Abstract
Depression results in a tremendous burden to individuals suffering from the disorder and to the global health economy. Available pharmacologic treatments for depression target monoamine levels and monoamine receptors. However, delayed onset of effect, partial or inadequate treatment response, and side-effects are significant limitations of current therapies. The search for a better understanding of mechanisms of depression and for new treatment targets has turned attention to intracellular mediators. Phosphodiesterases (PDEs) are enzymes that break down the intracellular second messenger mononucleotides cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP). Recent data from animal and human studies indicate that PDEs may play a role in depression and in related stress conditions. PDE genes have been linked directly to depression and to other genes associated with psychiatric disorders.
Introduction
Depression is a major cause of morbidity worldwide. The National Comorbidity Survey, conducted in the United States, estimated a 16% lifetime incidence of major depression and a 7% 12-month prevalence in 2001–02 Citation1. Since the arrival of safer medications with fewer side-effects in the late 1980s, rates of use of antidepressant medication have soared. According to the Centers for Disease Control's Chartbook on Trends in the Health of Americans, the percentage of adults who reported taking an antidepressant have tripled from the period 1988–1994 to 1999–2002, rising from 2.5% to 8% Citation2.
The arrival of newer medications has not resulted in improvements in efficacy and effectiveness of antidepressant therapy. Regardless of the specific antidepressant used, only half of patients improve with the first medication, and only one-third remit; recent data indicate that even after multiple trials, one-third of patients do not achieve remission Citation3, Citation4. Other limitations of current pharmacotherapies include delayed treatment response of 6 or more weeks Citation4, and intolerable side-effects for some people Citation5–7. There is clearly a need for on-going studies of underlying pathophysiological mechanisms in depression that can lead to the development of novel treatment approaches.
Current antidepressants share the same fundamental mechanism of action—manipulation of the levels of monoamines at the synaptic cleft. monoamine oxidase inhibitors MAOIs accomplish this by inhibiting the degradation of monoamines, thereby increasing their availability at the synapse; reuptake inhibitors increase synaptic monoamines by interfering with transporters into the presynaptic neuron, with particular medications targeting serotonin or norepinephrine to a greater or lesser degree. Some newer antidepressants also target specific monoaminergic receptors.
Key messages
One-third of people with depression do not achieve remission with current antidepressant treatment strategies.
Potential targets for the development of new treatments include intracellular second messenger systems.
Phosphodiesterases may play a role in therapeutic effects of antidepressants.
The relationship between changes in monoamine levels and therapeutic effects of antidepressant has not yet been fully elucidated. Increase in availability of monoamines at the synapse is immediate, yet treatment response is delayed. Downstream changes—in intracellular messaging systems, transcription, translation, and differential expression of receptors—are most likely mediators of symptom improvement. Postsynaptic receptors and the intracellular messaging systems to which they are coupled have become targets in the development of new therapeutics for depression Citation8. Of particular interest recently are phosphodiesterases (PDEs), enzymes that break down the important intracellular second messengers cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) Citation9, Citation10. cAMP and cGMP regulate the activity of enzymes, such as protein kinases, and transduce membrane signals to the nucleus to turn on and off particular genes. PDEs represent a key set of enzymes in this complex regulatory system, and they have become the target of much interest recently in psychiatric disorders and depression in particular. PDEs are expressed widely throughout the brain and thus are potential candidates for drug development. They are also involved in inflammatory diseases Citation11, Citation12. In this review, we will first briefly summarize the importance of cyclic nucleotides in the central nervous system (CNS) and then focus on PDEs, their structure and genetics, and on evidence from animal and human studies for the role of PDEs in depression.
Role of cyclic mononucleotides in the central nervous system
The second messengers cAMP and cGMP influence numerous physiological processes, such as inflammation, ion channel function, muscle contraction, differentiation, apoptosis, lipogenesis, glycogenolysis, gluconeogenesis, etc. Citation13, Citation14. These second messengers are also important regulators of central nervous system (CNS) functions. Hormone and neurotransmitter signaling are mediated by cAMP, and nitric oxide (NO) actions are mediated by cGMP. Cyclic mononucleotides mediate many other functions, such as learning, memory, mood, and nerve growth regeneration Citation12, Citation15, Citation16. Cyclic AMP also binds to a response-element binding protein (CREB) in the nucleus and affects the transcription of many genes, including brain-derived neurotrophic factor (BDNF), thought to be involved in long-term improvement of depression and depression-related brain changes Citation15, Citation17.
More is known about the biology of cAMP than cGMP. Transient fluctuations within a narrow range in intracellular cAMP concentrations modulate intracellular signaling, and maximal biological effect are caused by 2–3-fold changes in intracellular cAMP levels, though the cells have much larger potential for cAMP production Citation18, Citation19. Additionally, intracellular cAMP increases are transient in spite of continuous presence of the extracellular stimulus Citation20, Citation21. This restricted and transient nature of intracellular signaling transmission is essential for hormonal or neurotransmitter actions. Rapid and transient changes in cAMP and cGMP concentrations are the results of changes in both synthesis and degradation, which involve mechanisms at the receptor and postreceptor levels Citation22, Citation23. Accumulation of cyclic mononucleotides results from receptor uncoupling from G proteins and activity of synthetic enzymes cyclases, as well as rapid modulation of PDEs resulting in cAMP/cGMP degradation Citation24. Degradation most likely plays a larger role than synthesis in manipulating local concentrations, and compartmentalization further allows PDEs to fine-tune cAMP and cGMP levels, making them particularly attractive drug targets Citation25.
Structure and genetics of the PDEs
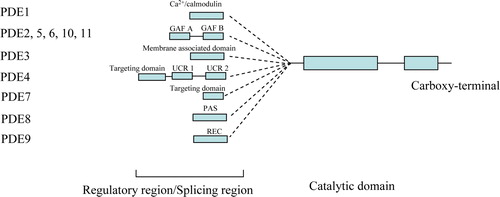
There are 11 families of PDEs, some of which metabolize cAMP, some cGMP, and some both. The PDE families are numbered, and most exist in several isoforms which are identified using letters, for example PDE4A-D; altogether, 21 genes make up the 11 PDE families, and alternative splicing leads to over 50 functional enzymes Citation26. PDEs contain a highly conserved catalytic domain and one or more N-terminal regulatory segments, and can also be grouped by the type of N-terminal domain Citation27 (); for example, the GAF PDEs, which are named after the first three classes of proteins recognized to contain this domain—mammalian cGMP-binding PDEs, Anabaena adenylyl cyclases, and Escherichia coli FhlA Citation28—have a high affinity for cGMP. The regulatory domain determines the subcellular compartment localization, substrate specificity, and activation/deactivation, therefore deciding the functional differentiation of PDEs. We will focus on two groups of PDEs that have shown promise as targets in depression: the GAF PDEs and the PDE4 Family.
The GAF PDEs
GAF domains are one of the largest families of small-molecule binding units initially discovered as the cGMP-binding regulatory domains of several cyclic nucleotide phosphodiesterases (PDEs) in mammals. Most of the GAF domain proteins identified in humans are PDEs Citation29, Citation30.
Five of the 11 PDE gene families, including PDE2, 5, 6, 10, and 11, have regulatory segments containing GAF domains. For PDE2 and PDE5, the binding of cGMP to at least one GAF domain in the regulatory segment is known to activate catalytic activity Citation31. The allosteric cGMP-binding function provided by the GAF domains in PDEs 2, 5, and 6 strongly prefers cGMP over cAMP Citation32. The two most recently discovered GAF-containing PDE families, PDE10 and 11, are still poorly characterized. A preliminary study suggests that PDE10A may contain a low-affinity cGMP-binding GAF domain Citation33, but no allosteric effect on the catalytic site has been established. Four N-terminal transcript variants encoding different PDE11A isoforms have been found Citation34, Citation35. PDE11A1, A2, and A3 have a catalytic domain and an N-terminus GAF domain, while the full-length form, PDE11A4, contains two GAF domains and a catalytic domain Citation34–37. PDE11A exhibits properties of a dual-substrate PDE Citation34, Citation35, Citation37.
Since rational drug design typically has been used to design competitive inhibitors of active sites, development of agonists and antagonists of PDEs activity based on binding to this GAF site might be possible Citation31. An antagonist might bind to the open (i.e. cGMP-free) conformation of the GAF domain and stabilize it in that state, blocking the natural ligand from binding and closing the GAF domain. The result would be an enzyme left in the inactive state. A GAF agonist, on the other hand, might bind more tightly to the closed conformation than the natural ligand, permanently activating the enzyme. The GAF domain makes this family of PDEs particularly attractive therapeutic targets for manipulation of cAMP and cGMP signaling.
The PDE4 family
The PDE4 family is the largest PDE family and has been extensively investigated for its structure, function, compartmentalization, and regulation Citation12. PDE4s are cAMP-specific PDEs, encoded by four genes, PDE4A, B, C, and D. Each of the PDE4 genes has numerous mRNA-splicing isoforms encoding long and short PDE4 isozymes at tissues and with cell type-specific profiles. The long PDE4 isozyme contains both upstream-conserved regions 1 and 2 (UCR1 and UCR2) in the N-terminal region, whereas the UCR1 is spliced out in short forms of PDE4 isozymes. The activity of PDE4 is mainly regulated through phosphorylation by protein kinase A and extracellular signal-related kinase (ERK) pathways Citation12, Citation26. The regulation of PDE4 has effects on cAMP response element-binding protein (CREB) signaling and brain-derived neurotrophic factor (BDNF) expression Citation38: the former has a key role in memory formation while the latter is critical for neuron survival. Thus, the inhibition of PDE4 has become a new therapeutic target in various clinical conditions, such as chronic obstructive pulmonary disease, depression, and neurodegenerative disease (COPD), depression, and neurodegenerative disease Citation10, Citation12, Citation39. PDE4 was shown to be specifically inhibited by the antidepressant agent rolipram (Ki=0.8 µM) and RO 20-1724, which provided a prototype for PDE inhibitor development Citation14.
Evidence for the role of PDEs in depression: animal studies
The regulation of cyclic nucleotide levels has important downstream consequences on signaling transduction cascades, gene expression, and resultant neuronal responses. Several lines of evidence from animal studies support a role for increased PDEs in mediating neuronal responses following chronic antidepressant treatment; for example, increases in PDE4A, PDE4B, and PDE4D gene expression, protein expression, and enzyme activity have been found after chronic antidepressant and electroconvulsive shock treatment in various brain areas including the cortex and hippocampus Citation40–47 (See ). While rolipram (a PDE4 inhibitor) displays antidepressant activity, sildenafil (a PDE5 inhibitor) and papaverine (a PDE10 inhibitor) display anxiogenic-like effects on behavioral tests of depression and anxiety, respectively Citation48–50. In the learned helplessness rodent model of depression, PDE4 and adenylate cyclase activity in frontal cortex and hippocampus were decreased in the acute depressive state, while PDE4 activity was increased in the delayed depressive state Citation51. Coadministration of rolipram with the tricyclic antidepressant imipramine was shown to decrease depressive behavior and increase CREB and BDNF in this model Citation52. BDNF is emerging as a key player in antidepressant-mediated hippocampal neurogenesis Citation53.
Table I. Effects of antidepressant treatment on phosphodiesterases (PDEs) in rodent brain.
PDE5 inhibition has been shown to prevent the antidepressant-like effect of venlafaxine, bupropion, and adenosine Citation54–56. Sildenafil has been shown to be anxiogenic in some animal models Citation49, Citation57. To investigate the possible pathway interactions further, Brink and colleagues demonstrated antidepressant-like effects of sildenafil only after central muscarinic receptor blockade with atropine, suggesting the importance of the interactions with cholinergic pathways Citation58.
The role of the PDE4D subtype in mediating the antidepressant activity of the PDE4 inhibitor rolipram has been studied by Zhang and colleagues Citation48. They examined the behavioral phenotype and desipramine, fluoxetine, and rolipram sensitivity of male PDE4D knock-out mice. Investigation of the behavioral phenotype of PDE4D knock-out mice found behavioral profiles of these mice that were antidepressant-like as revealed by decreased immobility time in the tail-suspension test as well as the forced swim test when compared to PDE4D wild types and heterozygotes. However, no significant differences between groups were found in the elevated plus maze test or in the multicompartment chamber test. While acute administration of either 20 mg/kg desipramine or 40 mg/kg fluoxetine 30 minutes prior to a forced swim test resulted in reduced immobility of three groups (PDE4D wild types, heterozygotes, and knock-outs), acute one-time administration of 0.5 mg/kg rolipram failed to decrease immobility in any of the groups. Repeated daily treatment with 0.5 mg/kg rolipram over 8 days was able to decrease immobility in wild-type mice only. Therefore, PDE4D knock-out mice showed a reduced sensitivity to the antidepressant-like effect of the PDE4 inhibitor rolipram on behavioral tests used to characterize depressive behavior. Knock-out mice deficient in PDEs show an antidepressant phenotype for PDE4D−/−, anxiogenic phenotype for PDE4B−/−, and no effects on depressive-like or anxiety-like behaviors for PDE1B−/− and PDE10A−/− Citation48, Citation59–61. In general, results from animal studies suggest that manipulating PDE activity can have an impact on depressive behaviors in animal models.
Evidence for the role of PDEs in depression: clinical studies in humans
As research in depression shifted from neurotransmitter levels and receptor binding to consideration of intracellular signaling, manipulation of second messengers has become increasingly of interest. The PDE4 family has been studied most extensively. Members of the PDE4 family are thought to contribute to the compartmentalization of cAMP within cells Citation12. In the human brain, three of the PDE4 isoforms, namely PDE4A, B, and D, are widely distributed, while PDE4C appears to be more limited to cortical areas, thalamic nuclei, and cerebellum Citation62.
PDE4-selective inhibitors have been considered as possible agents in the treatment of depression. The PDE4A inhibitor rolipram has been studied in the treatment of depression. Zeller and colleagues published the results of a phase II study of rolipram in treatment-refractory depression, finding a rapid antidepressant effect in 5 of 10 patients Citation63. A summary of early data on 150 patients from 20 centers indicated ‘good to very good’ effect of rolipram occurring within 1–10 days, a rapid response for antidepressant effect Citation64. Potentially limiting gastrointestinal side-effects were noted. Rolipram was equally effective as desipramine in a small randomized, double-blind, 28-day study of 35 patients with depression Citation65, and in that particular study rolipram was better tolerated due to the anticholinergic side-effects of desipramine. Laux et al. reported more equivocal results in a 28-day open-label study on 11 patients; 4 patients discontinued due to side-effects, and only 4 out 11 patients responded Citation66.
Unfortunately, side-effects such as nausea and emesis eventually prevented rolipram from reaching the market. However, other PDE4 inhibitors are being studied in the treatment of inflammatory disorders such as asthma, chronic obstructive pulmonary disease (COPD), allergic rhinitis, and atopic dermatitis Citation12, Citation67. Novel drugs are being developed to target the cognitive impairment of Alzheimer's disease Citation67.
Targeting subtypes of PDEs could be a way of dealing with intolerable side-effects. Studies in PDE4D knock-out mice have shown behaviors similar to wild-type mice treated with antidepressants, with reduced immobility on forced swim and tail-suspension tests Citation48, Citation68. However, PDE4D has been associated with emetic centers in the brain, which may limit this approach Citation26, Citation69.
PDE5 inhibitors are currently indicated for the treatment of erectile dysfunction and pulmonary arterial hypertension; initial evidence exists for a variety of disorders including Raynaud's phenomenon Citation70, altitude-induced hypoxemia Citation71, and cardiomyopathy associated with dystrophin deficiency Citation72. Vardenafil has been shown to improve mild depression compared to placebo in men with erectile dysfunction Citation73. Vardenafil does not appear to cross the blood–brain barrier, and so a direct effect is unlikely; the authors conclude that the effect is likely the result of improved sexual functioning and stress the importance of treating this symptom in depression. Sildenafil, however, does cross the blood–brain barrier and may also enhance memory and neurogenesis Citation74. Sildenafil has been successfully used to treat serotonin reuptake inhibitor-associated erectile dysfunction Citation75. In an open-label continuation of a placebo-controlled trial of sildenafil in antidepressant-related sexual dysfunction, the Hamilton depression score improved in individuals switched from placebo to sildenafil and showed no change in those already on sildenafil Citation76; erectile dysfunction improved in all subjects. Further studies need to be done to explore whether centrally acting PDE5 inhibitors have direct CNS effects that can lead to improvement in depressive symptoms.
Evidence for the role of PDEs in depression: human genetic studies
Our group recently reported an association of PDE genes with depression and with antidepressant treatment response Citation77. Single nucleotide polymorphisms (SNPs) were genotyped in 21 PDE genes in 284 depressed Mexican-Americans who participated in a prospective, double-blind, pharmacogenetic study of antidepressant treatment response and in 331 Mexican-American controls. A PDE11A haplotype was significantly associated with major depression, and remission on antidepressants was significantly associated with polymorphisms in PDE1A and PDE11A. However, a study of these two SNPs in a self-identified Hispanic population, a Caucasian population, and an African-American population treated with citalopram in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial failed to replicate the association between depression and PDE11A and PDE1A Citation78, showing only a nominal association between the tested PDE11A SNP and drug response in the African-American group. Further replications are needed to confirm these preliminary findings in ethnically defined populations. Interestingly, these findings support that dual-substrate or cGMP PDEs may be relevant to Major depression and suggest that drugs targeted to affect PDE function could represent a new treatment strategy for major depression and increasing the complexity of understanding the role cyclic mononucleotides in depression and antidepressant response.
Horvath et al. reported inactivating mutations disrupting the expression of PDE11A in three kindreds with adrenocortical hyperplasia Citation79. This was the first report linking a PDE gene to an inherited disorder associated with tumor formation. Mutation carriers present Cushing's syndrome early in life. This may have some relevance to depression, which is associated with glucocorticoid dysregulation Citation80.
PDE4B interacts directly with another major genetic locus that has been identified as a risk factor for depression, as well as for bipolar disorder and schizophrenia, known as DISC-1 (disrupted in schizophrenia-1) Citation81, Citation82. DISC-1 and PDE4B form complexes that may result in modulation of PDE4B activity; mutations in DISC-1 that result in abnormal phenotypes in animals occur in the binding sites for PDE4B. It is possible that these complexes may play a role in psychiatric illness. PDE10A inhibitors are also being investigated in the treatment of schizophrenia, with implications for other psychiatric disorders Citation83.
Recent results from a genome-wide linkage scan in the Genetics of Recurrent Early-Onset major depression (GenRED) study suggested evidence for linkage on chromosome 15q25-26 Citation84. Interestingly, the gene for PDE8A, a cAMP-specific PDE, falls under this linkage region (15q25.3); further investigation is needed of to clarify whether PDE8A could be indeed amongst the genes implicated in this chromosomal region in depression.
Finally, PDEs are expressed in immune and proinflammatory cells, and PDE inhibitors are being actively considered as candidates to treat disorders of chronic inflammation such as asthma, chronic obstructive pulmonary disease, rheumatoid arthritis, ankylosing spondylitis, Crohn's disease, and psoriasis Citation11, Citation12. They may also play a role in the neuroimmune endocrine axis that may mediate and modulate depressive and stress reactions Citation85, Citation86.
Conclusion
Treatment of major depression remains a challenge despite advances in pharmacology and neuroscience. Exploration of new pathways and drug candidates is essential to advance the field and offer hope to individuals for whom current treatments are ineffective. Evidence for involvement of PDEs in depression reviewed here suggests that these enzymes make attractive targets for drug development in depression. PDE antagonists have been active targets for current drug development efforts; but unfortunately, with the exception of PDE5 inhibitors, drugs are not yet commercially available to target most of these enzymes. Much research is needed to fully understand the impact of PDEs and their genetic variations in CNS function and stress response; presently, the role of PDEs in major depression and therapeutic effects of antidepressants is far from certain, but this research area represents a new promising frontier worthy of further investigation. As regulators of the ubiquitous second messengers cAMP and cGMP, PDE genetic variants may impact on several organ systems and their functions, providing a potential link between major depression, its systemic consequences, and comorbid stress-reactive conditions.
Acknowledgements
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003; 289: 3095–105
- National Center for Health Statistics. Health, United States, 2007. With Chartbook on Trends in the Health of Americans, Hyattsville, , MD: 2007.
- Kennedy SH, Giacobbe P. Treatment resistant depression—advances in somatic therapies. Ann Clin Psychiatry. 2007; 19: 279–87
- Rush AJ. Limitations in efficacy of antidepressant monotherapy. J Clin Psychiatry 2007; 68 Suppl 10: 8–10
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006; 163: 28–40
- Murphy GM, Jr, Kremer C, Rodrigues HE, Schatzberg AF. Pharmacogenetics of antidepressant medication intolerance. Am J Psychiatry. 2003; 160: 1830–5
- Papakostas GI, Fava M, Thase ME. Treatment of SSRI-resistant depression: a meta-analysis comparing within- versus across-class switches. Biol Psychiatry. 2008; 63: 699–704
- Mathew SJ, Manji HK, Charney DS. Novel drugs and therapeutic targets for severe mood disorders. Neuropsychopharmacology. 2008; 33: 2300
- Hebb AL, Robertson HA. Role of phosphodiesterases in neurological and psychiatric disease. Curr Opin Pharmacol. 2007; 7: 86–92
- Halene TB, Siegel SJ. PDE inhibitors in psychiatry—future options for dementia, depression and schizophrenia?. Drug Discov Today. 2007; 12: 870–8
- Giembycz MA, Smith SJ. Phosphodiesterase 7A: a new therapeutic target for alleviating chronic inflammation?. Curr Pharm Des. 2006; 12: 3207–20
- Houslay MD, Schafer P, Zhang KY. Keynote review: phosphodiesterase-4 as a therapeutic target. Drug Discov Today. 2005; 10: 1503–19
- Perry MJ, Higgs GA. Chemotherapeutic potential of phosphodiesterase inhibitors. Curr Opin Chem Biol. 1998; 2: 472–81
- Lugnier C. Cyclic nucleotide phosphodiesterase (PDE) superfamily: a new target for the development of specific therapeutic agents. Pharmacol Ther. 2006; 109: 366–98
- Tanis KQ, Duman RS. Intracellular signaling pathways pave roads to recovery for mood disorders. Ann Med. 2007; 39: 531–44
- Teng FY, Tang BL. Axonal regeneration in adult CNS neurons—signaling molecules and pathways. J Neurochem. 2006; 96: 1501–8
- Nair A, Vaidya VA. Cyclic AMP response element binding protein and brain-derived neurotrophic factor: molecules that modulate our mood?. J Biosci. 2006; 31: 423–34
- Soderling TR, Corbin JD, Park CR. Regulation of adenosine 3′,5′-monophosphate-dependent protein kinase. II. Hormonal regulation of the adipose tissue enzyme. J Biol Chem. 1973; 248: 1822–9
- Jarnaess E, Tasken K. Spatiotemporal control of cAMP signalling processes by anchored signalling complexes. Biochem Soc Trans. 2007; 35: 931–7
- Su YF, Johnson GL, Cubeddu L, Leichtling BH, Ortmann R, Perkins JP. Regulation of adenosine 3′:5′-monophosphate content of human astrocytoma cells: mechanism of agonist-specific desensitization. J Cyclic Nucleotide Res. 1976; 2: 271–85
- Barber R, Clark RB, Kelly LA, Butcher RW. A model of desensitization in intact cells. Adv Cyclic Nucleotide Res. 1978; 9: 507–16
- Hausdorff WP, Caron MG, Lefkowitz RJ. Turning off the signal: desensitization of beta-adrenergic receptor function. FASEB J. 1990; 4: 2881–9
- Lohse MJ. Molecular mechanisms of membrane receptor desensitization. Biochim Biophys Acta. 1993; 1179: 171–88
- Conti M, Nemoz G, Sette C, Vicini E. Recent progress in understanding the hormonal regulation of phosphodiesterases. Endocr Rev. 1995; 16: 370–89
- Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev. 2006; 58: 488–520
- Menniti FS, Faraci WS, Schmidt CJ. Phosphodiesterases in the CNS: targets for drug development. Nat Rev Drug Discov. 2006; 5: 660–70
- Charbonneau H, Prusti RK, LeTrong H, Sonnenburg WK, Mullaney PJ, Walsh KA, et al. Identification of a noncatalytic cGMP-binding domain conserved in both the cGMP-stimulated and photoreceptor cyclic nucleotide phosphodiesterases. Proc Natl Acad Sci U S A. 1990; 87: 288–92
- Aravind L, Ponting CP. The GAF domain: an evolutionary link between diverse phototransducing proteins. Trends Biochem Sci. 1997; 22: 458–9
- Letunic I, Copley RR, Pils B, Pinkert S, Schultz J, Bork P. SMART 5: domains in the context of genomes and networks. Nucleic Acids Res. 2006;34( Database issue):D257–60.
- Schulz DW, Mansbach RS, Sprouse J, Braselton JP, Collins J, Corman M, et al. CP-154,526: a potent and selective nonpeptide antagonist of corticotropin releasing factor receptors. Proc Natl Acad Sci U S A. 1996; 93: 10477–82
- Martinez SE, Beavo JA, Hol WG. GAF Domains: Two-billion-year-old molecular switches that bind cyclic nucleotides. Mol Interv. 2002; 2: 317–23
- Zoraghi R, Corbin JD, Francis SH. Properties and functions of GAF domains in cyclic nucleotide phosphodiesterases and other proteins. Mol Pharmacol. 2004; 65: 267–78
- Soderling SH, Bayuga SJ, Beavo JA. Isolation and characterization of a dual-substrate phosphodiesterase gene family: PDE10A. Proc Natl Acad Sci U S A. 1999; 96: 7071–6
- Hetman JM, Robas N, Baxendale R, Fidock M, Phillips SC, Soderling SH, et al. Cloning and characterization of two splice variants of human phosphodiesterase 11A. Proc Natl Acad Sci U S A. 2000; 97: 12891–5
- Yuasa K, Kotera J, Fujishige K, Michibata H, Sasaki T, Omori K. Isolation and characterization of two novel phosphodiesterase PDE11A variants showing unique structure and tissue-specific expression. J Biol Chem. 2000; 275: 31469–79
- Weeks JL, 2nd, Zoraghi R, Francis SH, Corbin JD. N-Terminal domain of phosphodiesterase-11A4 (PDE11A4) decreases affinity of the catalytic site for substrates and tadalafil, and is involved in oligomerization. Biochemistry. 2007; 46: 10353–64
- Fawcett L, Baxendale R, Stacey P, McGrouther C, Harrow I, Soderling S, et al. Molecular cloning and characterization of a distinct human phosphodiesterase gene family: PDE11A. Proc Natl Acad Sci U S A. 2000; 97: 3702–7
- Nibuya M, Nestler EJ, Duman RS. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci. 1996; 16: 2365–72
- Sturton G, Fitzgerald M. Phosphodiesterase 4 inhibitors for the treatment of COPD. Chest. 2002; 121: 192S–6S
- Suda S, Nibuya M, Ishiguro T, Suda H. Transcriptional and translational regulation of phosphodiesterase type IV isozymes in rat brain by electroconvulsive seizure and antidepressant drug treatment. J Neurochem. 1998; 71: 1554–63
- Takahashi M, Terwilliger R, Lane C, Mezes PS, Conti M, Duman RS. Chronic antidepressant administration increases the expression of cAMP-specific phosphodiesterase 4A and 4B isoforms. J Neurosci. 1999; 19: 610–8
- Ye Y, Jackson K, O'Donnell JM. Effects of repeated antidepressant treatment of type 4A phosphodiesterase (PDE4A) in rat brain. J Neurochem. 2000; 74: 1257–62
- Miro X, Perez-Torres S, Artigas F, Puigdomenech P, Palacios JM, Mengod G. Regulation of cAMP phosphodiesterase mRNAs expression in rat brain by acute and chronic fluoxetine treatment. An in situ hybridization study. Neuropharmacology. 2002; 43: 1148–57
- D'Sa C, Eisch AJ, Bolger GB, Duman RS. Differential expression and regulation of the cAMP-selective phosphodiesterase type 4A splice variants in rat brain by chronic antidepressant administration. Eur J Neurosci. 2005; 22: 1463–75
- Cho CH, Cho DH, Seo MR, Juhnn YS. Differential changes in the expression of cyclic nucleotide phosphodiesterase isoforms in rat brains by chronic treatment with electroconvulsive shock. Exp Mol Med. 2000; 32: 110–4
- Dlaboga D, Hajjhussein H, O'Donnell JM. Regulation of phosphodiesterase-4 (PDE4) expression in mouse brain by repeated antidepressant treatment: comparison with rolipram. Brain Res. 2006; 1096: 104–12
- Fujita M, Imaizumi M, D'Sa C, Zoghbi SS, Crescenzo MS, Hong J, et al. In vivo and in vitro measurement of brain phosphodiesterase 4 in rats after antidepressant administration. Synapse. 2007; 61: 78–86
- Zhang HT, Huang Y, Jin SL, Frith SA, Suvarna N, Conti M, et al. Antidepressant-like profile and reduced sensitivity to rolipram in mice deficient in the PDE4D phosphodiesterase enzyme. Neuropsychopharmacology. 2002; 27: 587–95
- Kurt M, Bilge SS, Aksoz E, Kukula O, Celik S, Kesim Y. Effect of sildenafil on anxiety in the plus-maze test in mice. Pol J Pharmacol. 2004; 56: 353–7
- Hebb AL, Robertson HA, Denovan-Wright EM. Phosphodiesterase 10A inhibition is associated with locomotor and cognitive deficits and increased anxiety in mice. Eur Neuropsychopharmacol. 2008; 18: 339–63
- Itoh T, Abe K, Tokumura M, Horiuchi M, Inoue O, Ibii N. Different regulation of adenylyl cyclase and rolipram-sensitive phosphodiesterase activity on the frontal cortex and hippocampus in learned helplessness rats. Brain Res. 2003; 991: 142–9
- Itoh T, Tokumura M, Abe K. Effects of rolipram, a phosphodiesterase 4 inhibitor, in combination with imipramine on depressive behavior, CRE-binding activity and BDNF level in learned helplessness rats. Eur J Pharmacol. 2004; 498: 135–42
- Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol. 2007; 18: 391–418
- Kaster MP, Rosa AO, Santos AR, Rodrigues AL. Involvement of nitric oxide-cGMP pathway in the antidepressant-like effects of adenosine in the forced swimming test. Int J Neuropsychopharmacol. 2005; 8: 601–6
- Dhir A, Kulkarni SK. Effect of addition of yohimbine (alpha-2-receptor antagonist) to the antidepressant activity of fluoxetine or venlafaxine in the mouse forced swim test. Pharmacology. 2007; 80: 239–43
- Dhir A, Kulkarni SK. Involvement of nitric oxide (NO) signaling pathway in the antidepressant action of bupropion, a dopamine reuptake inhibitor. Eur J Pharmacol. 2007; 568: 177–85
- Volke V, Wegener G, Vasar E. Augmentation of the NO-cGMP cascade induces anxiogenic-like effect in mice. J Physiol Pharmacol. 2003; 54: 653–60
- Brink CB, Clapton JD, Eagar BE, Harvey BH. Appearance of antidepressant-like effect by sildenafil in rats after central muscarinic receptor blockade: evidence from behavioural and neuro-receptor studies. J Neural Transm. 2008; 115: 117–25
- Zhang HT, Huang Y, Masood A, Stolinski LR, Li Y, Zhang L, et al. Anxiogenic-like behavioral phenotype of mice deficient in phosphodiesterase 4B (PDE4B). Neuropsychopharmacology. 2008; 33: 1611–23
- Siuciak JA, McCarthy SA, Chapin DS, Fujiwara RA, James LC, Williams RD, et al. Genetic deletion of the striatum-enriched phosphodiesterase PDE10A: evidence for altered striatal function. Neuropharmacology. 2006; 51: 374–85
- Siuciak JA, McCarthy SA, Chapin DS, Martin AN. Behavioral and neurochemical characterization of mice deficient in the phosphodiesterase-4B (PDE4B) enzyme. Psychopharmacology (Berl) 2008; 197: 115–26
- Perez-Torres S, Miro X, Palacios JM, Cortes R, Puigdomenech P, Mengod G. Phosphodiesterase type 4 isozymes expression in human brain examined by in situ hybridization histochemistry and[3H]rolipram binding autoradiography. Comparison with monkey and rat brain. J Chem Neuroanat. 2000; 20: 349–74
- Zeller E, Stief HJ, Pflug B, Sastre-y-Hernandez M. Results of a phase II study of the antidepressant effect of rolipram. Pharmacopsychiatry. 1984; 17: 188–90
- Horowski R, Satre-Y-Hernandez M. Clinical effects of the neurotropic selective cAMP phosphodiesterases inhibitor rolipram in depressed patients: global evaluation of the preliminary reports. Curr Ther Res. 1985; 38: 23–9
- Bobon D, Breulet M, Gerard-Vandenhove MA, Guiot-Goffioul F, Plomteux G, Sastre-y-Hernandez M, et al. Is phosphodiesterase inhibition a new mechanism of antidepressant action? A double blind double-dummy study between rolipram and desipramine in hospitalized major and/or endogenous depressives. Eur Arch Psychiatry Neurol Sci. 1988; 238: 2–6
- Laux G, Becker T, Kuhne G, Lesch KP, Riederer P, Beckmann H. Clinical and biochemical effects of the selective phosphodiesterase inhibitor rolipram in depressed inpatients controlled by determination of plasma level. Pharmacopsychiatry. 1988; 21: 378–9
- Clinical Trials.gov, M0952 in patients with mild-to-moderate Alzheimer's Disease, www.clinicaltrials.gov/ct2/show/NCT00362024 (accessed 10 August 2008).
- O'Donnell JM, Zhang HT. Antidepressant effects of inhibitors of cAMP phosphodiesterase (PDE4). Trends Pharmacol Sci. 2004; 25: 158–63
- Robichaud A, Stamatiou PB, Jin SL, Lachance N, MacDonald D, Laliberte F, et al. Deletion of phosphodiesterase 4D in mice shortens alpha(2)-adrenoceptor-mediated anesthesia, a behavioral correlate of emesis. J Clin Invest. 2002; 110: 1045–52
- Fries R, Shariat K, von Wilmowsky H, Bohm M. Sildenafil in the treatment of Raynaud's phenomenon resistant to vasodilatory therapy. Circulation. 2005; 112: 2980–5
- Richalet JP, Gratadour P, Robach P, Pham I, Dechaux M, Joncquiert-Latarjet A, et al. Sildenafil inhibits altitude-induced hypoxemia and pulmonary hypertension. Am J Respir Crit Care Med. 2005; 171: 275–81
- Khairallah M, Khairallah RJ, Young ME, Allen BG, Gillis MA, Danialou G, et al. Sildenafil and cardiomyocyte-specific cGMP signaling prevent cardiomyopathic changes associated with dystrophin deficiency. Proc Natl Acad Sci U S A. 2008; 105: 7028–33
- Rosen RC, Jackson G, Kostis JB. Erectile dysfunction and cardiac disease: recommendations of the Second Princeton Conference. Curr Urol Rep. 2006; 7: 490–6
- Uthayathas S, Karuppagounder SS, Thrash BM, Parameshwaran K, Suppiramaniam V, Dhanasekaran M. Versatile effects of sildenafil: recent pharmacological applications. Pharmacol Rep. 2007; 59: 150–63
- Fava M, Nurnberg HG, Seidman SN, Holloway W, Nicholas S, Tseng LJ, et al. Efficacy and safety of sildenafil in men with serotonergic antidepressant-associated erectile dysfunction: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2006; 67: 240–6
- Nurnberg HG, Fava M, Gelenberg AJ, Hensley PL, Paine S. Open-label sildenafil treatment of partial and non-responders to double-blind treatment in men with antidepressant-associated sexual dysfunction. Int J Impot Res. 2007; 19: 167–75
- Wong ML, Whelan F, Deloukas P, Whittaker P, Delgado M, Cantor RM, et al. Phosphodiesterase genes are associated with susceptibility to major depression and antidepressant treatment response. Proc Natl Acad Sci U S A. 2006; 103: 15124–9
- Teranishi KS, Slager SL, Garriock H, Kraft JB, Peters EJ, Reinalda MS, et al. Variants in PDE11A and PDE1A are not associated with citalopram response. Mol Psychiatry. 2007; 12: 1061–3
- Horvath A, Giatzakis C, Robinson-White A, Boikos S, Levine E, Griffin K, et al. Adrenal hyperplasia and adenomas are associated with inhibition of phosphodiesterase 11A in carriers of PDE11A sequence variants that are frequent in the population. Cancer Res. 2006; 66: 11571–5
- Gillespie CF, Nemeroff CB. Hypercortisolemia and depression. Psychosom Med. 67 Suppl 2005; 1: S26–8
- Millar JK, Mackie S, Clapcote SJ, Murdoch H, Pickard BS, Christie S, et al. Disrupted in schizophrenia 1 and phosphodiesterase 4B: towards an understanding of psychiatric illness. J Physiol. 2007; 584: 401–5
- Millar JK, Pickard BS, Mackie S, James R, Christie S, Buchanan SR, et al. DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science. 2005; 310: 1187–91
- Schmidt CJ, Chapin DS, Cianfrogna J, Corman ML, Hajos M, Harms JF, et al. Preclinical characterization of selective phosphodiesterase 10A inhibitors: a new therapeutic approach to the treatment of schizophrenia. J Pharmacol Exp Ther. 2008; 325: 681–90
- Holmans P, Weissman MM, Zubenko GS, Scheftner WA, Crowe RR, Depaulo JR, Jr, et al. Genetics of recurrent early-onset major depression (GenRED): final genome scan report. Am J Psychiatry. 2007; 164: 248–58
- Licinio J, Mastronardi C, Wong ML. Pharmacogenomics of neuroimmune interactions in human psychiatric disorders. Exp Physiol. 2007; 92: 807–11
- Licinio J, Wong ML. The role of inflammatory mediators in the biology of major depression: central nervous system cytokines modulate the biological substrate of depressive symptoms, regulate stress-responsive systems, and contribute to neurotoxicity and neuroprotection. Mol Psychiatry. 1999; 4: 317–27