Abstract
Background and aims. We aimed to replicate the previously observed association between acetylcholine receptor subtype M2 (CHRM2) gene polymorphisms and heart rate recovery (HRR) after exercise in patients with a recent acute myocardial infarction (AMI) and assess the prognostic significance of CHRM2 gene variants after AMI.
Methods. HRR was determined as the difference between maximal heart rate and heart rate at 1 minute after the symptom-limited bicycle exercise test in 192 post-AMI patients. Genetic variants at the CHRM2 locus in intron 5 (rs324640) and the 3’-UTR of exon 6 (rs8191992) were assessed.
Results. The rs324640 C/C and rs8191992 A/A homozygotes had more than a 3-fold risk of being in the lowest HRR quartile (≤ 8 bpm) compared to the T/T homozygotes (odds ratio (OR) 3.2, 95% confidence interval (CI) 1.2–8.6, P=0.017 and OR 3.8, 95% CI 1.3–11.1, P=0.016, respectively). In a larger sample of post-AMI patients (n=491), both C/C and A/A genotypes predicted cardiac mortality (11%) (adjusted relative risk (RR) 2.5, 95% CI 1.4–4.3, P=0.002 and RR 2.1, 95% CI 1.1–3.8, P=0.017, respectively) during a follow-up of 7.7±2.2 years.
Conclusions. DNA sequence variation at the CHRM2 locus is a determinant of cardiac autonomic function in the postexercise early recovery phase and predicts cardiac mortality after AMI.
Introduction
Impaired heart rate recovery (HRR) after physical exercise has been shown to be a powerful independent predictor of mortality in healthy subjects and in various patient populations Citation1–8. The interplay between vagal and sympathetic regulation mediates the decrease in heart rate (HR) during recovery from physical exercise. Acetylcholine released from cardiac vagal nerve endings triggers a negative chronotropic effect by decreasing HR Citation9, Citation10. Although the exact mechanisms of impaired HRR are not known, it has been suggested that delayed vagal reactivation following the termination of exercise is involved in impaired HRR Citation5, Citation11–15.
Muscarinic acetylcholine receptors play a fundamental role in cardiac function via vagally mediated regulation of the autonomic nervous system. The human heart expresses predominantly muscarinic acetylcholine receptor subtype 2 (CHRM2) Citation9, Citation10. Fisher et al. showed that vagally induced bradycardia responses are totally abolished in CHRM2-deficient mice in vivo Citation16, providing evidence for the functional significance of CHRM2 in HR regulation.
Abbreviations
Our previous study showed that DNA sequence variation at the CHRM2 locus is a potential modifier of HRR among healthy subjects Citation17. In a small substudy of a postacute myocardial infarction (AMI) population, we have previously reported that blunted HRR predicts short-term mortality in this cohort Citation2. Therefore, we set out to test two hypotheses: First, is there an association between the CHRM2 gene polymorphisms and HRR in post-AMI patients? Second, if there is such an association, do the genetic polymorphisms at CHRM2 predict mortality of post-AMI patients?
Methods
Patients
The study population consisted of 491 patients (during the period 1996–2000) with a recent (=7 days) MI (Division of Cardiology, University of Oulu, Finland), whose medical treatment was optimized according to the contemporary guide-lines. The demographic characteristics of the study cohort are presented in details in previous reports Citation18 and summarized in . The exclusion criteria were advanced age (>75 years), unstable angina at the time of recruitment, inability to perform the bicycle exercise test, dementia, alcoholism, drug abuse, non-sinus rhythm in electrocardiography, or any condition that could impair the subject's capacity to give informed consent. Almost all patients (97%) were on beta-blocking medication. All patients with markedly reduced left ventricular systolic function (<40%) were treated with angiotensin-converting enzyme inhibitors. All subjects were on aspirin or warfarin, unless contraindicated. Furthermore, all subjects with plasma cholesterol >5 mmol/L were on statins. The Ethical Committee of the Northern Ostrobothnia Hospital District, Oulu, Finland, approved the protocol, and all patients gave informed consent.
Key messages
The determinants of heart rate recovery (HRR) after exercise are not well known, although an attenuated HRR is an independent predictor of mortality among various patient populations.
The acetylcholine receptor subtype M2 (CHRM2) plays a key role in vagally induced bradycardiac responses.
DNA sequence variation in the CHRM2 locus is a potential modifier in the postexercise early recovery phase and predicts cardiac mortality after an acute myocardial infarction.
Table I. Clinical characteristics of the entire study group and in CHRM2 rs324640 and rs8191992 genotype groups.
Cardiac function measurements
Left ventricular systolic function was measured with echocardiography at 4–7 days after the acute MI. The left ventricle was divided into 16 segments, each of which was scored for its motion (-1 for dyskinesia, 0 for akinesia, 1 for hypokinesia, 2 for normokinesia, and 3 for hyperkinesia). Wall motion index is the mean score of all segments, and an estimate of the left ventricular ejection fraction was calculated by multiplying the wall motion index by 30 Citation19.
Exercise testing and heart rate recovery
The exercise testing data, which have been previously reported Citation2, were available for 192 patients. Systolic (SBP) and diastolic (DBP) blood pressures were assessed using an electronic sphygmomanometer (Dinamap Compact T, GE Medical Systems, Germany) at rest and during exercise testing. The subjects lay supine in a quiet room for at least 5 min before the resting blood pressure measurements. A symptom-limited maximal bicycle exercise test was performed by starting from a 25 W work-load and increasing the work-load by 25 W every 3 minutes. Blood pressures were assessed at the end of each work-load. The patients were encouraged to reach a symptom-limited maximal work-load. A real-time microprocessor QRS detector system (R-R Recorder, Polar Electro Ltd, Kempele, Finland) was used to record R-R data with a timing accuracy of 1 ms Citation20 during the exercise and for 10 minutes afterwards. HRR was determined as the difference between the maximal exercise HR and HR at 1 minute after exercise.
Determination of genotypes
Ethylenediamine tetra-acetic acid (EDTA) blood samples were obtained from all patients, and DNA was isolated. Two single-nucleotide polymorphisms (SNPs) were selected based on our previous findings of the associations between HRR and CHRM2 gene polymorphisms among healthy subjects Citation17: rs324640 in intron 5 (10,571 bp upstream of exon 6) and rs8191992 in the untranslated region of exon 6. The SNPs were genotyped using template-directed dye-terminator incorporation with the fluorescence polarization detection (FP-TDI) method. A DNA sequence containing a SNP was amplified with polymerase chain reaction (PCR), and after cleaning with shrimp alkaline phosphatase and exonuclease I, the PCR product was used as a template in the AcycloPrime-FP reaction. The SNP detection primers were designed so as to locate their 3’ end immediately upstream of the polymorphic (SNP) site. The SNP detection PCR reaction utilized a mutant thermostable polymerase and a pair of AcycloTerminators labeled with R110 and TAMRA (AcycloPrime-FP SNP kit, PerkinElmer Life Sciences, Boston MA), representing possible alleles for the SNP of interest. Allele detection was done by measuring the changes in fluorescence polarization after excitation of the samples by plane-polarized light using a Victor3 FP Plate Reader (PerkinElmer Life Sciences) on a 96-well plate format. Allele calling was performed using the SNPscorer genotyping software (SNPmacroVICTOR96 v. 7.0, PerkinElmer Life Sciences). Haplotypes were constructed using the SHEsis software package Citation21.
Follow-up and end-points
Patients were followed up for 7.7±2.2 years after their MI. Two independent end-point committees verified the mode of death in each case Citation18. The mortality statistics of the Central Statistical Office in Finland and the Causes of Death Register were used to confirm the mode of death. The quality of these registers has been validated previously Citation22–24. The end-points of this study were all-cause mortality and cardiac mortality.
Statistical analyses
A chi-square test was used to verify whether the observed genotype frequencies were in Hardy-Weinberg equilibrium, and pairwise linkage disequilibrium (LD) between the SNPs was assessed using the SHEsis software package Citation21. The normal Gaussian distribution of the data was verified by the Kolmogorov-Smirnov goodness-of-fit test. A chi-square test was also used for a comparison of the categorical parameters. The associations between the genotype groups, the end-point phenotypes, and the associations with the haplotypes were tested with an analysis of variance using the general linear model (GLM) procedure. The phenotypes were adjusted for age, sex, and body mass index (BMI). In addition, age, sex, BMI, and maximal HR were used as covariates for HRR. Our study group was divided into sex-specific quartiles according to HRR in order to verify whether the groups differed from each other in terms of the measured variables and genotypes. We used logistic regression analysis based on a binary dependent variable (lowest HRR quartile versus other quartiles) and calculated the odds ratio (OR) with 95% confidence interval (CI), for each genotype group, of having a poor HRR, i.e. being in the lowest HRR quartile while adjusting for age, BMI, and maximal HR (Model 1). We further adjusted Model 1 for New York Heart Association class (NYHA class) ≥ 2, medical treatment with aspirin or warfarin, changes in HR from rest to maximal exercise (ΔHR), and the maximal body weight-related work-load (W/kg) to exclude the possibility of these factors confounding our results (Model 2).
To assess the independent predictive value of CHRM2, multivariate Cox regression analysis was performed separately for genotype and haplotype, including age, sex, BMI, left ventricular systolic function, and diabetes as covariates. The results of the Cox regression analysis were expressed as relative risk (RR) with a 95% CI. Kaplan-Meier analysis with a log-rank test was used to compare survival curves between the genotype groups. A P-value <0.05 was considered statistically significant. The data were analyzed using the SPSS software (SPSS 12.0.1, SPSS Inc., USA).
Results
The frequencies of the CHRM2 rs324640 C and rs8191992 A alleles were 0.435 and 0.423, respectively. Pairwise linkage disequilibrium between the markers was r2=0.655 (D’=0.830). All genotype frequencies were in Hardy-Weinberg equilibrium. The genotype groups did not differ from each other in terms of clinical characteristics. Clinical characteristics according to rs324640 and rs8191992 genotype groups are presented in . Comparable characteristics were found also in the subset of 192 patients with exercise-testing data.
CHRM2 polymorphisms and heart rate recovery
HRR showed a non-significant tendency toward a difference between the CHRM2 genotypes, e.g. HRR was 11±8 bpm for the rs324640 C/C genotype, 13±6 bpm for C/T, and 14±7 bpm for T/T (P=0.138) (). Because of a possible non-linear association between the CHRM2 genotypes and HRR among the patients with cardiac disease, the cohort was divided into quartiles using sex-specific HRR cut-offs. The lowest quartile (≤ 8 bpm) was defined as the high-risk HRR group. The CHRM2 genotypes were separately analyzed in each quartile.
Table II. Associations between hemodynamic variables at rest and at maximal exercise according to CHRM2 rs324640 and rs8191992 genotype groups. Values are expressed as mean (SD). P-values refer to GLM procedure.
The patients in the lowest HRR quartile were older (63 years) compared to those in the other HRR quartiles (56–59 years, P=0.010). A total of 15% of the patients in the highest HRR quartile had NYHA class ≥2, whereas in the other HRR quartiles the number of patients with NYHA-class ≥2 varied from 33% to 40% (P=0.031). The medical treatment with aspirin or warfarin was lower (77%) among the patients in the lowest HRR quartile compared to patients in the other HRR quartiles (90%–100%, P=0.010). The maximal exercise HR, the increase in HR from rest to maximal exercise (chronotropic response), and the maximal body weight-related work-load (W/kg) were associated with HRR (P<0.0001, P<0.0001, and P=0.007, respectively) (). After adjustment for age, sex, and BMI, the statistical difference between the groups remained evident (P<0.0001, P=0.001, and P=0.066, respectively).
Table III. Associations between hemodynamic variables at rest and at maximal exercise in sex-specific quartiles according to heart rate recovery. Values are expressed as mean (SD). P-values refer to the GLM procedure.
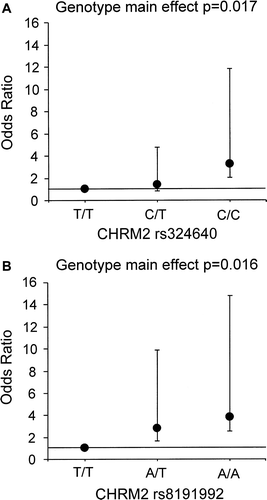
Logistic regression models using the lowest quartile versus the other three quartiles of HRR as a dependent variable showed both CHRM2 SNPs to be associated with HRR. The rs324640 C/C homozygotes had an over 3-fold risk of being in the lowest HRR quartile compared to the T/T homozygotes (OR 3.2; 95% CI 1.2–8.6, P=0.017) (A) after adjusting for age, BMI, and maximal HR (Model 1). Likewise, the risk of being in the lowest HRR quartile was 3.8 times higher among the rs8191992 A/A homozygotes than in the T/T homozygotes after adjusting for confounders (OR 3.8; 95% CI 1.3–11.1; P=0.016) (B, Model 1). After further adjustment for NYHA class ≥2, medical treatment with aspirin or warfarin, changes in HR from rest to maximal exercise, and the maximal body weight-adjusted work-load (Model 2), the risk for the minor allele genotypes of being in the lowest HRR quartiles compared to the common allele genotypes remained evident for both CHRM2 SNPs (rs324640: OR 3.7; 95% CI 1.3–10.1, P=0.012 and rs8191992: OR 4.1; 95% CI 1.3–12.5, P=0.013).
CHRM2 polymorphisms and risk of cardiac death
During the mean follow-up of 7.7±2.2 years, all-cause mortality (n=106) was 22% and cardiac mortality (n=52) was 11%. None of the CHRM2 genotypes were associated with all-cause mortality. However, both minor allele genotypes predicted cardiac death in univariate analysis (). The rs324640 C/C homozygotes had an over 2-fold risk for cardiac death compared to C/T and T/T genotypes (RR 2.2; 95% CI 1.3–3.9, P=0.005). Likewise, the risk for cardiac death was 1.8 times higher among the rs8191992 A/A homozygotes than for AT and T/T genotypes (RR 1.8; 95% CI 1.0–3.3; P=0.044). Both minor allele genotypes remained significant predictors of cardiac death in multivariate analysis after adjustment for age, sex, BMI, ejection fraction, and diabetes (rs324640 C/C: RR 2.5, 95% CI 1.4–4.3, P=0.002 and rs8191992 A/A: RR 2.1, 95% CI 1.1–3.8, P=0.017). Kaplan-Meier survival curves for CHRM2 rs324640 and rs8191992 genotypes as a predictor of cardiac death are presented in .
Figure 2. CHRM2 rs324640 (A) and rs8191992 (B) gene polymorphisms as predictors of cardiac death in Kaplan-Meier survival analysis. P-value=statistical significance of log rank.
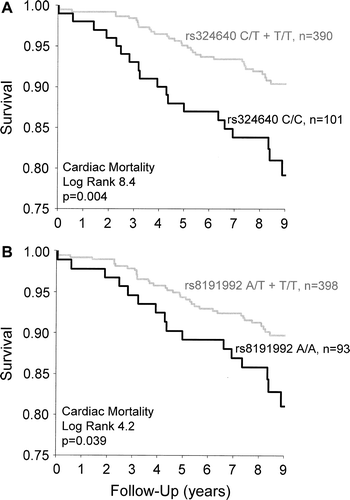
Table IV. The relative risk of death according to CHRM2 rs324640 C/C and rs8191992 A/A genotypes in relation to grouping of two remaining genotypes in uni- and multivariate Cox regression analysis including age, sex, body mass index, ejection fraction, and diabetes as covariates.
Haplotype analysis
The CHRM2 haplotypes were constructed using the ‘best’ option of the SHEsis software haplotyping function. The haplotype analyses confirmed the associations detected with individual SNPs, but it did not further strengthen the associations for HRR or risk of cardiac death. For example, the haplotype consisting of the minor alleles of the rs324640 and rs8191992 variants (CA) predicted cardiac death both under univariate (RR 2.3, 95% CI 1.3–4.2, P=0.007) and multivariate analysis (RR 2.3, 95% CI 1.3–4.3, P=0.006).
Discussion
We have shown that acetylcholine receptor M2 (CHRM2) gene polymorphisms are associated with the early HRR after maximal symptom-limited exercise and risk of cardiac death among patients with a recent MI. The minor alleles at CHRM2 rs324640 and rs8191992 SNPs were significantly more prevalent among MI patients with markedly impaired HRR after exercise. Moreover, CHRM2 rs324640 and rs8191992 minor allele genotypes showed increased risk for cardiac death. Since postexercise HRR has been shown to be an independent predictor of mortality among patients with a recent MI, the present results may provide insight into the molecular mechanisms underlying the regulation of HRR and its association with the pathophysiology of adverse cardiovascular events.
Role of CHRM2 in the regulation of heart rate recovery
Activation of cardiac vagal efferents leads to release of acetylcholine, which acts on cardiac CHRM2 to decrease HR Citation9, Citation10, Citation16. Several studies have shown that the main physiological mechanism underlying postexercise cardiodeceleration is vagal reactivation Citation3, Citation4, Citation11–13. A recent study showed that acetylcholine receptor resistance in rats resulted in reduced cardiac muscarinic receptor function, leading to cardiovagal insufficiency Citation25. Furthermore, mongrel dogs vulnerable to ventricular fibrillation had reduced HRR compared to dogs resistant to malignant arrhythmias. The differences in HRR and cardiac vagal activity were eliminated by administration of atropine, which confirmed the dominant role of vagal activity in postexercise control of HR Citation15. Moreover, we previously reported that, among healthy individuals, CHRM2 gene polymorphisms are potential modifiers of HRR both in a sedentary state and after an endurance training program Citation17. In the present study, impaired HRR was shown to be associated with the same SNPs of the CHRM2 gene even among post-MI patients. Taken together, these findings suggest that attenuated HRR in the early recovery phase and increased risk for cardiac death in the presence of CHRM2 minor alleles define a pathophysically distinct clinical cohort of MI patients with preserved HRR who are likely to be heterozygotes or homozygotes for the major alleles at these two SNPs of the CHRM2 gene.
Heart rate recovery and risk of cardiac death among patients with myocardial infarction
Cardiovascular autonomic regulation during exercise, consisting of an increase in sympathetic activity and a decrease in vagal activity, is altered in patients with MI. Despite the increased sympathetic activation, exercise-induced increments in HR are markedly attenuated Citation26. This blunted chronotropic response to exercise is most likely to be due to blunted beta-adrenoceptor responsiveness Citation27 with a limited capacity to further withdraw vagal activity during exercise Citation28. In the present study, the patients were on beta-blocking medication, which inhibits sympathetic activation. We speculate that this may have contributed to the magnitude of HRR after maximal exercise. In addition to beta-blocking medication and genotype, complex interactions between hemodynamic, clinical, therapeutic, autonomic dysregulation and psychological factors may influence HRR among post-AMI patients.
We observed that the proportion of patients with NYHA class ≥2 was lowest in the highest quartile of HRR compared to the other quartiles. This may be associated with better exercise capacity, which was seen as a tendency for higher maximal work-load and augmented HR response to exercise in the highest quartile compared to the other quartiles. Furthermore, the medical treatment with aspirin or warfarin was lower in the lowest quartile of HRR compared to the other quartiles. One possible explanation for this is polypharmacy Citation29. If patients consume many drugs, it is possible that the medical treatment is not the same for all. The notion of higher risk for non-optimized therapy has been previously reported among elderly patients Citation29. Our subjects in the lowest quartile of HRR were older compared to patients in the other quartiles. However, after adjustment for potential confounders, the higher incidence of the minor allele genotypes at the CHRM2 gene among patients with very poor HRR remained evident. This implies a functional link between DNA sequence variation at the CHRM2 locus and HRR.
The cut-off value of HRR <12 bpm used in a previous study has been found to provide prognostic information about MI patients Citation2. This is also the most common cut-off value defining normal versus abnormal HRR Citation4, Citation30. A recent study by Wolk and colleagues showed that heart failure patients with the lowest HRR (≤6 bpm) represented a cohort with greater impairment in terms of echocardiographic, neurohormonal, and hemodynamic characteristics Citation31. Furthermore, they also found that 50% of patients with the lowest HRR had heart failure of ischemic etiology, in contrast to patients with the highest HRR (≥13), in whom the prevalence of ischemic heart failure was only 7%. Furthermore, very poor HRR (<6.5 bpm) has also been shown to provide additional prognostic information about heart failure patients Citation32. The present results showed that poor HRR (≤8 bpm) among post-MI patients is also related to impaired exercise capacity and a reduced chronotropic response to exercise, which is in line with the previous findings on heart failure patients with delayed HRR Citation31.
The associations between genotype and cardiac death were ranged from 1.8- to 2.2-fold in univariate analysis in the present study. Interestingly, the degree of association remained stable (2.1- to 2.5-fold) in multivariate analysis after adjustment for demographic variables, and established risk factors including left ventricular systolic function and diabetes. This provides support for the hypothesis that the CHRM2 gene locus makes a true biological contribution to impaired cardiac function, which emphasizes its relevance for predicting cardiac mortality and providing additional prognostic information in the clinical setting. Furthermore, since the CHRM2 gene variants predicted specifically cardiac death and not all-cause mortality, a causal relationship is suggested between these gene variants and progressive impairment of cardiac responsiveness and/or risk of fatal events after AMI.
Limitations
The hall-marks of a strong genetic association study include accurate and comprehensive genotyping, evaluation of a large number of carefully phenotyped subjects, and replication of significant associations. We recognize that the modest number of patients in the present study is a limiting factor. Therefore, replication in an independent population is clearly warranted. Furthermore, the focus of the present study could have been sharpened if it had been based on tag single nucleotide polymorphisms, which represent independent haplotype blocks. It would have made it possible to explore more thoroughly the associations between the entire store of genetic variation at the CHRM2 locus, and HRR and survival among patients with MI. This is an important issue and one that will need to be considered if future studies are to yield more precise definition of the high- and low-risk genotype groups.
Finally, although our results suggest some form of causality, it should be recognized that they are based on association only. Complex statistical models can never substitute for true experimental data or randomized control trials.
Conclusions and implications
These data suggest that, among patients with a recent MI, the minor alleles of two SNPs at the CHRM2 locus are more prevalent in those with low HRR, and are associated with increased risk for cardiac death. This may reflect a genetic predisposition to impaired autonomic cardiovascular regulation. Since postexercise HRR has been shown to be an independent predictor of mortality both in healthy subjects and in various patient groups, the present results provide suggestive evidence on the molecular mechanisms underlying the regulation of HRR and its association with adverse cardiovascular events among patients with MI. The identification of specific genetic markers related to autonomic dysfunction would improve risk stratification in the general population, as well as in different patient groups.
Acknowledgements
This study was supported by grants from the Finnish Ministry of Education and the Medical Council of the Academy of Finland, Helsinki, Finland. C. Bouchard is partially funded by the George A. Bray Endowed Chair in Nutrition. The authors express their gratitude to Päivi Kastell, RN, Pirkko Huikuri, RN, and Päivi Karjalainen, RN. Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Cheng YJ, Lauer MS, Earnest CP, Church TS, Kampert JB, Gibbons LW, et al. Heart rate recovery following maximal exercise testing as a predictor of cardiovascular disease and all-cause mortality in men with diabetes. Diabetes Care. 2003; 26: 2052–7
- Nissinen SI, Makikallio TH, Seppanen T, Tapanainen JM, Salo M, Tulppo MP, et al. Heart rate recovery after exercise as a predictor of mortality among survivors of acute myocardial infarction. Am J Cardiol. 2003; 91: 711–4
- Jouven X, Empana JP, Schwartz PJ, Desnos M, Courbon D, Ducimetiere P. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med. 2005; 352: 1951–8
- Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999; 341: 1351–7
- Cole CR, Foody JM, Blackstone EH, Lauer MS. Heart rate recovery after submaximal exercise testing as a predictor of mortality in a cardiovascularly healthy cohort. Ann Intern Med. 2000; 132: 552–5
- Watanabe J, Thamilarasan M, Blackstone EH, Thomas JD, Lauer MS. Heart rate recovery immediately after treadmill exercise and left ventricular systolic dysfunction as predictors of mortality: the case of stress echocardiography. Circulation. 2001; 104: 1911–6
- Vivekananthan DP, Blackstone EH, Pothier CE, Lauer MS. Heart rate recovery after exercise is a predictor of mortality, independent of the angiographic severity of coronary disease. J Am Coll Cardiol. 2003; 42: 831–8
- Diller GP, Dimopoulos K, Okonko D, Uebing A, Broberg CS, Babu-Narayan S, et al. Heart rate response during exercise predicts survival in adults with congenital heart disease. J Am Coll Cardiol. 2006; 48: 1250–6
- Brodde OE, Michel MC. Adrenergic and muscarinic receptors in the human heart. Pharmacol Rev. 1999; 51: 651–90
- Caulfield MP. Muscarinic receptors—characterization, coupling and function. Pharmacol Ther. 1993; 58: 319–79
- Arai Y, Saul JP, Albrecht P, Hartley LH, Lilly LS, Cohen RJ, et al. Modulation of cardiac autonomic activity during and immediately after exercise. Am J Physiol. 1989; 256: H132–41
- Pierpont GL, Stolpman DR, Gornick CC. Heart rate recovery post-exercise as an index of parasympathetic activity. J Auton Nerv Syst. 2000; 80: 169–74
- Imai K, Sato H, Hori M, Kusuoka H, Ozaki H, Yokoyama H, et al. Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J Am Coll Cardiol. 1994; 24: 1529–35
- Curfman GD, Hillis LD. A new look at cardiac exercise testing. N Engl J Med. 2003; 348: 775–6
- Smith LL, Kukielka M, Billman GE. Heart rate recovery after exercise: a predictor of ventricular fibrillation susceptibility after myocardial infarction. Am J Physiol Heart Circ Physiol. 2005; 288: H1763–9
- Fisher JT, Vincent SG, Gomeza J, Yamada M, Wess J. Loss of vagally mediated bradycardia and bronchoconstriction in mice lacking M2 or M3 muscarinic acetylcholine receptors. FASEB J. 2004; 18: 711–3
- Hautala AJ, Rankinen T, Kiviniemi AM, Makikallio TH, Huikuri HV, Bouchard C, et al. Heart rate recovery after maximal exercise is associated with acetylcholine receptor M2 (CHRM2) gene polymorphism. Am J Physiol Heart Circ Physiol. 2006; 291: H459–66
- Huikuri HV, Tapanainen JM, Lindgren K, Raatikainen P, Mäkikallio TH, Juhani Airaksinen KE, et al. Prediction of sudden cardiac death after myocardial infarction in the beta-blocking era. J Am Coll Cardiol. 2003; 42: 652–8
- Berning J, Steensgaard-Hansen F. Early estimation of risk by echocardiographic determination of wall motion index in an unselected population with acute myocardial infarction. Am J Cardiol. 1990; 65: 567–76
- Ruha A, Sallinen S, Nissila S. A real-time microprocessor QRS detector system with a 1-ms timing accuracy for the measurement of ambulatory HRV. IEEE Trans Biomed Eng. 1997; 44: 159–67
- Shi YY, He L. SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell Res. 2005; 15: 97–8
- Pajunen P, Koukkunen H, Ketonen M, Jerkkola T, Immonen-Raiha P, Karja-Koskenkari P, et al. The validity of the Finnish Hospital Discharge Register and Causes of Death Register data on coronary heart disease. Eur J Cardiovasc Prev Rehabil. 2005; 12: 132–7
- Mahonen M, Salomaa V, Torppa J, Miettinen H, Pyorala K, Immonen-Raiha P, et al. The validity of the routine mortality statistics on coronary heart disease in Finland: comparison with the FINMONICA MI register data for the years 1983–1992. Finnish multinational MONItoring of trends and determinants in CArdiovascular disease. J Clin Epidemiol 1999; 52: 157–66
- Rapola JM, Virtamo J, Korhonen P, Haapakoski J, Hartman AM, Edwards BK, et al. Validity of diagnoses of major coronary events in national registers of hospital diagnoses and deaths in Finland. Eur J Epidemiol. 1997; 13: 133–8
- Padley JR, Overstreet DH, Pilowsky PM, Goodchild AK. Impaired cardiac and sympathetic autonomic control in rats differing in acetylcholine receptor sensitivity. Am J Physiol Heart Circ Physiol. 2005; 289: H1985–92
- Colucci WS, Ribeiro JP, Rocco MB, Quigg RJ, Creager MA, Marsh JD, et al. Impaired chronotropic response to exercise in patients with congestive heart failure. Role of postsynaptic beta-adrenergic desensitization. Circulation. 1989; 80: 314–23
- Izawa K, Tanabe K, Omiya K, Yamada S, Yokoyama Y, Ishiguro T, et al. Impaired chronotropic response to exercise in acute myocardial infarction patients with type 2 diabetes mellitus. Jpn Heart J. 2003; 44: 187–99
- Binkley PF, Nunziata E, Haas GJ, Nelson SD, Cody RJ. Parasympathetic withdrawal is an integral component of autonomic imbalance in congestive heart failure: demonstration in human subjects and verification in a paced canine model of ventricular failure. J Am Coll Cardiol. 1991; 18: 464–72
- Makikallio TH, Barthel P, Schneider R, Bauer A, Tapanainen JM, Tulppo MP, et al. Frequency of sudden cardiac death among acute myocardial infarction survivors with optimized medical and revascularization therapy. Am J Cardiol. 2006; 97: 480–4
- Gibbons RJ. Abnormal heart-rate recovery after exercise. Lancet. 2002; 359: 1536–7
- Wolk R, Somers VK, Gibbons RJ, Olson T, O'Malley K, Johnson BD. Pathophysiological characteristics of heart rate recovery in heart failure. Med Sci Sports Exerc. 2006; 38: 1367–73
- Arena R, Guazzi M, Myers J, Peberdy MA. Prognostic value of heart rate recovery in patients with heart failure. Am Heart J 2006; 151:181: e7–13