö Jahnsson Foundation, the Lydia Maria Julin foundation, and the Maud Kuistila Foundation.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988; 37: 1595–607
- Smoak CG, Burke GL, Webber LS, Harsha DW, Srinivasan SR, Berenson GS. Relation of obesity to clustering of cardiovascular disease risk factors in children and young adults. The Bogalusa Heart Study. Am J Epidemiol. 1987; 125: 364–72
- Bao W, Srinivasan SR, Wattigney WA, Berenson GS. Persistence of multiple cardiovascular risk clustering related to syndrome X from childhood to young adulthood. The Bogalusa Heart Study. Arch Intern Med. 1994; 154: 1842–7
- Chen W, Bao W, Begum S, Elkasabany A, Srinivasan SR, Berenson GS. Age-related patterns of the clustering of cardiovascular risk variables of syndrome X from childhood to young adulthood in a population made up of black and white subjects: the Bogalusa Heart Study. Diabetes. 2000; 49: 1042–8
- Ford ES, Giles WH, Mokdad AH. Increasing prevalence of the metabolic syndrome among U.S. Adults. Diabetes Care. 2004; 27: 2444–9
- Raitakari OT, Porkka KV, Räsänen L, Rönnemaa T, Viikari JS. Clustering and six year cluster-tracking of serum total cholesterol, HDL-cholesterol and diastolic blood pressure in children and young adults. The Cardiovascular Risk in Young Finns Study. J Clin Epidemiol. 1994; 47: 1085–93
- Goodman E, Daniels SR, Meigs JB, Dolan LM. Instability in the diagnosis of metabolic syndrome in adolescents. Circulation. 2007; 115: 2316–22
- DiPietro L, Mossberg HO, Stunkard AJ. A 40-year history of overweight children in Stockholm: life-time overweight, morbidity, and mortality. Int J Obes Relat Metab Disord. 1994; 18: 585–90
- Lee IM, Manson JE, Hennekens CH, Paffenbarger RS, Jr. Body weight and mortality. A 27-year follow-up of middle-aged men. JAMA. 1993; 270: 2823–8
- Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics 2002; 109: 45–60
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006; 295: 1549–55
- Juonala M, Raitakari M, Viikari SA, Raitakari OT. Obesity in youth is not an independent predictor of carotid IMT in adulthood. The Cardiovascular Risk in Young Finns Study. Atherosclerosis. 2006; 185: 388–93
- Freedman DS, Khan LK, Dietz WH, Srinivasan SR, Berenson GS. Relationship of childhood obesity to coronary heart disease risk factors in adulthood: the Bogalusa Heart Study. Pediatrics. 2001; 108: 712–8
- Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004; 350: 2362–74
- Åkerblom HK, Viikari J, Uhari M, Räsänen L, Byckling T, Louhivuori K, et al. Atherosclerosis precursors in Finnish children and adolescents. I. General description of the cross-sectional study of 1980, and an account of the children's and families’ state of health. Acta Paediatr Scand Suppl. 1985; 318: 49–63
- Telama R, Viikari J, Välimäki I, Siren-Tiusanen H, Åkerblom HK, Uhari M, et al. Atherosclerosis precursors in Finnish children and adolescents. X. Leisure-time physical activity. Acta Paediatr Scand Suppl. 1985; 318: 169–80
- Alberti KG, Zimmet P, Shaw J. The metabolic syndrome—a new worldwide definition. Lancet. 2005; 366: 1059–62
- Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005; 112: 2735–52
- Balkau B, Charles MA. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR). Diabet Med. 1999; 16: 442–3
- Leino M, Raitakari OT, Porkka KV, Helenius HY, Viikari JS. Cardiovascular risk factors of young adults in relation to parental socioeconomic status: the Cardiovascular Risk in Young Finns Study. Ann Med. 2000; 32: 142–51
- Porkka KV, Raitakari OT, Leino A, Laitinen S, Rasanen L, Ronnemaa T, et al. Trends in serum lipid levels during 1980–1992 in children and young adults. The Cardiovascular Risk in Young Finns Study. Am J Epidemiol. 1997; 146: 64–77
- Juonala M, Viikari JS, Hutri-Kahonen N, Pietikainen M, Jokinen E, Taittonen L, et al. The 21-year follow-up of the Cardiovascular Risk in Young Finns Study: risk factor levels, secular trends and east-west difference. J Intern Med. 2004; 255: 457–68
- Herbert V, Lau KS, Gottlieb CW, Bleicher SJ. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965; 25: 1375–84
- Juonala M, Viikari JS, Rönnemaa T, Taittonen L, Marniemi J, Raitakari OT. Childhood C-reactive protein in predicting CRP and carotid intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. Arterioscler Thromb Vasc Biol. 2006; 26: 1883–8
- Raitakari OT, Juonala M, Kahonen M, Taittonen L, Laitinen T, Maki-Torkko N, et al. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA. 2003; 290: 2277–83
- Lautala P, Åkerblom HK, Viikari J, Louhivuori K, Uhari M, Dahlstrom S, et al. Atherosclerosis precursors in Finnish children and adolescents. VII. Serum immunoreactive insulin. Acta Paediatr Scand Suppl. 1985; 318: 127–33
- Rönnemaa T, Knip M, Lautala P, Viikari J, Uhari M, Leino A, et al. Serum insulin and other cardiovascular risk indicators in children, adolescents and young adults. Ann Med. 1991; 23: 67–72
- Vanhala MJ, Vanhala PT, Keinanen-Kiukaanniemi SM, Kumpusalo EA, Takala JK. Relative weight gain and obesity as a child predict metabolic syndrome as an adult. Int J Obes Relat Metab Disord. 1999; 23: 656–9
- Srinivasan SR, Myers L, Berenson GS. Predictability of childhood adiposity and insulin for developing insulin resistance syndrome (syndrome X) in young adulthood: the Bogalusa Heart Study. Diabetes. 2002; 51: 204–9
- Lemieux I, Pascot A, Couillard C, Lamarche B, Tchernof A, Almeras N, et al. Hypertriglyceridemic waist: A marker of the atherogenic metabolic triad (hyperinsulinemia; hyperapolipoprotein B; small, dense LDL) in men?. Circulation. 2000; 102: 179–84
- Raitakari OT, Porkka KV, Rönnemaa T, Knip M, Uhari M, Åkerblom HK, et al. The role of insulin in clustering of serum lipids and blood pressure in children and adolescents. The Cardiovascular Risk in Young Finns Study. Diabetologia. 1995; 38: 1042–50
- Bao W, Srinivasan SR, Berenson GS. Persistent elevation of plasma insulin levels is associated with increased cardiovascular risk in children and young adults. The Bogalusa Heart Study. Circulation. 1996; 93: 54–9
- Reynisdottir S, Angelin B, Langin D, Lithell H, Eriksson M, Holm C, et al. Adipose tissue lipoprotein lipase and hormone-sensitive lipase. Contrasting findings in familial combined hyperlipidemia and insulin resistance syndrome. Arterioscler Thromb Vasc Biol. 1997; 17: 2287–92
- Ginsberg HN. New perspectives on atherogenesis: role of abnormal triglyceride-rich lipoprotein metabolism. Circulation. 2002; 106: 2137–42
- Rowe JW, Young JB, Minaker KL, Stevens AL, Pallotta J, Landsberg L. Effect of insulin and glucose infusions on sympathetic nervous system activity in normal man. Diabetes. 1981; 30: 219–25
- DeFronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM. A balanced overview. Diabetes Care. 1992; 15: 318–68
- Sun SS, Grave GD, Siervogel RM, Pickoff AA, Arslanian SS, Daniels SR. Systolic blood pressure in childhood predicts hypertension and metabolic syndrome later in life. Pediatrics. 2007; 119: 237–46
- Yang X, Telama R, Viikari J, Raitakari OT. Risk of obesity in relation to physical activity tracking from youth to adulthood. Med Sci Sports Exerc. 2006; 38: 919–25
- Wisse BE. The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J Am Soc Nephrol. 2004; 15: 2792–800
- Willerson JT, Ridker PM. Inflammation as a cardiovascular risk factor. Circulation. 2004; 109: II2–10
- Ford ES, Ajani UA, Mokdad AH. The metabolic syndrome and concentrations of C-reactive protein among U.S. youth. Diabetes Care. 2005; 28: 878–81
- de Ferranti SD, Gauvreau K, Ludwig DS, Newburger JW, Rifai N. Inflammation and changes in metabolic syndrome abnormalities in US adolescents: findings from the 1988–1994 and 1999–2000 National Health and Nutrition Examination Surveys. Clin Chem. 2006; 52: 1325–30
- Ventura AK, Loken E, Birch LL. Risk profiles for metabolic syndrome in a nonclinical sample of adolescent girls. Pediatrics. 2006; 118: 2434–42
- Yang WS, Yang YC, Chen CL, Wu IL, Lu JY, Lu FH, et al. Adiponectin SNP276 is associated with obesity, the metabolic syndrome, and diabetes in the elderly. Am J Clin Nutr. 2007; 86: 509–13
- Gomez-Abellan P, Hernandez-Morante JJ, Lujan JA, Madrid JA, Garaulet M. Clock genes are implicated in the human metabolic syndrome. Int J Obes (Lond) 2008; 32: 121–8
- Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002; 288: 2709–16
- Ninomiya JK, L'Italien G, Criqui MH, Whyte JL, Gamst A, Chen RS. Association of the metabolic syndrome with history of myocardial infarction and stroke in the Third National Health and Nutrition Examination Survey. Circulation. 2004; 109: 42–6
- Mattsson N, Ronnemaa T, Juonala M, Viikari JS, Jokinen E, Hutri-Kahonen N, et al. Arterial structure and function in young adults with the metabolic syndrome: the Cardiovascular Risk in Young Finns Study. Eur Heart J. 2008; 29: 784–91
- Juonala M, Jarvisalo MJ, Maki-Torkko N, Kahonen M, Viikari JS, Raitakari OT. Risk factors identified in childhood and decreased carotid artery elasticity in adulthood: the Cardiovascular Risk in Young Finns Study. Circulation. 2005; 112: 1486–93
- Juonala M, Viikari JS, Ronnemaa T, Helenius H, Taittonen L, Raitakari OT. Elevated blood pressure in adolescent boys predicts endothelial dysfunction: the cardiovascular risk in young Finns study. Hypertension. 2006; 48: 424–30
- Morrison JA, Friedman LA, Gray-McGuire C. Metabolic syndrome in childhood predicts adult cardiovascular disease 25 years later: the Princeton Lipid Research Clinics Follow-up Study. Pediatrics. 2007; 120: 340–5