Abstract
Background. Helicobacter pylori causes chronic gastritis, peptic ulcer disease, and is the most important risk factor for non-cardia gastric cancer, and has been shown to upregulate matrix metalloproteinases (MMPs) in infected gastric mucosa. MMPs are proteolytic enzymes regulated by tissue inhibitors of metalloproteinases (TIMPs).
Aims. We set up this study to find out whether H. pylori gastritis induces systemic MMP response.
Methods. Serum samples were collected from patients undergoing gastroscopy; 26 patients had H. pylori gastritis and 18 were H. pylori-negative controls with normal gastric mucosa. Serum MMP levels were analysed by enzyme-linked immunosorbent assay.
Results. Significantly elevated serum levels of collagenase-2 (MMP-8), gelatinase B (MMP-9), neutrophil elastase (NE), and myeloperoxidase (MPO), and reduced serum levels of gelatinase A (MMP-2) and TIMP-1 were demonstrated in patients with H. pylori gastritis as compared to H. pylori-negative controls. No significant differences were shown in serum matrilysin-1 (MMP-7) levels.
Conclusions. For the first time, we show enhanced MMP-8 response in H. pylori infection together with other neutrophil degranulation products (MMP-9, MPO, NE). Elevated circulating neutrophil degranulation product levels in serum of H. pylori-positive patients reflect accelerated proteolysis and oxidative stress, and may contribute to extraintestinal sequelae, such as cardiovascular diseases.
Introduction
Helicobacter pylori causes gastritis, and, although the infection is asymptomatic in most cases, some infected persons develop severe gastric diseases and may be at risk for some extragastric disorders as well. H. pylori is considered the most important single risk factor in peptic ulcer disease and non-cardiac gastric cancer Citation1. Despite the activated immune response of the host, as shown for example by abundant polymorphonuclear and mononuclear leukocyte infiltration in the gastric mucosa as well as high levels of specific H. pylori antibodies Citation2, the chronic infection persists for decades unless actively eradicated Citation3. Neither the reasons for chronicity nor those for the development of serious sequelae of the infection are completely clarified.
Matrix metalloproteinases (MMPs) are genetically distinct but structurally related zinc-dependent metalloendopeptidases, which can be classified based on their primary structures and substrate specificities into different groups, such as collagenases (MMP-1, -8, -13), gelatinases (MMP-2, -9), stromelysins (MMP-3, -10, -11, -19), matrilysins (MMP-7, -26), membrane-type MMPs (MT-MMPs) (MMP-14, -15, -16, -17, -24, -25), and other MMPs Citation4, Citation5. MMPs can collectively degrade almost all components of extracellular matrix and basement membrane, as well as process serpins, growth factors, pro- and anti-inflammatory cytokines and chemokines, as well as apoptotic signals to modulate immune response Citation4–7. They have also been shown to be important in malignant pathological processes Citation8. The excess activity of MMPs leads to host damage by causing connective tissue break-down Citation5, Citation7. In host defence against bacteria, leukocytes migrate to the site of inflammation and, due to infectious or inflammatory stimuli, MMP-8 and MMP-9 are released and locally activated Citation5, Citation7, Citation9. The cleavages of non-matrix cytokine and chemokine substrates of MMP-8 and MMP-9 can be decisive and subsequently can direct the pro- and anti-inflammatory actions of the neutrophil-derived MMPs Citation5, Citation7, Citation9. In this regard, MMP-8 and MMP-9 have recently been shown to modify immune response by modulating chemokinesis and to exert defensive and anti-inflammatory properties Citation5, Citation6. Recently the role of MMPs in immune response has been under keen investigation in various infectious diseases Citation7. The activity of MMPs is regulated by antiproteases called tissue inhibitors of metalloproteinases (TIMPs) Citation10 and recently some specific TIMPs, including TIMP-1, were demonstrated to be locally upregulated in the gastric mucosa of H. pylori patients Citation11. Neutrophil elastase (NE) is a serine protease which is degranulated from azurophilic granules of neutrophils when exposed to inflammatory stimuli and microbial virulence factors. NE can degrade almost all extracellular matrix and plasma proteins as well as activate proMMPs and inactivate TIMP-1 Citation5, Citation12, Citation13. Degranulation of neutrophils releases also antimicrobial substances such as myeloperoxidase (MPO). MPO, by generating oxidants such as hypochlorous acid, is able not only to oxidatively inactivate pathogenic microbes but also to inactivate TIMP-1 as well as to activate latent proMMPs Citation5, Citation12, Citation14. Thus, MPO oxidatively and NE proteolytically can potentiate the destructive MMP cascades.
Key messages
For the first time, we show enhanced MMP-8 response in Helicobacter pylori infection together with other neutrophil degranulation products (MMP-9, myeloperoxidase, neutrophil elastase).
Elevated circulating neutrophil degranulation product levels in serum of H. pylori-positive patients reflect accelerated proteolysis and oxidative stress, and may contribute to extraintestinal sequelae, such as cardiovascular diseases.
The role of MMPs in gastrointestinal tract diseases, such as gastric ulcer Citation15 and possibly cancer Citation16, Citation17, has been suggested. As gastric ulcer and cancer are important late gastric sequelae of a chronic H. pylori infection, the association of MMPs with H. pylori infection has recently gained a lot of interest Citation7. In fact, regarding H. pylori infection, increased expression of MMP-2 Citation18–22, MMP-7 Citation19, Citation20, Citation23, Citation24, and MMP-9 Citation18–22, Citation25, Citation26 has been demonstrated by cell and gastric biopsy experiments whereas the role of MMP-8 is practically unknown Citation11. The present study was set up to find out whether H. pylori gastritis induces an enhanced MMP-8 response and if the local gastric infection is also reflected in a systemic MMP-2, -7, -9, TIMP-1, NE, and MPO response by measuring circulating levels in serum of these patients.
Methods
Patients
A total of 26 Caucasian patients (age range 42–82, median 64 years; 18 females) with histologically proven H. pylori gastritis and 18 H. pylori-negative Caucasian patients (age range 62–85, median 72 years; 15 females) with macroscopically and histologically proven normal gastric mucosa were included. Both the patients and the controls were gastroscopied at Herttoniemi Hospital in 2004–2005. shows the underlying diseases of the patients. The indications for and macroscopical and histological findings of the H. pylori-positive patients are shown in . The gastroscopy indications for the controls were the following: reflux (seven patients), gastric pain (five patients), anaemia (three patients), weight loss (one patient), and suspected coeliac disease (two patients). The H. pylori-negative controls were older than the H. pylori-positive patients (P=0.0241, Mann-Whitney test).
Table I. Underlying diseases of the Helicobacter pylori-positive patients and the H. pylori-negative controls. No statistically significant differences in the presence of any of the underlying diseases were found between the two groups.
Table II. Characteristics of Helicobacter pylori-positive gastroscopied patients.
The Ethics Committee of the Hospital District of Helsinki and Uusimaa approved the study, and all the participants gave their written informed consent.
Gastric histology
The gastric biopsies (two from antrum and two from corpus) were stained with haematoxylin-eosin, Alcian Blue (pH 2.5)-periodic acid-Schiff, and modified Giemsa stains. Grade of gastritis () was assessed by one experienced pathologist according to the updated Sydney system Citation27.
Serum
Serum samples were collected from patients in context with the endoscopy and were stored at −20°C until analysed. MMP-2, -7, -8, -9, NE, MPO, and TIMP-1 analyses were carried out by enzyme-linked immunosorbent assay as earlier described Citation28, Citation29. MMP-2, MMP-7, MMP-8, MMP-9, and TIMP-1 concentrations were determined using commercially available enzyme-linked immunosorbent assay (ELISA) kits. Biotrak ELISA systems (Amersham Biosciences UK Ltd, Buckinghamshire, UK) were used for MMP-2, MMP-8, and MMP-9 according to the manufacturer's protocol. DuoSet ELISA development Systems (R&D Systems, Minneapolis, USA) for TIMP-1 and Quantakine MMP-7 (total) immunoassay for MMP-7 were used correspondingly. All samples were analysed in duplicate. The used MMP- and TIMP-1 ELISAs detect as informed by the manufacturer active, pro-, complexed and fragmented forms of the studied MMPs and TIMP-1. MPO and NE levels were measured according to manufacturer's instructions by commercial kits purchased from Immunodiagnostic AG (Bensheim, Germany) and Bender MedSystems mbH (Vienna, Austria), respectively. The secondary antibody in each kit was conjugated with horseradish peroxidase, and tetramethyl benzidine was used as a substrate. The absorbance was measured at 450 nm using Labsystems Multiskan RC (Thermo Bioanalysis Corporation, Santa FE, USA). The levels of MMPs, TIMP-1, MPO, and NE were expressed as ng per mL, and for calculation of MMP/TIMP-1 ratios the levels were converted to mol per L.
Serum C-reactive protein (CRP) levels (≥0.3 mg/L) were measured by routine methods.
Statistical analysis
Data were analysed by using GraphPad Prism version 4.0c (GraphPad Inc., San Diego, California, USA). Data of two groups were compared by the Mann-Whitney test. Data are presented as median (25%–75% percentiles). Correlations were studied using Spearman's rank correlation test. A P-value less than 0.05 was considered statistically significant.
Results
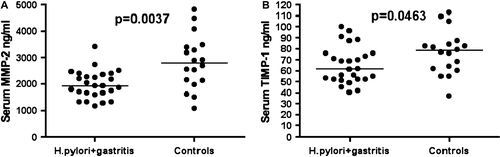
The serum levels of sensitive CRP did not significantly differ (P=0.5828) between the H. pylori-positive gastritis patients (median 1.0, range, <0.3–7.0 mg/L) and the H. pylori-negative controls with normal gastric mucosa (median 0.95, range <0.3–12.0 mg/L). However, MMP-8, MMP-9, NE, and MPO serum levels of patients with H. pylori gastritis were significantly elevated when compared to the serum levels of H. pylori-negative control patients (). The levels were 27.6 (21.2–39.6) versus 11.3 (8.05–14.5) ng of MMP-8 per mL, P<0.0001 (A), and 170 (132–251) versus 112 (73.5–201) ng of MMP-9 per mL, P=0.0290 (B). For NE and MPO, the corresponding levels were 169.5 (120.5–225.0) versus 89.0 (69.50–133.5) ng of NE per mL, P=0.0004 (C), and 81.50 (60.50–109.5) versus 46.50 (35.00–68.00) ng of MPO per mL, P=0.0075 (D), respectively. The serum MMP-2 levels of H. pylori gastritis patients were significantly decreased when compared to those of H. pylori-negative control patients (1921 (1616–2369) versus 2787 (2040–3420) ng of MMP-2 per mL, P=0.004) (A). Furthermore, serum levels of TIMP-1 were significantly lower in H. pylori-positive patients 61.50 (52.00–76.00) than in H. pylori-negative controls 78.00 (61.00–85.50) ng per mL, P=0.0463 (B). In addition, the MMP-8/TIMP-1 and MMP-9/TIMP-1 ratios for H. pylori-positive patients were significantly higher than those for H. pylori-negative controls (P<0.0001 and P=0.0047, respectively). On the contrary, serum levels of MMP-7 between H. pylori-positive and -negative subjects did not significantly differ (P=0.242). MMP-7 levels correlated with acute inflammation (r=0.47, P=0.016, scores for antrum and corpus together), but the levels of other biomarkers tested did not correlate either with acute or chronic or total inflammation scored either separately for antrum and corpus or both together. Among the H. pylori-negative control patients, the levels of MMP-7 (r=0.67, P=0.0022), MMP-9 (r=0.47, P=0.0480), and TIMP-1 (r=0.55, P=0.0185) correlated with increasing age.
Figure 1. Serum levels of neutrophilic degranulation markers matrix metalloproteinase (MMP)-8 (A), MMP-9 (B), neutrophil elastase (NE) (C), and myeloperoxidase (MPO) (D) of 26 Helicobacter pylori gastritis patients and 18 H. pylori-negative controls with normal gastric mucosa. Data of two groups compared by the Mann-Whitney test. The horizontal line indicates median.
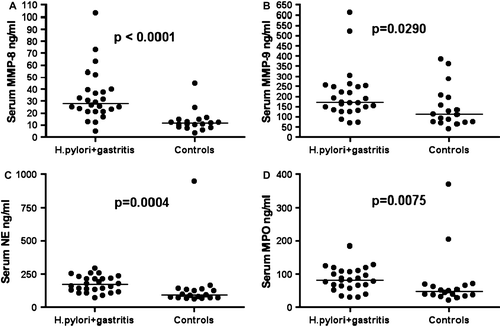
Figure 2. Serum matrix metalloproteinase (MMP)-2 (A) and tissue inhibitor of metalloproteinase (TIMP)-1 (B) levels of 26 Helicobacter pylori gastritis patients and 18 H. pylori-negative controls with normal gastric mucosa. Data of two groups compared by the Mann-Whitney test. The horizontal line indicates median.
Discussion
We found significantly elevated serum levels of neutrophilic degranulation markers MMP-8, MMP-9, NE, and MPO in patients with H. pylori gastritis as compared to H. pylori-negative controls with macroscopically and histologically normal gastric mucosa. In addition, significantly decreased levels of serum MMP-2 and TIMP-1 were detected in H. pylori gastritis patients when compared to H. pylori-negative controls. These findings indicate, for the first time, that H. pylori infection is associated with an enhanced MMP-8 response. The finding strongly suggests enhanced neutrophil degranulation further supported by elevated serum levels of NE and MPO in H. pylori-positive patients as compared to H. pylori-negative controls. Elevated MPO may indeed potentiate the oxidative activation of both MMP-8 and MMP-9 as well as oxidatively inactivate TIMP-1 Citation12, Citation14. Enhanced levels of MMP-8 and MPO indicate increased degranulation of specific granules of neutrophils. NE, a serine protease, can also accelerate MMP-cascades by activating latent proMMPs and inactivating TIMP-1 Citation5, Citation12. The lower serum levels of TIMP-1 and higher MMP-8/TIMP-1 ratios in H. pylori gastritis patients as compared to H. pylori-negative individuals suggest that the proteolytic activity of MMP-8 may be relatively unopposed by TIMP-1 in chronic H. pylori gastritis or that the higher TIMP-1 levels in controls were due to the older age.
Earlier studies have shown an increased production of MMP-9, another MMP from C-particles or tertiary granules of neutrophils Citation5, Citation12, in H. pylori-infected gastric and other cells Citation18, Citation20–22, Citation25, Citation26, Citation30 and in gastric biopsies from H. pylori-positive patients Citation18, Citation25. In the present study, we could confirm and further extend the association of H. pylori infection with elevated MMP-9 production, and we could show, for the first time, also a systemic elevation of MMP-9 in serum of patients with chronic H. pylori gastritis. In addition to MMP-9, we also assayed the serum levels of MMP-2, both of which can degrade type IV collagen abundant in basement membranes. Earlier studies have demonstrated in H. pylori infection that local expression of MMP-2 in gastric biopsies or in infected cell lines is upregulated Citation18–22 or there is no effect at all Citation25, Citation31. In the present study, the serum MMP-2 levels were found to be lower in H. pylori-positive patients as compared to H. pylori-negative subjects with normal gastric mucosa. Recently McQuibban et al. Citation32 showed that MMP-2 cleaved monocyte chemoattractant protein-3, which resulted in chemokine antagonism and reduced inflammation. In our study, the systemic ‘downregulation’ of MMP-2 in H. pylori infection may reflect, at least to some extent, enhanced inflammation against H. pylori infection.
MMP-7 expression levels have been shown to be enhanced in H. pylori-infected cell lines or in gastric biopsies Citation19, Citation20, Citation23, Citation24, and MMP-7 has been found to be capable of activating MMP-8 Citation33. However, in the present study, the serum levels of MMP-7 did not significantly differ between H. pylori-positive patients and controls with normal gastric mucosa.
Although it is generally considered that H. pylori has no proven role in extraintestinal sequelae, except probably for unexplained iron deficiency anaemia and idiopathic thrombocytopenic purpura Citation1, a recent meta-analysis indicated an association between more virulent CagA-positive (positive for protein coded by cytotoxin-associated gene) H. pylori infection and ischaemic heart disease Citation34. H. pylori Citation18, Citation19, Citation30 and especially CagA-positive strains Citation20, Citation22, Citation25 have been shown to upregulate MMP-9 production. Furthermore, according to a recent finding, successful therapy, in contrast to unsuccessful treatment, of H. pylori infection results in downregulation of MMP-9 in gastric biopsies taken from patients after therapy Citation35. There is increasing evidence supporting an association of MMPs, especially MMP-9, with the pathogenesis of cardiovascular diseases Citation36. Recent studies have shown that circulating MMP-8 and MMP-9 concentrations together with an elevated MMP-8/TIMP-1 ratio were significantly associated with cardiovascular disease deaths, indicating that elevated circulating MMP-8, MMP-9 and MMP-8/TIMP-1 can be regarded as important prognostic factors in patients with cardiovascular diseases Citation37–39. We found in this study that chronic H. pylori infection, lasting for decades unless eradicated, induces a systemic host response by increasing the serum levels of two functionally distinct MMPs, a collagenase MMP-8 and a gelatinase MMP-9, both of which may have a role in cardiovascular diseases Citation37–39. In addition, serum MMP-8/TIMP-1 and MMP-9/TIMP-1 ratios were significantly elevated. Overall, this suggests that, by increasing MMP-8 and MMP-9 (together with other tissue-destructive neutrophil degranulation products, MPO and NE) in systemic circulation where the activity is only moderately opposed by low levels of TIMP-1, chronic H. pylori infection may predispose to cardiovascular diseases. This may be especially possible in combination with elevated levels of sensitive serum CRP. Nevertheless, in our study, there was no significant difference in the serum CRP levels between the H. pylori-positive and -negative subjects.
The reasons why only a small number of H. pylori-infected individuals develop serious gastric and extragastric sequelae are not known, but bacterial, host, and environmental factors are thought to be involved Citation1. MMPs are enzymes which influence the host immune response in infectious diseases Citation7 and contribute to the pathogenesis of gastric ulcers Citation15 and possibly cancer Citation16, Citation17, Citation40. Recently in a work by Hellmig and co-workers, patients with certain genetic variants of MMP-9 had a higher risk for gastric ulcers than others Citation15. Furthermore, elevated plasma levels of MMP-9 have been demonstrated in patients with gastric cancer Citation41. In the present study, none of the Helicobacter-positive gastritis patients had duodenal ulcer but three of them had gastric ulcer. The MMP-9 levels of these three gastric ulcer patients were clearly above the median of all patients, and close or above the 75% percentile value (data not shown). In addition to MMP-9, the efficient collagen-degrading protease MMP-8 has recently been suggested to have a role in gastrointestinal diseases such as gastric cancer Citation40. Thus, it is not surprising that elevated levels of MMP-8, mostly originating from neutrophils, were detected in H. pylori gastritis in the present study. MMP-8 expression and levels are mainly regulated by selective release of the contents of specific granules upon neutrophil degranulation Citation5, Citation7, Citation12. Prepacked and presynthesized MMP-8 is released by triggered neutrophils without significant de novo synthesis Citation12. This also concerns two other neutrophilic degranulation products studied, i.e. MPO and NE. Therefore, the systemic elevated level of MMP-8 (and that of MPO and NE) in H. pylori infection as shown in the present study is not eventually reflected by local gastric production of MMP-8 mRNA Citation11.
Our main interest was to see if local H. pylori gastritis could associate with a systemic MMP response as demonstrated by circulating serum MMP and neutrophilic degranulation product (MPO and NE) levels, and thus it was crucial to include a control group with endoscopically verified normal gastric mucosa. Our control patients were all referred to upper endoscopy for various reasons and could have other diseases or infectious processes than H. pylori that may increase the MMP levels. However, no significant differences were detected either in the presence of underlying diseases or in the serum CRP levels between the H. pylori-positive gastritis patients and the controls.
In conclusion, in H. pylori gastritis the serum levels of MMP-8, MMP-9, MMP-8/TIMP-1, MMP-9/TIMP-1, MPO, and NE are elevated, but those of MMP-2 and TIMP-1 decreased as compared to the serum levels in H. pylori-negative individuals with healthy gastric mucosa. Our results for the first time suggest the association of H. pylori infection with a systematically enhanced neutrophil-derived proteolytic and oxidative response which may contribute to extraintestinal sequelae, such as cardiovascular diseases. Our novel findings raise the question whether we should search and treat H. pylori infection more actively, not only because of abnormalities in the gastrointestinal tract but also considering extraintestinal sequelae.
Acknowledgements
This study was in part presented at the XXth International Workshop on Helicobacter and Related Bacteria in Chronic Digestive Inflammation, European Helicobacter Study Group, Istanbul, Turkey, 20–22 September 2007. This work has been supported by grants from the Academy of Finland and Helsinki University Central Hospital Research (EVO) Funds. Declaration of interest: Dr Pentti Sipponen has served as a speaker, a consultant, and an advisory board member for Biohit Plc (a company that produces laboratory pipettes and develops and markets laboratory tests), and as a speaker for meetings and congresses of many pharmaceutical companies. In the last 10 years, he has not received any funds from any companies or organizations, except the funding from the University Hospital. Dr Sipponen owns stocks and shares in Biohit Plc, including some other companies on the stock-market in Helsinki. Dr Sipponen does not own any personal patents. The other authors have no competing interests. The authors alone are responsible for the content and writing of the paper.
References
- Malfertheiner P, Megraud F, O'Morain C, Bazzoli F, El-Omar E, Graham D, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007; 56: 772–81
- Sobala G, Crabtree J, Dixon M, Schorah C, Taylor J, Rathbone B, et al. Acute Helicobacter pylori infection: clinical features, local and systemic immune response, gastric mucosal histology, and gastric juice ascorbic acid concentrations. Gut. 1991; 32: 1415–8
- Kosunen TU, Aromaa A, Knekt P, Salomaa A, Rautelin H, Lohi P, et al. Helicobacter antibodies in 1973 and 1994 in the adult population of Vammala, Finland. Epidemiol Infect. 1997; 119: 29–34
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002; 2: 161–74
- Sorsa T, Tjaderhane L, Konttinen YT, Lauhio A, Salo T, Lee HM, et al. Matrix metalloproteinases: contribution to pathogenesis, diagnosis and treatment of periodontal inflammation. Ann Med. 2006; 38: 306–21
- McMillan SJ, Kearley J, Campbell JD, Zhu XW, Larbi KY, Shipley JM, et al. Matrix metalloproteinase-9 deficiency results in enhanced allergen-induced airway inflammation. J Immunol. 2004; 172: 2586–94
- Elkington PTG, O'Kane CM, Friedland JS. The paradox of matrix metalloproteinases in infectious disease. Clin Exp Immunol. 2005; 142: 12–20
- Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol. 2002; 3: 207–14
- Hartog CM, Wermelt JA, Sommerfeld CO, Eichler W, Dalhoff K, Braun J. Pulmonary matrix metalloproteinase excess in hospital-acquired pneumonia. Am J Respir Crit Care Med. 2003; 167: 593–8
- Baker AH, Edwards DR, Murphy G. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci. 2002; 115: 3719–27
- Bodger K, Ahmed S, Pazmany L, Pritchard DM, Michael A, Khan AL, et al. Altered gastric corpus expression of tissue inhibitors of metalloproteinases in human and murine Helicobacter infection. J Clin Pathol. 2008; 61: 72–8
- Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989; 320: 365–76
- Geraghty P, Rogan MP, Greene CM, Boxio RMM, Poiriert T, O'Mahony M, et al. Neutrophil elastase up-regulates cathepsin B and matrix metalloproteinase-2 expression. J Immunol. 2007; 178: 5871–8
- Wang Y, Rosen H, Madtes DK, Shao B, Martin TR, Heinecke JW, et al. Myeloperoxidase inactivates TIMP-1 by oxidizing its N-terminal cysteine residue. J Biol Chem. 2007; 282: 31826–34
- Hellmig S, Ott S, Rosenstiel P, Fölsch UR, Hampe J, Schreiber S. Genetic variants in matrix metalloproteinase genes are associated with development of gastric ulcer in H. pylori infection. Am J Gastroenterol. 2006; 101: 29–35
- Mrena J, Wiksten JP, Nordling S, Kokkola A, Ristimäki A, Haglund C. MMP-2 but not MMP-9 associated with COX-2 and survival in gastric cancer. J Clin Pathol. 2006; 59: 618–23
- Zheng H, Takahashi H, Murai Y, Cui Z, Nomoto K, Niwa H, et al. Expression of MMP-2, MMP-9 and VEGF are closely linked to growth, invasion, metastasis and angiogenesis of gastric cancer. Anticancer Res. 2006; 26: 3579–83
- Bergin PJ, Edebo A, Wen S, Johnsson E, Andersson J, Lönroth H, et al. Increased production of matrix metalloproteinases in Helicobacter pylori-associated human gastritis. Helicobacter. 2004; 9: 201–10
- Koyama S. Significance of cell-surface expression of matrix metalloproteinases and their inhibitors on gastric epithelium and infiltrating mucosal lymphocytes in progression of Helicobacter pylori-associated gastritis. Scand J Gastroenterol. 2004; 39: 1046–53
- Yanagisawa N, Geironson L, Al-Soud WA, Ljungh Å. Expression of matrix metalloproteinase-2, -7, -9 on human colon, liver and bile duct cell lines by enteric and gastric Helicobacter species. FEMS Immunol Med Microbiol. 2004; 44: 197–204
- Kundu P, Mukhopadhyay AK, Patra R, Banerjee A, Berg DE, Swarnakar S. Cag pathogenicity island-independent up-regulation of matrix metalloproteinases-9 and -2 secretion and expression in mice by Helicobacter pylori infection. J Biol Chem. 2006; 281: 34651–62
- Oliveira MJ, Costa AC, Costa AM, Henriques L, Suriano G, Atherton J, et al. Helicobacter pylori induces gastric epithelial cell invasion in a c-Met and type IV secretion system-dependent manner. J Biol Chem. 2006; 281: 34888–96
- Bebb JR, Letley DP, Thomas RJ, Aviles F, Collins HM, Watson SA, et al. Helicobacter pylori upregulates matrilysin (MMP-7) in epithelial cells in vivo and in vitro in a Cag dependent manner. Gut. 2003; 52: 1408–13
- Crawford HC, Krishna US, Israel DA, Matrisian LM, Washington MK, Peek RM. Helicobacter pylori strain-selective induction of matrix metalloproteinase-7 in vitro and within gastric mucosa. Gastroenterology. 2003; 125: 1125–36
- Mori N, Sato H, Hayashibara T, Senba M, Geleziunas R, Wada A, et al. Helicobacter pylori induces matrix metalloproteinase-9 through activation of nuclear factor (B. Gastroenterology. 2003; 124: 983–92
- Wu CY, Wang CJ, Tseng CC, Chen HP, Wu MS, Lin JT, et al. Helicobacter pylori promote gastric cancer cell invasion through a NF-kappaB and COX-2-mediated pathway. World J Gastroenterol. 2005; 11: 3197–203
- Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney system. International workshop on the histopathology of gastritis, Houston 1994. Am J Surg Pathol. 1996; 20: 1161–81
- Bergmann U, Michaelis J, Oberhoff R, Knauper V, Beckmann R, Tschesche H. Enzyme linked immunosorbent assays (ELISA) for the quantitative determination of human leukocyte collagenase and gelatinase. J Clin Chem Clin Biochem. 1989; 27: 351–9
- Lauhio A, Konttinen YT, Tschesche H, Nordstrom D, Salo T, Lahdevirta J, et al. Reduction of matrix metalloproteinase 8-neutrophil collagenase levels during long-term doxycycline treatment of reactive arthritis. Antimicrob Agents Chemother. 1994; 38: 400–2
- Bergin PJ, Wen S, Pan-Hammarström Q, Quiding-Järbrink M. Secretion of matrix metalloproteinase-9 by macrophages, in vitro, in response to Helicobacter pylori. FEMS Immunol Med Microbiol. 2005; 45: 159–69
- Yokoyama T, Otani Y, Kurihara N, Sakurai Y, Kameyama K, Suzuki H, et al. Matrix metalloproteinase expression in cultured human gastric wall fibroplasts—interactions with Helicobacter pylori isolated from patients with ulcers. Aliment Pharmacol Ther. 2000; 14: 193–8
- McQuibban GA, Gong JH, Tam EM, McCulloch CA, Clark-Lewis I, Overall CM. Inflammation dampened by gelatinase A cleavage of monocyte chemoattractant protein-3. Science. 2000; 289: 1202–6
- Dozier S, Escobar GP, Lindsey ML. Matrix metalloproteinase (MMP)-7 activates MMP-8 but not MMP-13. Med Chem. 2006; 2: 523–6
- Pasceri V, Patti G, Cammarota G, Pristipino C, Richichi G, Di Sciascio G. Virulent strains of Helicobacter pylori and vascular diseases: a meta analysis. Am Heart J. 2006; 151: 1215–22
- Danese S, Papa A, Ricci R, Maggiano N. Helicobacter pylori eradication down-regulates matrix metalloproteinase-9 expression in chronic gastritis and gastric ulcer. Gastroenterology. 2004; 126: 369–71
- Kai H, Ikeda H, Yasukawa H, Kai M, Seki Y, Kuwahara F, et al. Peripheral blood levels of matrix metalloproteases-2 and -9 are elevated in patients with acute coronary syndromes. J Am Coll Cardiol. 1998; 32: 368–72
- Blankenberg S, Rupprecht HJ, Poirier O, Bickel C, Smieja M, Hafner G, et al. Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease. Circulation. 2003; 107: 1579–85
- Lint VP, Libert C. Matrix metalloproteinase-8: cleavage can be decisive. Cytokine Growth Factor Rev. 2006; 17: 217–23
- Tuomainen AM, Nyyssönen K, Laukkanen JA, Tervahartiala T, Tuomainen TP, Salonen JT, et al. Serum matrix metalloproteinase-8 concentrations are associated with cardiovascular outcome in men. Arterioscl Tromb Vasc Biol 2007; 27: 2722–8
- Kubben FJGM, Sier CFM, Meijer MJW, van den Berg M, van der Reijden JJ, Griffioen G, et al. Clinical impact of MMP and TIMP gene polymorphisms in gastric cancer. Br J Cancer. 2006; 95: 744–51
- Torii A, Kodera Y, Uesaka K, Hirai T, Yasui K, Morimoto T, et al. Plasma concentration of matrix metalloproteinase 9 in gastric cancer. Br J Surg. 1997; 84: 133–6