Abstract
Background and aims. Prospective data on development of venous obstruction after electrode implantation are limited. We performed a prospective study on 150 patients undergoing first pacemaker implantation.
Methods. Venographies at base-line and 6 months postimplantation in all patients, 50 patients included into a long-term follow-up of a mean of 2.4 years after implantation.
Results. At 6 months 14% had obstructions, but only 1 patient (0.7%) developed acute symptomatic upper extremity venous thrombosis. Pulmonary embolism (PE) was encountered in 5 (3.3%).
After 6 months only 2 patients experienced pain in ipsilateral arm, but none had edema of arm, neck or head, or clinical PE. The 5 patients with total venous occlusion (TVO) at 6 months had no localized symptoms. Late venographic abnormalities developed in 5 (10%) patients: 4 TVOs and 1 stenosis. Two of the new lesions developed among 25 patients with normal 6-month venograms. Overall, TVO was detected in 9 of 150 patients. No factors emerged as independent predictors of total occlusion in multiple regression analysis.
Conclusions. TVO is not uncommon after pacemaker implantation, and mostly occurs without any localizing symptoms. Most venous lesions seem to develop during the first months postimplantation, but late and unpredictable TVO may also occur.
Introduction
Venous obstruction and thrombosis have been reported to develop in 20%–60% of patients after pacemaker (PM) implantation Citation1–6. Prospective data on the development of these venous changes are limited. Our venography-based prospective study revealed an incidence of 14% of venous obstructive lesions at 6 months after PM or implantable cardioverter-defibrillator (ICD) implantation Citation7. Because the time-course and risk factors for the development of venous lesions are still unclear, we report the prospective long-term follow-up of our patient cohort together with detailed data on all pacemaker-related complications during the follow-up.
Material and methods
Study protocol and patient selection
This study is part of a wider protocol in progress in our institutions to assess thrombotic and bleeding complications of cardiac procedures Citation8. This prospective study initially recruited a total of 150 consecutive adult patients scheduled for an implantation of their first permanent PM or ICD and without contraindications for venography Citation7. The recruitment of study patients was initiated in November 2003 and completed in February 2005. Details of the initial study group from the first 6 months have been described previously Citation7, and the key findings are summarized in .
Table I. Acute and subacute complications after pacemaker or implantable cardioverter-defibrillator implantation in 150 patients.
Our study was conducted in two hospitals in south-western Finland and approved by the ethical committees and hospital administrations of both institutions. Informed, written consent was obtained from all patients willing to participate. All patients were followed for complications during the initial base-line hospital stay and upon follow-up at 3 and 6 months. An intravenous contrast venography (ICV) and transthoracic echocardiography were conducted at base-line and at 6 months postoperatively. Data on patient history, medications, and symptoms were collected at base-line and at subsequent follow-up visits.
Abbreviations
For the long-term follow-up, we aimed to restudy half of the 150 patients who participated in the 6-month follow-up. Thus, 75 patients were initially aimed for the follow-up beyond the first 6 months. The selection was conducted in two cohorts: 1) all patients with venographic abnormalities at 6 months in order to evaluate the long-term fate of the lesions, and 2) a sample of patients with no venographic abnormalities at 6 months. However, 25 patients in total were excluded or dropped out due to following reasons: death (n=5), refusal to participate (n=6), elevated creatinine (n=1), an allergic reaction from previous venography (n=1), current in-patient treatment for unrelated serious disorders (n=3), and logistic difficulties due to geographical distance (n=6). Further two patients were excluded due to a failure of obtaining a venous access for venography, and one patient due a technical failure of digital storage of the venography. Thus, a total of 50 patients (mean age 66.5 years, 55% males) were included in the final long-term follow-up. Among these 50 patients the previous 6-month ICV had shown new venous abnormalities in 23 cases (46%), and in the remaining 27 (54%) the findings were unchanged compared to base-line ICV. The former group includes patients with venous stenosis (n=7), total occlusions (n=3), and small non-occlusive thrombi (n=13). Implanted device types were 30 (60%) dual-chamber, 8 (16%) single-chamber, 5 (10%) biventricular pacemakers (number of leads: 2–3, mean 2.4), and 7 (14%) ICDs (number of leads: 1–2, mean 1.3). The long-term follow-up visits and venographies were conducted at a mean of 2.4±0.3 years after the device implantation.
Key messages
This is one of the few prospective studies conducted on the development of venous pathology after pacemaker implantation.
Venous thrombosis and obstructions are relatively common after transvenous pacing lead implantation, but most of the lesions are asymptomatic.
Most cases of venous pathology seem to develop during the first months after device implantation, but late occlusions may also occur.
Clinical evaluation
All follow-ups included an interview to ascertain the patients’ symptoms, and a chart review for possible PM-related and other outpatient or inpatient hospital treatments, current antithrombotic medications, and potential symptoms located in the implantation area or in the ipsilateral upper extremity. Physical examination included an inspection of possible superficial venous collaterals and swelling of the upper extremity or neck.
Venographic technique
ICV was performed via an intravenous cannula inserted into the medial antecubital vein ipsilateral to the side of the device. In order to calibrate images for subsequent diameter measurements, a section of radio-opaque tape measure or a standard-length steel rod was placed on the skin overlying the imaged area as a measurement reference standard. A single 20 mL bolus of radiographic contrast dye (Hexabrix®, Guerbet, Roissy, France) was injected to image an area including the veins from proximal sections of upper extremity veins to the superior vena cava (SVC). During venography patients were instructed to breathe quietly without breath-holding to avoid the Valsalva effect. The ICVs were obtained in a single plane (anterior-posterior) and stored on CD-ROM disks for subsequent analysis and measurements. All ICVs were conducted in equal fashion.
Venographic analysis
At base-line, the narrowest and widest points of the target vessels for lead placement were identified by visual inspection to obtain minimum (Dmin) and maximum (Dmax) venous diameters, and measurements from two to three individually calibrated frames were averaged to express the final diameters. A publicly available digital image measurement software program (ImageJ®, US National Institute of Health) was utilized. Diameter measurements were repeated from the same venous segments in the follow-up ICVs in identical fashion. Base-line venographies were also analyzed for potential obstructions and malformations Citation7. The follow-up ICVs were analyzed for the presence of stenosis, complete occlusion, and/or non-flow-limiting thrombi. Definition of a new stenosis at 6 months had to meet the following requirements: 1) a diameter reduction of at least 50% compared to base-line ICV in a venous segment identified visually as the narrowest point in the follow-up ICV, and 2) no significant stenosis at the same location at base-line. The late ICVs were compared against both base-line and 6-month ICVs, and the same criteria for stenosis were utilized. Total occlusion was defined as a complete interruption of venous flow with or without new regional collateral veins. Non-flow-limiting thrombi were defined as venous filling defects attached to PM leads or vessel walls, but did not block flow, or narrowed the lumen less than 50%. Thus, only patients in whom both the base-line and the 6-month venograms were successfully completed (n=136; 91%) could be included in the determination of a new venous stenosis. All successful 6-month ICVs (n=140, 93%) were assessed for total occlusions and for potential non-obstructive filling defects suggestive of thrombus formation. The late venographies were compared with those obtained at base-line and at 6 months postimplantation to perform measurements at the same venous locations, and to assess for possible new areas of venous luminal narrowing or filling defects.
Statistical analysis
The absolute and relative frequencies were calculated for categorical variables, and statistically tested with chi-square or Fisher's exact tests. Continuous variables are expressed as mean±SD and tested for statistical significance with the Mann-Whitney U-test. The Wilcoxon signed rank test was used to calculate the significance of the changes over time in continuous variables. Independent predictors of end-points were searched by logistic regression analysis.
Results
Clinical complications
Acute complications after PM implantation developed in 8 (5.3%) of the 150 patients. These included two cases (1.3%) of pericardial effusion, one of which mandated drainage, and one case (0.7%) of pneumothorax (). During the 6-month follow-up, mechanical complications included three cases (2.0%) of atrial lead dislodgement, one (0.7%) patient with a lead failure due to subclavian crush, and one (0.7%) patient with an impending skin perforation requiring surgical revision ().
Symptom presentation
During the first 6 months of follow-up, only one patient (0.7%) was diagnosed with a symptomatic upper extremity deep venous thrombosis (venous Doppler ultrasound 10 days postoperatively). The patient was anticoagulated with warfarin for 3 months, resulting in complete resolution of the symptoms, and a fully patent venogram at 6 months and at 2 years. Symptomatic pulmonary embolism was encountered in a total of 5 (3.3%) of the 150 patients (). All cases of pulmonary embolism (PE) were diagnosed by a nuclear ventilation-perfusion scan. Symptoms of PE developed 2 months after implantation in one patient, and in the remaining four the diagnosis was made due to symptom-presentation at the 6-month follow-up visit. Two of the patients with PE had a TVO in the 6-month ICV, and two had patent veins, but no venography was available in the remaining one patient due to technical CD-ROM failure. The five patients with a TVO in 6-month venography had no localized symptoms in the ipsilateral upper extremity or in the region of the pacemaker pocket.
After 6 months no patients experienced swelling of the upper extremity, neck or head, or clinical pulmonary embolism. Only two (4%) patients expressed complaints of intermittent pain in the ipsilateral upper extremity, both of whom also presented with an abnormal late venogram. On inspection, none were found to have obvious superficial cutaneous collateral veins. Eleven patients had needed hospital admissions after the 6-month follow-up visit, which were PM-related in only two cases. Most of the patients (n=44, 86%) in the late follow-up were treated with an antithrombotic medication: either warfarin (n=23) or aspirin (n=21).
Venography at base-line and at 6 months
A successful diagnostic base-line venography was available in 145 (96.7%) patients. Base-line abnormalities were seen in ten (6.7%) venograms including seven (4.8%) cases of stenosis Citation7. No changes in the base-line abnormalities were seen in the majority of these patients at 6 months, but one patient with a base-line stenosis showed significant progression of the lesion, and one patient with a persistent left superior vena cava developed a new stenosis. In total, there were 14 (10.2%) cases of new stenosis and 5 (3.6%) total venous occlusions (TVO) at 6 months, detailed data on which have been reported previously Citation7. In addition to these obstructive lesions, there were new small non-flow limiting filling defects suggestive of thrombus formation in 20 (14%) of the 140 cases with a successful 6-month venogram. In total, 39 (28%) patients had an abnormal finding in the 6-month ICV, 23 (59%) of whom were included in the late follow-up.
Late venography
Serial venographies (base-line, 6 months, and 2 years) were available in all 50 patients. The mean venographic minimum and maximum diameters did not change significantly between the 6-month and late venographies (). New abnormalities were discovered in five (10%) patients. These included three confirmed cases of TVO, and one patient with borderline stenosis. The remaining one patient had multiple new collateral veins, but no obstructive lesion could be documented. This patient was symptomatic with intermittent upper extremity pain, but, unfortunately, in his somewhat underexposed venography the distal axillary vein was masked by the PM generator, and the brachial vein was outside the imaged area. However, the presence of abundant collaterals was regarded as a sign of significant functional obstruction, and the patient was thus determined to be a fourth case of TVO. The three confirmed TVOs were localized either in the subclavian (n=2) or the axillary (n=1) veins ipsilateral to the implanted device. Two male patients with confirmed TVO had presented with an abnormal venogram already at 6 months: one with a mild venous stenosis () and the other with a non-occlusive lead-associated thrombus (). One of the patients with a confirmed TVO (male, age 77) had experienced symptoms potentially related to the lesion (intermittent upper extremity pain), whereas the other two were asymptomatic.
Figure 1. Venographic images prior to implantation of a dual chamber pacemaker (A), and 6 months (B) and 25 months (C) postoperatively on a 57-year-old male patient. A stenosis (arrow) of the subclavian vein was seen in the 6-month study (B), and complete occlusion of the same vessel with collateral venous flow after 2 years (C).
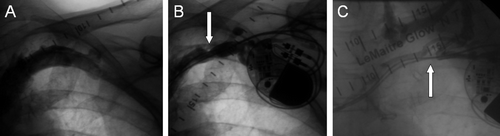
Figure 2. Panels A and B depict a filling defect suggestive of thrombus formation (arrows) in the 6-month venography of an 81-year-old male patient implanted with a biventricular pacemaker. Late venography (C) 29 months after implantation shows complete occlusion of the axillary vein (arrow) and several new collateral vessels.
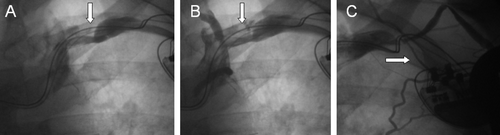
Table II. Venographic findings in 50 patients at base-line, 6 months, and at 2 years.
There were 25 patients in the late follow-up in whom no abnormalities had been discovered in the preimplant or in the 6-month venogram, 2 (8%) of whom had developed a new lesion on the late venography (1 TVO and 1 borderline stenosis). Two of the patients with abnormalities in base-line venography (n=10, data published previously Citation7) were included in the long-term follow-up, both of whom had venous stenosis and showed no progression of the base-line lesions at 6 months or in the late follow-up ICV ().
Six-month venography was abnormal in 23 (46%) of the 50 late follow-up patients. In the majority (83%) of these cases, the late ICV revealed no progression or resolution of their lesions, although TVO developed in three (17%) patients. The small filling defects interpreted as lead-associated thrombi at 6 months (n=13) had resolved in the late venography in only two patients and were unchanged in ten ().
Predictors for total venous occlusion
Associations with TVO-development were searched from multiple clinical patient-related variables as well as from aspects of implantation, devices, and leads. A larger proportion of patients who were in atrial fibrillation (n=29) at the time of the device implantation developed TVO compared to patients (n=121) who were in sinus rhythm (17.2% versus 3.3%, P=0.014). The only feature of the implanted electrodes showing some association with TVO was lead insulation: the proportion of patients with at least one polyurethane-coated lead was more common among patients with total venous occlusion (33% versus 7%, P=0.032). Venous access type, surgical aspects of the implantation, cardiac rhythm at base-line, or lead insulation did not emerge as independent predictors of venous occlusion in multiple regression analysis.
Discussion
The current prospective study evaluated serial venous changes in a cohort of 50 patients followed for a mean of 2.4 years after PM or ICD implantation. This was a subset from a previously published prospective series of 150 consecutive unselected patients implanted with a first PM or ICD, who underwent venographies at base-line and after 6 months of follow-up Citation7. Our present findings suggest that most of the venous irritation and damage leading to obstruction is set into motion relatively early in the postoperative phase. However, in some patients the process seems to continue longer or may even start later because late complete venous occlusion may occur in presumably normal veins at 6 months after implantation.
The development of late venous obstruction in patients with previously normal venograms obtained at shorter follow-up is a unique feature of our study. To our knowledge, the only other prospective venography-based study with serial short- and longer-term venographies (up to 18 months) showed no new obstructions after the first 6–12 months in 26 patients with previously normal venograms Citation1. At the other end of the spectrum, the only patient in our series with a symptomatic early acute venous thrombosis was repeatedly found to have normal venograms during later follow-up. Most of the total venous occlusions were clinically silent, although pulmonary embolism was diagnosed in two of these patients based on careful symptom history during the follow-up visit. It was disappointing that no clinical predictors for the venous complications could be revealed by a careful consideration of various clinical and procedure-related factors.
After late venography, the number of patients with TVO amounts to a total of 9 cases or 6% in our entire series of 150 patients. This figure is lower than in previously published series, but is probably an underestimate of the true incidence of occlusions, because only one-third of the original study group was included in the late follow-up. Should a similar relative rate of TVO occur among the 100 remaining patients not followed venographically beyond 6 months, approximately 10 additional cases of TVO would be expected to develop, yielding a hypothetical incidence of 13%. There are only limited earlier data on the time-course of lead-induced venous changes in the literature. In an early cross-sectional series of 100 patients from a pacemaker follow-up clinic, venographies were performed at 44±10 months after pacemaker implantation, and total occlusion was revealed in 15% of the patients Citation9. In a small prospective venography-based study of 40 patients with no base-line venography, an early (1–6 months) total occlusion was observed in 8% with no further abnormalities discovered at 18–24-months follow-up Citation1. Oginosawa et al. Citation3 performed digital subtraction angiography prior to PM implantation on 131 patients and after a mean follow-up of 44 months re-studied 60% of the patients, observing asymptomatic total occlusion in 10 (13%) of 79 patients. In recent small cross-sectional studies, where venograms were performed in conjunction with device or lead replacement at widely ranging time intervals from initial device implantations, the prevalence of total venous occlusion has ranged from 9% to 25% Citation2, Citation6, Citation10, Citation11. However, selection bias, wide variation in follow-up times, and e.g. pacemaker infection Citation10 are likely to have contributed to these figures.
One of the unique features of our study is that the changes in venous calibers were serially assessed in a quantitative fashion. A small, but statistically significant, reduction in the mean venous diameters from the preimplantation phase to the 6-month follow-up was observed Citation7. In the late follow-up the venous diameters were measured in identical fashion at the same reference points, and no significant further changes in the mean venous diameters were found to have occurred in the group as a whole after the 6-month follow-up. Small filling defects suggestive of non-flow-limiting thrombi seen in 14% of the 6-month venograms are likely to be clinically insignificant as they exhibited no progression in the late venograms.
Limitations of this study include the fact that only one-third of the initial group of patients were followed beyond the first 6 months. Furthermore, the selection of patients with pre-existing lesions may affect the incidence of venous lesions in the late follow-up. Thus, the incidence of TVO developing later than the first 6 months post-PM implantation, as reported in the current study, should be interpreted with caution.
In conclusion, our prospective study shows that complete venous obstruction is not an uncommon complication, even with the modern transvenous pacing leads, and occurs without any localizing symptoms in most of the cases. Most of the obstructive lesions and changes in venous calibers appear to develop during the first months of the postimplantation period, but late and unpredictable complete venous occlusion may also occur. Unfortunately, no significant predictive factors for the development of venous lesions could be identified. One clinical implication from the study is that one should always perform a venogram of the upper extremity before upgrading or for other reasons implanting new leads to the same side of a previous pacemaker.
Acknowledgements
This study was supported by research grants from the Finnish Foundation for Cardiovascular Research (Helsinki, Finland), South-Western Finland Hospital District Research Fund (Turku, Finland), and Finnish Cardiac Society (Helsinki, Finland). Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Antonelli D, Turgeman Y, Kaveh Z, Artoul S, Rosenfeld T. Short-term thrombosis after transvenous permanent pacemaker insertion. Pacing Clin Electrophysiol. 1989; 12: 280–2
- Goto Y, Abe T, Sekine S, Sakurada T. Long-term thrombosis after transvenous permanent pacemaker implantation. Pacing Clin Electrophysiol. 1998; 21: 1192–5
- Oginosawa Y, Abe H, Nakashima Y. The incidence and risk factors for venous obstruction after implantation of transvenous pacing leads. Pacing Clin Electrophysiol. 2002; 25: 1605–11
- Da Costa SS, Scalabrini Neto A, Costa R, Caldas JG, Martinelli Filho M. Incidence and risk factors of upper extremity deep vein lesions after permanent transvenous pacemaker implant: a 6-month follow-up prospective study. Pacing Clin Electrophysiol. 2002; 25: 1301–6
- van Rooden CJ, Molhoek SG, Rosendaal FR, Schalij MJ, Meinders AE, Huisman MV. Incidence and risk factors of early venous thrombosis associated with permanent pacemaker leads. J Cardiovasc Electrophysiol. 2004; 15: 1258–62
- Haghjoo M, Nikoo MH, Fazelifar AF, Alizadeh A, Emkanjoo Z, Sadr-Ameli MA. Predictors of venous obstruction following pacemaker or implantable cardioverter-defibrillator implantation: a contrast venographic study on 100 patients admitted for generator change, lead revision, or device upgrade. Europace. 2007; 9: 328–32
- Korkeila P, Nyman K, Ylitalo A, Koistinen J, Karjalainen P, Lund J, et al. Venous obstruction after pacemaker implantation. Pacing Clin Electrophysiol. 2007; 30: 199–206
- Karjalainen PP, Porela P, Ylitalo A, Vikman S, Nyman K, Vaittinen MA, et al. Safety and efficacy of combined antiplatelet-warfarin therapy after coronary stenting. Eur Heart J. 2007; 28: 726–32
- Mitrovic V, Thormann J, Schlepper M, Neuss H. Thrombotic complications with pacemakers. Int J Cardiol. 1983; 2: 363–74
- Bracke F, Meijer A, Van Gelder B. Venous occlusion of the access vein in patients referred for lead extraction: influence of patient and lead characteristics. Pacing Clin Electrophysiol. 2003; 26: 1649–52
- Lickfett L, Bitzen A, Arepally A, Nasir K, Wolpert C, Jeong KM, et al. Incidence of venous obstruction following insertion of an implantable cardioverter defibrillator. A study of systematic contrast venography on patients presenting for their first elective ICD generator replacement. Europace. 2004; 6: 25–31