Abstract
Treatment of bipolar disorder (BD) has traditionally focused on alleviation of acute symptoms and prevention of future recurrences. Current treatment guide-lines advocate more or less similar treatment algorithms for all patients. Such approach largely ignores the clinical, genetic, and pathophysiological heterogeneity of BD, which makes certain patients more (or less) likely to respond to specific treatments. Variables such as family history, comorbidity, course of illness, quality and duration of previous remissions, physical and medical comorbidity, and side-effects may help in selecting the most effective treatment for an individual patient, yet their value is not recognized by current algorithms. As well, polymorphisms of specific genes may prove useful in predicting treatment outcome and/or understanding the pharmacological mechanisms of mood stabilization. Novel molecular targets have recently emerged from studies of mechanisms of action of available mood stabilizers. They include inhibitors of protein kinase C, inhibitors of glycogen synthase kinase, or medications modulating glutamatergic neurotransmission. As well, treatment targets are moving beyond acute symptoms and prevention of mood episodes. Cognitive deficits, persistence of residual symptoms, and increased mortality of BD are recognized as important for outcome of BD, yet are not always adequately addressed by traditional treatments.
Introduction
Bipolar disorder (BD) is a recurrent major psychiatric illness with a lifetime prevalence of 1%. Central to BD is periodic mood dysregulation manifesting as episodes of depression and mania in BD type I, or depression and hypomania in BD type II.
Diagnosis of BD is based exclusively on clinical signs and symptoms—history of episodes of mania and depression. The hall-marks of mania are elevated, expansive, or irritable mood, increased goal-directed activity, pleasure-seeking behavior, and impaired judgment. Patients with mania have a diminished need for sleep and are frequently hyperactive and easily distracted. They are often talkative and excited and tend to have an unrestrained and accelerated flow of ideas.
Anhedonia and depressed mood are key symptoms of depression. Patients often describe their depressed mood as having a distinct quality that distinguishes it from the normal emotion of sadness or grief. Depressed patients frequently describe social withdrawal, lack of energy and motivation, as well as disturbances in sleep and appetite, decreased concentration and impaired memory. Both depression and mania are associated with a risk of suicide. In severe episodes patients experience symptoms of psychosis in the form of delusions or hallucinations. Such symptoms are often congruent with the underlying mood, for instance delusions of grandiosity and omnipotence in manic patients, or delusions of guilt during depressive episodes.
The clinical course of BD varies considerably between patients. Some experience discrete episodes of mania or depression followed by spontaneous remissions, others suffer from a chronic persisting illness. In certain subjects the timing of episodes is more or less regular, even suggesting a seasonal pattern, in many others the course appears completely random.
Key messages
Bipolar disorder is a life-long complex disorder. Untreated it leads to major cost in terms of high disability and mortality. Successful treatment of bipolar disorder is usually multimodal, including pharmacotherapy, education, psychotherapy, as well as adjustment of life-style.
Many patients can be treated successfully if attention is paid to clinical and genetic factors that allow prediction of treatment response. Study of genetic variation of candidate genes may help in selecting optimal treatments and in identifying novel molecular targets.
Symptoms other than mood dysregulation are common and play a significant role in the outcome of the illness. Most significant are physical morbidity and neurocognitive impairment, both not addressed sufficiently by current treatments.
Mood dysregulation is the core feature of BD, but manifestations of the illness occur in other psychiatric and physical domains as well. A substantial proportion of BD patients can be diagnosed with additional psychiatric conditions such as panic disorder, social phobia, eating disorders, or substance abuse. Medical comorbidity is common, largely attributable to the higher prevalence of modifiable risk factors for metabolic syndrome and cardiovascular disease—obesity, insulin resistance and type II diabetes mellitus, hypertension and dyslipidemia Citation1, Citation2. All-cause mortality in BD is 2--3 times and suicide mortality at least 15 times that of the general population Citation3; the leading cause of death is cardiovascular disease followed by suicide.
In summary, BD is a complex disorder with adverse consequences in multiple domains. Similar to other complex (and chronic) conditions that occupy much of today's medicine, treatment of BD is multimodal. Pharmacotherapy is the cornerstone, but education, psychotherapy, and life-style modification all play important roles. In this paper we first review briefly the current standards of treatment. Then we focus on three areas: 1) optimizing treatments with existing medications by matching them to specific patient profiles, including pharmacogenetic approaches; 2) review of novel treatment modalities; and 3) improving outcomes by addressing previously neglected clinical domains such as residual psychopathology, neurocognitive impairment, and high mortality of BD.
Abbreviations
Table I. Key features of bipolar disorder
Treatment of bipolar disorder in 2008
The treatment of BD currently focuses on controlling acute phases of the illness (depressive, manic, and mixed episodes, and rapid cycling) as well as on long-term prevention of recurrences.
Acute treatment
Mania represents the most conspicuous face of BD. Yet it is less frequent than depression and it can usually be treated effectively. The more challenging aspect is establishing the therapeutic alliance necessary to treat a manic patient. First-line medications include lithium, valproate, and atypical antipsychotics (AAPs), either alone or in combination. Additional treatments such as carbamazepine, oxcarbazepine, clozapine, or electroconvulsive therapy (ECT) may be used alone or in combination with one of the first-line treatments, if those are unsuccessful Citation4.
Relatively little attention is given to treatment of mixed states of BD. Such patients experience symptoms of both mania and depression, either concurrently or rapidly alternating. In the US, carbamazepine and most of AAPs (olanzapine, risperidone, aripiprazole, and ziprasidone) have all received Food and Drug Administration (FDA) approval for treatment of mixed states. However, valproate and lamotrigine are also commonly used, the latter one especially if depressive symptoms dominate the clinical picture. Some guide-lines recommend that antidepressants be avoided in the treatment of mixed states, due to the risk of worsening intraepisodic mood lability Citation5.
Depression remains the most taxing aspect of BD Citation6, Citation7. Patients spend considerably more time depressed than manic, and, while a number of treatment options are available, their efficacy is comparatively low. Antidepressants, the mainstay of treatment of major depressive disorder, can be effective in bipolar depression but need to be used with caution. They can inadvertently lead to a switch into mania, induce rapid cycling, but also worsen the depression. The risks of antidepressant use are recognized in treatment guide-lines, which usually recommend either mood stabilizers alone or cautious use of antidepressants combined with mood stabilizers Citation4, Citation8. Conventional mood stabilizers, such as lithium and lamotrigine alone or in combination remain popular often first-line therapy of bipolar depression, even though a recent review of lamotrigine trials raised some doubts Citation9. Only two treatments have been approved by FDA for bipolar depression—the olanzapine-fluoxetine combination and quetiapine. Selective serotonin re-uptake inhibitors, venlafaxine, and bupropion are indicated when mood stabilizers are ineffective and for ‘breakthrough’ depressions Citation10. Tricyclic antidepressants are now largely avoided, as they have been considered most likely to induce rapid cycling or switch to mania. Among older antidepressants, monoamine oxidase inhibitors are an overlooked but often effective treatment for bipolar depression.
The treatment of rapid cycling (defined as the presence of at least four mood episodes in a year) involves reducing or stopping any possible cycling-promoting agents, such as antidepressants, and adding or optimizing mood stabilizers. Some patients with rapid cycling BD benefit from lithium, valproate, lamotrigine, carbamazepine, high doses of thyroid hormones, or AAPs Citation11, although rapid cycling in general is difficult to treat.
Long-term treatment
The main goal of long-term treatment of BD is prevention of acute episodes of the illness. Traditionally, prophylactic treatment used to be recommended if there was any evidence of high recurrence risk judged by the number and frequency of previous episodes. More recently the trend has shifted towards treating early, even after the first manic episode Citation12. For bipolar II disorder extrapolation of such recommendation could be problematic, and the individual recommendation may depend more on the frequency and severity of both hypomanic and depressive episodes.
Lithium has well established efficacy in the prevention of mania and likely in the prevention of depression and suicide. All current treatment guide-lines recommend lithium or valproate for long-term prophylaxis, although the evidence for prophylactic efficacy of valproate is less firm; in the US valproate is currently not approved by FDA. Controlled studies suggest that AAPs (in particular olanzapine, aripiprazole, and quetiapine) have mood-stabilizing properties and might become a standard alternative for long-term therapy. Lamotrigine has been shown efficacious in preventing both manic and depressive episodes with a more robust effect on the latter ones Citation13, Citation14. A systematic review of randomized controlled trials of lithium, lamotrigine, olanzapine, and valproate demonstrated that, compared with placebo, all were more effective at preventing relapse of either polarity Citation15. Carbamazepine may provide effective prophylaxis for mixed states or for those with atypical features Citation16.
Long-term treatment of BD should include strategies to improve treatment compliance, such as providing education to patients and their families, including recognition of early signs of relapse as well as prodromal and subsyndromal symptoms, and management of stressors. Specific psychotherapies such as interpersonal and social rhythms therapy Citation17 and family-focused therapy Citation18 grew from systematic long-term studies. Cognitive therapy, found useful in major depression, has been used successfully with less severely ill bipolar patients in particular Citation19.
Most classes of drugs for BD have been available for a long time—lithium for almost 60 years Citation20 and typical antipsychotics for over 50 years. First reports of the possible effectiveness of the antiepileptic drugs (AEDs) carbamazepine and valproate appeared in the late 1960s and were followed by confirmatory controlled studies in subsequent decades. The last two decades saw a rise in the use of lamotrigine and AAPs that have largely replaced the older typical antipsychotics. However, the increasing number of treatment options has not yet translated into better outcomes Citation21. This could be for a variety of reasons, but particularly relevant seems to be the suggestion made by some authors that an expansion of diagnostic boundaries of BD and inclusion of an increasingly heterogeneous group of conditions into a single diagnostic category have produced a diagnosis that is losing its clinical and predictive utility Citation22, Citation23.
Optimizing the use of current treatments
Taking heterogeneity of BD into account
Converging evidence from clinical and genetic research supports the view of BD as a heterogeneous condition. Consequently, not all patients benefit from the same treatment. At the clinical level, certain features of the illness tend to co-occur suggesting a specific pattern and an existence of BD subtypes. For instance, patients with early onset have higher prevalence of rapid cycling, increased comorbidity (anxiety, non-affective disorders, mood lability, impulsivity, substance abuse, and high rates of suicide), and they are less likely to respond to long-term treatment Citation24–27. Patients with episodic (non-chronic) clinical course typically have low rates of comorbid conditions and family history of bipolar disorder; if they experience psychosis, its symptoms are usually mood-congruent, present only during episodes of abnormal mood Citation22, Citation28.
Further evidence of heterogeneity exists at the genetic level. BD is a largely heritable condition, with correlation in relatives largely due to genetic factors Citation29. The transmission of BD appears to be genetically complex, consistent with an oligogenic model, assuming multiple genes of pleiotropic effects and leading to a broad spectrum of phenotypes. Susceptibility regions (but no specific genes) for BD have been mapped using genetic linkage studies. These have demonstrated an overlap of linkage findings between BD and other diagnostic categories (BD and unipolar depression, depression as part of schizophrenia, and schizophrenia as part of the bipolar spectrum in lithium non-responders), suggesting heterogeneity and fuzzy diagnostic boundaries of BD Citation30. Familiality of specific traits, such as lithium responsiveness Citation31 or concordance of clinical course between affected parents and children Citation32, provides further evidence for heterogeneity.
Specific subtypes of BD also differ with respect to molecular genetic findings. For instance, the linkage findings in the 18q region are prominent in families with comorbid panic disorder, rapid mood switching, and higher rates of bipolar II rather than bipolar I illness Citation33. On the other hand, several linkage studies have indicated an overlap of findings in BD and schizophrenia, particularly in the 13q and 22q regions. These results were especially robust in families where mood disorders co-occurred with psychotic (and mood-incongruent) features Citation34, Citation35.
Algorithmic or targeted treatment?
Although generally acknowledged, heterogeneity is not given consideration in the current treatment guide-lines. Arguably, evaluation of the relevant predictors requires detailed and time-consuming assessment and review of past and collateral histories. Instead, most guide-lines recommend an algorithmic approach to treatment or prophylaxis of mood states based on broad diagnostic criteria. This approach, perhaps with some exaggeration, could be described as ‘Start with treatment A—if not effective, switch to B or add C—and if either fails, proceed to treatment D’. This approach largely ignores the possibility that certain patients respond preferentially to specific treatments. While there are only few randomized trials that address this issue directly, data from comparisons of responders to specific mood stabilizers as well as systematic observations from specialized clinics suggest that this could indeed be the case.
For instance, lithium responders typically have a fully remitting, episodic course of illness, lower risk of comorbid conditions, and a family history of BD but not other conditions Citation28, Citation36, Citation37. Conversely, lamotrigine responders have more rapid cycling and higher rates of comorbid conditions, particularly in the anxiety-panic disorder spectrum, with a family history of anxiety and major depression, but not BD Citation36. Indeed, treatment response in individual patients appears to be specific as several predictors of poor response to lithium (manic severity, anxiety and dysphoria, rapid cycling, and negative family history of mood disorders) were found associated with good response to carbamazepine Citation38 or valproate Citation39. Similarly, in a large German study patients with less typical BD, for instance those with BD II or with mood-incongruent psychosis, responded better to carbamazepine than to lithium Citation40.
Thus, after a careful assessment a patient could start a treatment targeted to his clinical profile rather than running through all consecutive options until finding an effective one.
In summary, useful factors to consider in selecting the most effective treatment for an individual patient include clinical presentation, family history, comorbidity, course of illness, quality and duration of previous remissions, residual symptoms, physical/medical comorbidity, and side-effects Citation28, Citation41. Consequently, psychiatry needs to move beyond check-lists of symptoms in current diagnostic criteria in order to develop a more rational approach to the systematic long-term treatment. Potentially, outcome could be improved by examining the distinguishing features of patient phenotypes and matching them to specific medication using pharmacogenetics.
Table II. Factors to consider for selecting optimal long-term treatment
Pharmacogenetics
The recognition of heterogeneity of BD leads further to the notion that allelic variants of specific genes are associated with response (or non-response) to individual medications. Clinical experience has suggested that, for instance, response to antidepressants or lithium is similar among relatives. This has been supported by several case series and later by a few observational studies Citation37, Citation42. Similarly, Grof et al. have shown that response to prophylactic lithium treatment is familial with relatives of responders having 3.7 times higher likelihood of responding compared to unselected BD patients Citation31.
Family history of specific psychiatric conditions alone can be a useful guide to selecting long-term treatment. For instance, responders to lithium typically have a stronger family history of BD, while non-responders have more relatives with schizophrenia. A family history of anxiety disorders (as well as comorbid anxiety) is more common in responders to lamotrigine.
A number of studies have tested individual candidate genes for an association with response to lithium in particular. The most promising findings are related to the promoter polymorphism of the serotonin transporter gene, with non-responders showing higher frequency of the short (low transcription activity) allele Citation43. Based on the observed effects of lithium on glycogen synthase kinase 3β (GSK 3β) Adli et al. found an association between GSK 3β gene promoter variant and response to lithium augmentation in patients with major depression Citation44, but the same polymorphism has not been associated with response to long-term lithium Citation45. Of further interest are the studies of inositol monophosphatase gene on chromosome 18 Citation46, the recently reported association of lithium response with the Stargazin gene Citation47, as well as association of the gene XBP1, which is related to endoplasmic reticulum stress response, with poor response to lithium Citation48.
Arguably, attempts to match specific treatment to specific phenotypic characteristics or genetic markers are still in their early stages. However, with further advance and replication, they will lead to a more rational use of current and future treatments.
Novel pharmacological targets
Many patients can benefit from an optimal use of today's treatments Citation21, Citation49. For others who do not respond to or tolerate them, we will need medications based on different mechanisms of action.
The pharmacodynamic properties of early medications to treat BD do not dramatically differ from recent ones. Both old and new antipsychotics block postsynaptic D2 receptors and both old and new antidepressants increase synaptic levels of serotonin and/or norepinephrine. Yet advances in molecular biology have clearly demonstrated that it is likely the postsynaptic activity, and ultimately changes in gene expression, which may be more closely implicated in the pathophysiology and treatment of mood disorders. The fact that sites of action of commonly used antidepressants are upstream from the main source of dysregulation may explain the relative lack of efficacy of these medications, as well as their potential benefit in a broad range of conditions. Furthermore, in light of new findings regarding changes in signal transduction, modulating activity at the receptor level through second messenger systems, with the goal of affecting targets further downstream, may be ineffective (see also ). It is encouraging that finally, after more than 50 years, medications with new mechanisms of action start to make their way to psychiatry.
Figure 1. A simplified diagram of the most important pathways regulated by mood-stabilizing medications. Full lines indicate stimulatory and dotted lines inhibitory effects. A number of these pathways converge on regulatory proteins and transcription factors, which through fine-tuning of balance between pro- and antiapoptotic mechanisms regulate neurogenesis, cell survival, and resilience to damage through corticosteroids etc. The effects of available medications, however, affect these regulatory proteins/transcription factors indirectly, mostly through actions on release of neurotransmitters. Novel medications should interfere with these pathways further ‘downstream’.
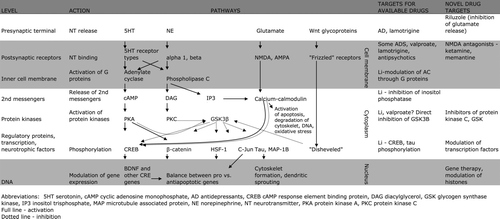
Phosphatidylinositol pathway
A number of new molecular targets stem from the study of effects of lithium, which is one of the most bioactive elements and an as yet unsurpassed mood stabilizer. Lithium acts through several second messenger pathways. The best studied is its effect on the phosphatidylinositol pathway. A number of G protein-coupled receptors (subtypes of serotonergic, cholinergic, dopaminergic, and adrenergic receptors) activate phospholipase C after binding of ligand. This enzyme cleaves membrane phospholipid phosphatidylinositol-4,5-bisphosphate (PIP2) to inositol 1,4,5-triphosphate (IP3) and diacylglycerol (DAG). DAG activates protein kinase C (PKC). IP3 activates calcium/calmodulin-dependent processes. IP3 is recycled back to PIP2 by inositol monophosphate phosphatase and inositol polyphosphatase phosphatase; both are directly inhibited by lithium. Through depletion of free inositol, lithium dampens the activation of signaling pathways in neurons Citation50.
Tamoxifen, an antiestrogen used in the treatment of breast cancer, is also a relatively selective PKC inhibitor and readily crosses the blood brain barrier. Two clinical studies in manic patients recently showed relatively good antimanic effects of tamoxifen at a dose of 20–140 mg.
Glycogen synthase kinase
Glycogen synthase kinase (GSK 3β) is an important protein kinase directly inhibited by lithium. A number of signal transduction pathways, including the Wnt signaling pathway, as well as actions of serotonergic, dopaminergic, and glutamatergic neurotransmitter systems, converge on GSK 3β. GSK 3β regulates about 40 molecular targets related among others to cell division, stem cell renewal and differentiation, apoptosis, neural plasticity, circadian rhythms, and insulin action Citation51. Selective brain permeable GSK 3β inhibitors exist and show antidepressant as well as antimanic effects in animal models Citation52–54. Modulation of one molecular target, affecting both poles of the illness, is of marked interest, as antidepressant and antimanic properties of available mood stabilizers are typically not well balanced. No clinical trials with GSK inhibitors in human subjects so far exist. Of some concern is the fact that the main GSK modulator, the Wnt pathway, is dysregulated in many forms of cancer, and interfering with the balance between pro- and antiapoptotic processes might promote oncogenesis. However, Li treatment does not seem to increase the risk of malignancy, and no oncogenic effects of GSK 3β inhibitors have been demonstrated in animal models.
AMPA/NMDA throughput
Glutamate is the main excitatory neurotransmitter and, together with GABA (gamma-amino butyric acid), accounts for 98% of neurotransmitters in the brain. Not surprisingly, abnormalities of the glutamatergic system have been implicated in a number of neurological and psychiatric disorders. Glutamate acts on both ionotropic (ligand-gated ion channels) as well as metabotropic (receptors bound to G protein) receptors. Three major classes of ionotropic glutamatergic receptors are expressed in mammalian brain, including NMDA (N-methyl D-aspartic acid), AMPA (α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid), and kainate receptors. During various adverse conditions (ischemia, high levels of corticosteroids), excessive release of glutamate and excessive activation of NMDA receptors may lead to excitotoxicity and activation of proapoptotic processes. There are several ways of modulating the glutamatergic system.
Riluzole, an FDA-approved treatment for amyotrophic lateral sclerosis, inhibits release of glutamate through both the inhibition of voltage-dependent sodium and P-type calcium channels. Recently, open-label trials of riluzole revealed antidepressant effects in patient with major depression Citation55. Riluzole was also beneficial when added to lithium in the treatment of bipolar depression Citation56 and when added to conventional antidepressants in alleviating treatment-resistant depression Citation57.
Ketamine is a high-affinity NMDA antagonist. Through blockade of presynaptic NMDA receptors, it increases glutamate release. The released glutamate predominantly acts on AMPA receptors due to blockade of postsynaptic NMDA receptors, thus increasing AMPA throughput. A single dose of ketamine has been shown to alleviate depressive-like behavior in animal models—an effect which typically requires chronic administration of conventional antidepressants. Two small double-blind, placebo-controlled, cross-over trials of unipolar, treatment-resistant depressive patients found a robust, rapid (within 2 hours of administration), and relatively sustained (1 week) response to a single dose of ketamine. The improvement associated with ketamine infusion reflected a change in core depressive symptoms, which was temporarily unrelated to ketamine-induced euphoria and psychotomimetic symptoms Citation58, Citation59. No other available drug has yet shown a similarly rapid and relatively sustained response after a single dose.
There are a number of other potential molecular targets, including corticoliberin, bcl2, melatonin, and antioxidants, which are beyond the scope of this review.
Novel clinical targets
Treatment of mood disorders has traditionally focused on alleviating symptoms and preventing recurrence of episodes. Treatment of other aspects of BD has attracted comparatively less attention. Particularly relevant for outcome of the illness are 1) cognitive impairment associated with BD, 2) persisting residual symptoms, and 3) excessive mortality of patients with mood disorders.
Neurocognitive deficits
Patients with BD exhibit a range of neuropsychological deficits during both euthymic and acute phases of the illness Citation60; the severity of neurocognitive impairment appears to correlate with the number or duration of previous mood episodes Citation61. These deficits negatively influence functional outcome Citation62–64. Some of the cognitive domains (verbal learning and memory) decline as a function of past illness burden Citation65. Interestingly, some of the neuroanatomical changes which also become more pronounced with increasing illness burden appear in regions subserving memory function (hippocampus and temporal lobes). Corticosteroid-mediated hippocampal damage may be one of the links between repeated mood episodes, particularly depressive, and neurocognitive deficits Citation66.
Both preclinical and clinical studies have shown that hypercortisolemia is associated with impairment of memory and hippocampal damage, possibly through hyperactivation of NMDA receptors (excitotoxicity). Hypercortisolemia and impaired HPA feedback appear in 40%–60% of patients with mood disorders. Decreased hippocampal volume is a relatively consistent finding among patients with unipolar depression (although less so among bipolar patients) and has been associated with an increased burden of illness. Treatment of hypercortisolemia Citation67, use of antidepressants Citation68 or lithium Citation69–71, has led to increases in hippocampal or gray matter volume, neuronal viability, and improvements in memory in human clinical populations.
Residual symptoms
Psychiatric interventions have traditionally focused on treatment or prevention of syndromes. Remission of illness in psychiatry is typically defined as a score below a particular (and arbitrary) threshold on a scale rating severity of symptoms. For instance, the threshold defining remission in studies of cognitive functioning ranges between 6 and 14 points on the Hamilton Depression Rating Scale. Yet, residual symptoms are very common among patients with mood disorders. Prospective studies in bipolar I patients showed sustained symptomatic morbidity (mostly depressed-dysthymic-dysphoric) 30%–50% of time (for review see Citation72). Reliance on syndromal recovery is suboptimal for other reasons as well, since it does not typically correspond with functional recovery. For example, deficits in psychosocial functioning in bipolar patients seem to persist for as long as 2 years following hospitalization or syndromal recovery Citation73–75. Subsyndromal symptoms may also influence functional outcome. For example, depressive symptoms in patients with bipolar disorder were negatively correlated with Global Assessment of Functioning (GAF) scores in a sample in which none of the patients met the criteria for major depression Citation76. Another study described an increase in work absenteeism in patients with as few as two symptoms of depression Citation77. Furthermore, residual subsyndromal symptoms are negatively associated with cognitive functioning Citation78, and specific residual symptoms, such as persistent sleep dysregulation, may contribute to an increased risk of diabetes and coronary heart disease Citation79, Citation80. In light of the functional consequences of residual symptoms, their underlying pathophysiology and means of treatment should be studied in more detail.
Increased mortality of bipolar disorder
Mood disorders are potentially lethal conditions, not only due to suicide, but also due to somatic causes, perhaps with the exception of malignancy Citation81. Cardiovascular mortality is responsible for the largest number of excess death in BD Citation3, Citation82. This is likely caused by a markedly increased prevalence of cardiovascular (CV) risk factors including obesity, diabetes mellitus, hypertension, and metabolic syndrome among patients with mood disorders Citation83. It is not clear whether this increase in CV risk factors is related to treatment, life-style, socio-economic status, presence of atypical depressive symptoms (hypersomnia and hyperphagia with carbohydrate craving), endocrine dysregulation (hypercortisolemia), immunological effects (overexpression of inflammatory markers), hemostasis-related factors (platelet activation), or autonomic nervous system abnormalities Citation84. There may also be a correlation between pathophysiology of mood disorders and diabetes mellitus (through dysregulation of Wnt, the GSK pathway, or as a result of brain-derived neurotrophic factor (BDNF) abnormalities) Citation85, Citation86.
Lithium is the only established medication that decreases mortality in mood disorders by reducing the risk of suicide Citation87 and cardiovascular mortality Citation88 through mechanisms that are not entirely clear. Interestingly, insulin, similar to lithium, is an inhibitor of GSK signaling. Dysregulation of GSK seems to underlie insulin resistance in type II diabetes Citation86, as well as being implicated in the pathophysiology of mood disorders. Some of the novel molecular targets affecting the GSK system might thus prove to be effective in treating the metabolic syndrome and ultimately decreasing mortality in mood disorders.
Conclusion
Treatment of BD remains one of the major challenges in psychiatry. In almost 60 years since the modern use of lithium, the number of treatment options has increased considerably but is not matched by substantially better outcomes. The complex nature of the illness as well as changing diagnostic boundaries of BD Citation89 are just a few of the possible reasons. The way forward may lie in the developments outlined in our paper—a better appreciation of the heterogeneity of the illness to match patient features with specific treatment, development of treatments targeting novel molecular mechanisms, and considering not only mood changes but the whole range of symptoms relevant for disease outcome.
Acknowledgements
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Fagiolini A, Frank E, Scott JA, Turkin S, Kupfer DJ. Metabolic syndrome in bipolar disorder: findings from the Bipolar Disorder Center for Pennsylvanians. Bipolar Disord. 2005; 7: 424–30
- Newcomer JW. Medical risk in patients with bipolar disorder and schizophrenia. J Clin Psychiatry 2006; 67(Suppl 9)25–30
- Osby U, Brandt L, Correia N, Ekbom A, Sparen P. Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry. 2001; 58: 844–50
- Yatham LN, Kennedy SH, O'Donovan C, Parikh S, Macqueen G, McIntyre R, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) guidelines for the management of patients with bipolar disorder: consensus and controversies. Bipolar Disord 2005; 7(Suppl 3)5–69
- Kruger S, Young LT, Braunig P. Pharmacotherapy of bipolar mixed states. Bipolar Disord. 2005; 7: 205–15
- Judd LL, Akiskal HS, Schettler PJ, Endicott J, Maser J, Solomon DA, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002; 59: 530–7
- Judd LL, Akiskal HS, Schettler PJ, Coryell W, Endicott J, Maser JD, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003; 60: 261–9
- Young LT, Joffe RT, Robb JC, MacQueen GM, Marriott M, Patelis-Siotis I. Double-blind comparison of addition of a second mood stabilizer versus an antidepressant to an initial mood stabilizer for treatment of patients with bipolar depression. Am J Psychiatry. 2000; 157: 124–6
- Calabrese JR, Huffman RF, White RL, Edwards S, Thompson TR, Ascher JA, et al. Lamotrigine in the acute treatment of bipolar depression: results of five double-blind, placebo-controlled clinical trials. Bipolar Disord. 2008; 10: 323–33
- Thase ME. Pharmacotherapy of bipolar depression: an update. Curr Psychiatry Rep. 2006; 8: 478–88
- Schneck CD. Treatment of rapid-cycling bipolar disorder. J Clin Psychiatry 2006; 67(Suppl 11)22–7
- Goodwin GM. Evidence-based guidelines for treating bipolar disorder: recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2003; 17: 149–73
- Vieta E, Rosa AR. Evolving trends in the long-term treatment of bipolar disorder. World J Biol Psychiatry. 2007; 8: 4–11
- McAllister-Williams RH. Relapse prevention in bipolar disorder: a critical review of current guidelines. J Psychopharmacol. 2006; 20: 12–6
- Smith LA, Cornelius V, Warnock A, Bell A, Young AH. Effectiveness of mood stabilizers and antipsychotics in the maintenance phase of bipolar disorder: a systematic review of randomized controlled trials. Bipolar Disord. 2007; 9: 394–412
- Greil W, Kleindienst N, Erazo N, Muller-Oerlinghausen B. Differential response to lithium and carbamazepine in the prophylaxis of bipolar disorder. J Clin Psychopharmacol. 1998; 18: 455–60
- Frank E, Kupfer DJ, Thase ME, Mallinger AG, Swartz HA, Fagiolini AM, et al. Two-year outcomes for interpersonal and social rhythm therapy in individuals with bipolar I disorder. Arch Gen Psychiatry. 2005; 62: 996–1004
- Miklowitz DJ, George EL, Richards JA, Simoneau TL, Suddath RL. A randomized study of family-focused psychoeducation and pharmacotherapy in the outpatient management of bipolar disorder. Arch Gen Psychiatry. 2003; 60: 904–12
- Scott J, Paykel E, Morriss R, Bentall R, Kinderman P, Johnson T, et al. Cognitive-behavioural therapy for severe and recurrent bipolar disorders: randomised controlled trial. Br J Psychiatry. 2006; 188: 313–20
- Cade JFJ. Lithium salts in the treatment of psychotic excitement. Med J Aust. 1949; 2: 349–51
- Garnham J, Munro A, Slaney C, MacDougall M, Passmore M, Duffy A, et al. Prophylactic treatment response in bipolar disorder: Results of a naturalistic observation study. J Affect Disord. 2007; 104: 185–90
- Baldessarini RJ. A plea for integrity of the bipolar disorder concept. Bipolar Disord. 2000; 2: 3–7
- Alda M. The phenotypic spectra of bipolar disorder. Eur Neuropsychopharmacol 2004; 14(Suppl 2)S94–9
- Young LT, Cooke RG, Robb JC, Levitt AJ, Joffe RT. Anxious and non-anxious bipolar disorder. J Affect Disord. 1993; 29: 49–52
- Feske U, Frank E, Mallinger AG, Houck PR, Fagiolini A, Shear MK, et al. Anxiety as a correlate of response to the acute treatment of bipolar I disorder. Am J Psychiatry. 2000; 157: 956–62
- Henry C, Van den Bulke D, Bellivier F, Etain B, Rouillon F, Leboyer M. Anxiety disorders in 318 bipolar patients: prevalence and impact on illness severity and response to mood stabilizer. J Clin Psychiatry. 2003; 64: 331–5
- Rihmer Z, Szadoczky E, Furedi J, Kiss K, Papp Z. Anxiety disorders comorbidity in bipolar I, bipolar II and unipolar major depression: results from a population-based study in Hungary. J Affect Disord. 2001; 67: 175–9
- Grof P, Alda M, Grof E, Fox D, Cameron P. The challenge of predicting response to stabilising lithium treatment. The importance of patient selection. Br J Psychiatry 1993; 163(Suppl 21)16–9
- Duffy A, Grof P, Robertson C, Alda M. The implications of genetic studies of major mood disorders for clinical practice. J Clin Psychiatry. 2000; 61: 630–7
- Grof P, Alda M, Grof E, Zvolsky P, Walsh M. Lithium response and genetics of affective disorders. J Affect Disord. 1994; 32: 85–95
- Grof P, Duffy A, Cavazzoni P, Grof E, Garnham J, MacDougall M, et al. Is response to prophylactic lithium a familial trait?. J Clin Psychiatry. 2002; 63: 942–7
- Duffy A, Alda M, Kutcher S, Cavazzoni P, Robertson C, Grof E, et al. A prospective study of the offspring of bipolar parents responsive and nonresponsive to lithium treatment. J Clin Psychiatry. 2002; 63: 1171–8
- MacKinnon DF, Zandi P, Cooper J, Potash JB, Simpson SG, Gershon E, et al. Comorbid bipolar disorder and panic disorder in families with a high prevalence of bipolar disorder. Am J Psychiatry. 2002; 159: 30–5
- Potash JB, Zandi PP, Willour VL, Lan TH, Huo Y, Avramopoulos D, et al. Suggestive linkage to chromosomal regions 13q31 and 22q12 in families with psychotic bipolar disorder. Am J Psychiatry. 2003; 160: 680–6
- Berrettini WH. Are schizophrenic and bipolar disorders related? A review of family and molecular studies. Biol Psychiatry. 2000; 48: 531–8
- Passmore M, Garnham J, Duffy A, MacDougall M, Munro A, Slaney C, et al. Phenotypic spectra of bipolar disorder in responders to lithium versus lamotrigine. Bipolar Disord. 2003; 5: 110–4
- Duffy A, Alda M, Milin R, Grof P. A consecutive series of treated affected offspring of parents with bipolar disorder: is response associated with the clinical profile?. Can J Psychiatry. 2007; 52: 369–76
- Post RM, Uhde TW, Roy-Byrne PP, Joffe RT. Correlates of antimanic response to carbamazepine. Psychiatr Res. 1987; 21: 71–83
- Bowden CL. Spectrum of effectiveness of valproate in neuropsychiatry. Expert Rev Neurother. 2007; 7: 9–16
- Kleindienst N, Greil W. Differential efficacy of lithium and carbamazepine in the prophylaxis of bipolar disorder: results of the MAP study. Neuropsychobiology 2000; 42(Suppl 1)2–10
- Gelenberg AJ, Pies R. Matching the bipolar patient and the mood stabilizer. Ann Clin Psychiatry. 2003; 15: 203–16
- Franchini L, Serretti A, Gasperini M, Smeraldi E. Familial concordance of fluvoxamine response as a tool for differentiating mood disorder pedigrees. J Psychiatr Res. 1998; 32: 255–9
- Serretti A, Lilli R, Mandelli L, Lorenzi C, Smeraldi E. Serotonin transporter gene associated with lithium prophylaxis in mood disorders. Pharmacogenomics J. 2001; 1: 71–7
- Adli M, Hollinde DL, Stamm T, Wiethoff K, Tsahuridu M, Kirchheiner J, et al. Response to lithium augmentation in depression is associated with the glycogen synthase kinase 3-beta -50T/C single nucleotide polymorphism. Biol Psychiatry. 2007; 62: 1295–302
- Szczepankiewicz A, Rybakowski JK, Suwalska A, Skibinska M, Leszczynska-Rodziewicz A, Dmitrzak-Weglarz M, et al. Association study of the glycogen synthase kinase-3beta gene polymorphism with prophylactic lithium response in bipolar patients. World J Biol Psychiatry. 2006; 7: 158–61
- Sjoholt G, Ebstein RP, Lie RT, Berle JO, Mallet J, Deleuze JF, et al. Examination of IMPA1 and IMPA2 genes in manic-depressive patients: association between IMPA2 promoter polymorphisms and bipolar disorder. Mol Psychiatry. 2004; 9: 621–9
- Silberberg G, Levit A, Collier D, St Clair D, Munro J, Kerwin RW, et al. Stargazin involvement with bipolar disorder and response to lithium treatment. Pharmacogenet Genomics. 2008; 18: 403–12
- Masui T, Hashimoto R, Kusumi I, Suzuki K, Tanaka T, Nakagawa S, et al. A possible association between the -116C/G single nucleotide polymorphism of the XBP1 gene and lithium prophylaxis in bipolar disorder. Int J Neuropsychopharmacol. 2006; 9: 83–8
- Grof P. Selecting effective long-term treatment for bipolar patients: monotherapy and combinations. J Clin Psychiatry 2003; 64(Suppl 5)53–61
- Silverstone PH, McGrath BM, Kim H. Bipolar disorder and myo-inositol: a review of the magnetic resonance spectroscopy findings. Bipolar Disord. 2005; 7: 1–10
- Jope RS, Roh MS. Glycogen synthase kinase-3 (GSK3) in psychiatric diseases and therapeutic interventions. Curr Drug Targets. 2006; 7: 1421–34
- Kozikowski AP, Gaisina IN, Yuan H, Petukhov PA, Blond SY, Fedolak A, et al. Structure-based design leads to the identification of lithium mimetics that block mania-like effects in rodents. Possible new GSK-3beta therapies for bipolar disorders. J Am Chem Soc. 2007; 129: 8328–32
- Einat H, Manji HK. Cellular plasticity cascades: Genes-to-behavior pathways in animal models of bipolar disorder. Biol Psychiatry. 2006; 59: 1160–71
- Gould TD, Zarate CA, Manji HK. Glycogen synthase kinase-3: a target for novel bipolar disorder treatments. J Clin Psychiatry. 2004; 65: 10–21
- Zarate CA, Jr, Payne JL, Quiroz J, Sporn J, Denicoff KK, Luckenbaugh D, et al. An open-label trial of riluzole in patients with treatment-resistant major depression. Am J Psychiatry. 2004; 161: 171–4
- Zarate CA, Jr, Quiroz JA, Singh JB, Denicoff KD, De Jesus G, Luckenbaugh DA, et al. An open-label trial of the glutamate-modulating agent riluzole in combination with lithium for the treatment of bipolar depression. Biol Psychiatry. 2005; 57: 430–2
- Sanacora G, Kendell SF, Levin Y, Simen AA, Fenton LR, Coric V, et al. Preliminary evidence of riluzole efficacy in antidepressant-treated patients with residual depressive symptoms. Biol Psychiatry. 2007; 61: 822–5
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000; 47: 351–4
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006; 63: 856–64
- Robinson LJ, Thompson JM, Gallagher P, Goswami U, Young AH, Ferrier IN, et al. A meta-analysis of cognitive deficits in euthymic patients with bipolar disorder. J Affect Disord. 2006; 93: 105–15
- Emilien G, Septien L, Brisard C, Corruble E, Bourin M. Bipolar disorder: how far are we from a rigorous definition and effective management?. Prog Neuropsychopharmacol Biol Psychiatry. 2007; 31: 975–96
- Atre-Vaidya N, Taylor MA, Seidenberg M, Reed R, Perrine A, Glick-Oberwise F. Cognitive deficits, psychopathology, and psychosocial functioning in bipolar mood disorder. Neuropsychiatry Neuropsychol Behav Neurol. 1998; 11: 120–6
- Martinez-Aran A, Vieta E, Colom F, Reinares M, Benabarre A, Torrent C, et al. Neuropsychological performance in depressed and euthymic bipolar patients. Neuropsychobiology 2002; 46(Suppl1)16–21
- Laes JR, Sponheim SR. Does cognition predict community function only in schizophrenia?: a study of schizophrenia patients, bipolar affective disorder patients, and community control subjects. Schizophr Res. 2006; 84: 121–31
- Savitz J, Solms M, Ramesar R. Neuropsychological dysfunction in bipolar affective disorder: a critical opinion. Bipolar Disord. 2005; 7: 216–35
- Hoschl C, Hajek T. Hippocampal damage mediated by corticosteroids—a neuropsychiatric research challenge. . Eur Arch Psychiatry Clin Neurosci 2001; 251(Suppl 2)II81–8
- Starkman MN, Giordani B, Gebarski SS, Berent S, Schork MA, Schteingart DE. Decrease in cortisol reverses human hippocampal atrophy following treatment of Cushing's disease. Biol Psychiatry. 1999; 46: 1595–602
- Vermetten E, Vythilingam M, Southwick SM, Charney DS, Bremner JD. Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol Psychiatry. 2003; 54: 693–702
- Moore GJ, Bebchuk JM, Hasanat K, Chen G, Seraji-Bozorgzad N, Wilds IB, et al. Lithium increases N-acetyl-aspartate in the human brain: in vivo evidence in support of bcl-2's neurotrophic effects?. Biol Psychiatry. 2000; 48: 1–8
- Moore GJ, Bebchuk JM, Wilds IB, Chen G, Manji HK. Lithium-induced increase in human brain grey matter. Lancet. 2000; 356: 1241–2
- Yucel K, McKinnon MC, Taylor VH, Macdonald K, Alda M, Young LT, et al. Bilateral hippocampal volume increases after long-term lithium treatment in patients with bipolar disorder: a longitudinal MRI study. Psychopharmacology (Berl) 2007; 195: 357–67
- Huxley N, Baldessarini RJ. Disability and its treatment in bipolar disorder patients. Bipolar Disord. 2007; 9: 183–96
- Coryell W, Scheftner W, Keller M, Endicott J, Maser J, Klerman GL. The enduring psychosocial consequences of mania and depression. Am J Psychiatry. 1993; 150: 720–7
- Keck PE, Jr, McElroy SL, Strakowski SM, West SA, Sax KW, Hawkins JM, et al. 12-month outcome of patients with bipolar disorder following hospitalization for a manic or mixed episode. Am J Psychiatry. 1998; 155: 646–52
- Tohen M, Hennen J, Zarate CM, Jr, Baldessarini RJ, Strakowski SM, Stoll AL, et al. Two-year syndromal and functional recovery in 219 cases of first-episode major affective disorder with psychotic features. Am J Psychiatry. 2000; 157: 220–8
- Altshuler LL, Gitlin MJ, Mintz J, Leight KL, Frye MA. Subsyndromal depression is associated with functional impairment in patients with bipolar disorder. J Clin Psychiatry. 2002; 63: 807–11
- Johnson J, Weissman MM, Klerman GL. Service utilization and social morbidity associated with depressive symptoms in the community. JAMA. 1992; 267: 1478–83
- Clark L, Iversen SD, Goodwin GM. Sustained attention deficit in bipolar disorder. Br J Psychiatry. 2002; 180: 313–9
- Yaggi HK, Araujo AB, McKinlay JB. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care. 2006; 29: 657–61
- Ayas NT, White DP, Manson JE, Stampfer MJ, Speizer FE, Malhotra A, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003; 163: 205–9
- Laursen TM, Munk-Olsen T, Nordentoft M, Mortensen PB. Increased mortality among patients admitted with major psychiatric disorders: a register-based study comparing mortality in unipolar depressive disorder, bipolar affective disorder, schizoaffective disorder, and schizophrenia. J Clin Psychiatry. 2007; 68: 899–907
- Angst F, Stassen HH, Clayton PJ, Angst J. Mortality of patients with mood disorders: follow-up over 34–38 years. J Affect Disord. 2002; 68: 167–81
- Birkenaes AB, Opjordsmoen S, Brunborg C, Engh JA, Jonsdottir H, Ringen PA, et al. The level of cardiovascular risk factors in bipolar disorder equals that of schizophrenia: a comparative study. J Clin Psychiatry. 2007; 68: 917–23
- Taylor V, MacQueen G. Associations between bipolar disorder and metabolic syndrome: A review. J Clin Psychiatry. 2006; 67: 1034–41
- Krabbe KS, Nielsen AR, Krogh-Madsen R, Plomgaard P, Rasmussen P, Erikstrup C, et al. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia. Diabetologia. 2007; 50: 431–8
- Meijer L, Flajolet M, Greengard P. Pharmacological inhibitors of glycogen synthase kinase 3. Trends Pharmacol Sci. 2004; 25: 471–80
- Tondo L, Hennen J, Baldessarini RJ. Lower suicide risk with long-term lithium treatment in major affective illness: a meta-analysis. Acta Psychiatr Scand. 2001; 104: 163–72
- Ahrens B, Muller-Oerlinghausen B, Schou M, Wolf T, Alda M, Grof E, et al. Excess cardiovascular and suicide mortality of affective disorders may be reduced by lithium prophylaxis. J Affect Disord. 1995; 33: 67–75
- Grof P, Alda M. Discrepancies in the efficacy of lithium. Arch Gen Psychiatry. 2000; 57: 191