Abstract
Aims. Because sudden cardiac death (SCD) is due to cardiac electrical instability, we postulated that prediction of this mode of death by exercise capacity will be enhanced by combined assessment with T-wave alternans (TWA), an index of repolarization abnormality.
Material and methods. The Finnish Cardiovascular Study enrolled consecutive patients (n=2,044) with a routine clinically indicated exercise test. Exercise capacity was measured in metabolic equivalents (METs) and TWA by time-domain modified moving average method.
Results. During 47.2±12.8-month follow-up (mean±SD) 120 patients died; 58 were cardiovascular deaths, and 29 were SCD. In multivariate analysis after adjustment for sex, age, smoking, use of β-blockers, as well as other common coronary risk factors, the relative risk of patients whose exercise capacity was depressed (MET < 8) was 8.8 (95% CI 2.0–38.9, P=0.004) for SCD. The combination of low exercise capacity (MET < 8) and elevated TWA (≥65 µV) yielded relative risks for SCD of 36.1 (6.3–206.0, P<0.001), for cardiovascular mortality of 21.1 (6.7–66.2, P<0.001), and for all-cause mortality of 7.8 (3.5–17.4, P<0.001) over patients with neither factor.
Conclusions. Reduced exercise capacity, particularly in combination with heightened TWA, indicating enhanced cardiac electrical instability, powerfully predicts risk for SCD in patients referred for exercise testing.
Introduction
Exercise capacity predicts all-cause and cardiac mortality in men more powerfully than do other cardiac risk factors Citation[1]. This prognostic utility is similar among women Citation[2], Citation[3] and across racial groups Citation[4]. Surprisingly, its potential to estimate risk specifically for sudden cardiac death (SCD) has not been investigated. This is an important gap in our knowledge, as reduced exercise capacity could reflect the extent of heart disease, including myocardial scarring and poor myocardial perfusion that create a heterogeneous myocardial substrate with increased susceptibility to lethal re-entrant arrhythmias. Combined testing with T-wave alternans (TWA), a marker of cardiac electrical instability, during routine symptom-limited exercise tests could demonstrate an association with SCD as well as increase the strength of prediction.
Key messages
Reduced exercise capacity powerfully predicts risk for sudden cardiac death in a general population of patients referred for a clinical exercise test.
T-wave alternans, an indicator of ventricular electrical instability, adds significantly to the prognostic strength of reduced exercise capacity.
The literature provides clues supporting the rationale for combined analysis of exercise capacity with TWA. Tapanainen and co-workers Citation[5] demonstrated in postmyocardial infarction patients that inability to achieve a target heart rate of 105–110 beats/min, as required for spectral TWA testing, was itself predictive of cardiovascular mortality. More recently, it has been advised that spectral TWA tests previously considered ‘indeterminate’ based on not achieving a target heart rate should be classified as ‘abnormal’ or ‘non-negative’ Citation[6]. However, target heart rate is not a reliable measure of exercise capacity, as patients’ inability to increase heart rate may be influenced by many factors including sinus node responsiveness or medications, particularly beta-adrenergic blocking agents (β-blockers), as well as by mechanical function of the heart. Metabolic equivalents (METs) have been shown to be a more reliable measure of exercise capacity Citation[7].
Abbreviations
Based on extensive evidence that SCD is due to cardiac electrical instability, we postulated that prediction of this mode of death by exercise capacity will be enhanced by combined assessment with TWA, an index of repolarization abnormality. We applied the time-domain modified moving average method for TWA analysis Citation[8], which, because of its intrinsic flexibility, permits TWA analysis during routine symptom-limited exercise protocols in which exercise capacity can be measured. The method has undergone extensive validation and performs at a resolution of 1 microvolt, equivalently to the spectral method Citation[9], Citation[10]. We tested its utility to improve SCD risk stratification in a general population of patients referred for a clinical exercise test.
Material and methods
Study cohort
All consecutive patients coming for an exercise test at Tampere University Hospital and willing to participate were enrolled in the Finnish Cardiovascular Study (FINCAVAS). Between October 2001 and the end of 2004, a study population of 2,212 patients (1,400 men and 812 women) was recruited. Results of analysis of TWA alone in about half of the patients (1,037) have been reported Citation[11]. A total of 2,044 patients (1,305 men and 739 women) had technically successful exercise tests (92.4% of all tests) and were studied in the current investigation (). A test was technically adequate if storing hemodynamic data and continuous digital electrocardiogram as well as TWA assessment and exercise capacity recording in METs were successful.
Table I. Patient characteristics and unadjusted percentage of beta-adrenergic blocking agent (β-blocker) users as well as prevalence of cardiovascular diseases, symptoms, and risk factors for all participants (n=2,044) divided by gender and separately for men with sudden cardiac death (SCD) (n=28). The differences between men with and without SCD were tested by using t test for independent samples for continuous variables and chi-square test for categorical variables.
The main indications for exercise testing were suspicion of coronary heart disease (46%), palpitation or sense of arrhythmia (21%), evaluation of work capacity (18%) and adequacy of coronary heart disease treatment (16%), as well as obtaining an exercise test profile prior to an invasive operation (14%) or after myocardial infarction (8%); some patients had more than one indication. The Ethics Committee of Tampere University Hospital District of Pirkanmaa, Finland, approved the study protocol, and all patients gave informed consent prior to the interview and measurements, as stipulated in the Declaration of Helsinki.
Study flow
After an informed consent was signed, the medical history of each patient was collected with a computer-based questionnaire form. Thereafter, the exercise test was performed.
Exercise test protocol
Prior to the exercise stress test, subjects lay down in the supine position for 10 minutes, and the resting electrocardiogram was digitally recorded. Exercise testing was performed using a bicycle ergometer with electrical brakes. The lead system used was the Mason-Likar modification of the standard 12-lead system Citation[12]. The initial work-load varied from 20 watts (W) to 30 W, and the load was increased stepwise by 10–30 W every minute. Continuous electrocardiograms were digitally recorded at 500 hertz with CardioSoft exercise system (Version 4.14, GE Healthcare, Freiburg, Germany).
Heart rate was continuously registered with electrocardiograms during the tests, while systolic and diastolic arterial pressures were measured with a brachial cuff every 2 minutes. Exercise capacity in METs was estimated on the standardized basis of maximum work-load and weight of the patient, with 1 MET equivalent to 3.5 mL oxygen uptake/kilogram/min.
Measurement of TWA
TWA was analyzed fully automatically by investigators blinded to clinical outcomes with the released version of GE Healthcare Modified Moving Average software. Modified moving average analysis Citation[8] calculates and compares separate average morphologies of odd and even beats. Continuous updating for every incoming beat by a weighting factor of 1/8 of the difference between the on-going average and the new incoming beat produces continuous moving averages of odd and even beats. This approach is intrinsically robust and is suitable for TWA analysis during periods of activity or fluctuating heart rates Citation[13]. Algorithms have also been incorporated to decrease the influence of noise and artifacts, such as those caused by pedaling and respiration Citation[14]. The following steps were taken to ensure quality control of TWA values. Throughout the analysis, beat-labeling was performed to exclude the suspect and preceding beat based on noise and prematurity according to several criteria. These included: beats with >20 microvolts of noise, which was measured during the isoelectric segments; regions with >25% of noisy beats; and ventricular premature beats.
Standard precordial leads (V1 to V6) were recorded continuously during the entire exercise test. The highest TWA value in any lead during the exercise-phase at heart rates <125 beats/min was derived. This heart rate limit was set, as inaccuracies in TWA measurement can result at heart rates exceeding this range Citation[15]. TWA results in limb leads were excluded as these leads are subject to significant motion artifact, as confirmed by visual inspection of templates of superimposed electrocardiograms in the GE Healthcare system. Precordial leads have also been shown to be optimum for TWA measurement Citation[16], Citation[17].
The TWA values obtained by the modified moving average method are 4- to 6-fold higher than the values reported by the spectral method. This difference is due primarily to the fact that the time-domain modified moving average method calculates the maximum difference in amplitudes of successive T-waves, while the spectral method computes an average value from its spectra, which are determined across the entire T-wave and across 128 beats.
Ejection fraction
Measurement of left ventricular ejection fraction is not routine for patients referred for a clinical exercise test. However, ejection fraction was determined for 1,117 (55%) of study patients with echocardiography or isotope techniques within 6 months of exercise testing.
Follow-up
Death certificates listing causes of death using the tenth revision of the International Classification of Diseases (ICD-10) were received from the Causes of Death Register, maintained by Statistics Finland, in April 2007; this source has been shown to be reliable Citation[18]. Diagnosis numbers and certificate texts were used to classify deaths as all-cause, cardiovascular, or SCD, i.e., cardiovascular death within 24 hours after onset of symptoms. Autopsy rate was 40% for all deaths and 60% for patients with SCD.
Statistical analysis
Predictivity for SCD and for cardiovascular and all-cause mortality by METs with and without elevated TWA was analyzed using Cox proportional hazards models. Analyses of exercise capacity in METs were performed with the cut-point of <8, which has been used in studies in women Citation[19], Citation[20] but to our knowledge not in studies with men. In subgroup analyses in women, the cut-point of <5 Citation[2] was also used. The Pearson correlations between maximum heart rate and exercise capacity in METs and between TWA magnitude and maximum heart rate were calculated.
For analyses of TWA, the cut-point of 65 microvolts (µV) in precordial leads was used, because it had the best prognostic power in our previous study Citation[11]. Low exercise capacity and TWA were combined in one categorical variable with three different groups of patients: MET ≥ 8 and TWA < 65 µV; MET < 8 or TWA ≥ 65 µV; and MET < 8 and TWA ≥ 65 µV. Thereafter, risk for all-cause and cardiovascular death as well as for SCD was estimated with Cox regression analysis using the following covariates Citation[21]: sex, age, body mass index, daily smoking (yes/no), use of β-blockers (yes/no), as well as prior diagnoses of coronary heart disease (yes/no), myocardial infarction (yes/no), and diabetes (yes/no) (). Use of β-blockers was defined as ‘no’ if the patient did not use β-blockers or if the pause in β-blocker use before the test was 3 days or more. Sensitivity and specificity as well as positive and negative predictive values were calculated for exercise capacity alone and in combination with TWA compared to patients with neither factor ().
Table II. Adjusted relative risks for all-cause mortality, cardiovascular mortality, and sudden cardiac death according to exercise capacity in metabolic equivalents (MET) and T-wave alternans (TWA), adjusted by covariates used in the Cox regression models (n=2,044).
Table III. Sensitivity (Sn), specificity (Sp), as well as positive (PPV) and negative (NPV) predictive values for sudden cardiac death and for cardiovascular and all-cause mortality (n=2,044).
Statistical analyses were performed with the SPSS release 15.0 for Windows (SPSS Inc., Chicago, Illinois). All statistical tests were two-tailed and used an alpha level of 0.05.
Results
Among the 2,044 enrolled patients, 120 deaths (5.9%) occurred over the succeeding 47.2±12.8 months (mean±SD). Of those, 58 (2.8%) were categorized as cardiovascular deaths and 29 (1.4%) further as SCD. Thus, the cardiovascular mortality of the present patients was 0.71%/year. Prevalence of all-cause death, cardiovascular death, and SCD in women was 3.8% (n=28), 1.1% (n=8), and 0.1% (n=1), respectively; in men, the prevalence was 7.0% (n=92), 3.8% (n=50), and 2.1% (n=28), respectively. Left ventricular ejection fraction, reported for 1,117 patients, was 66±14% (mean±SD). Of these, 103 patients (9.2%) presented with ejection fraction <50%, 39 patients (1.9%) with ejection fraction <40%, and 10 patients (0.9%) with ejection fraction <30%. Among the 29 SCD cases, ejection fraction was reported for 15 (52%) and was 60.5±15.7 (mean±SD). Only 24 patients (1.2%) had an implantable cardioverter defibrillator. Male patients with SCD reached lower percent of expected heart rate, more frequently had coronary heart disease and prior myocardial infarction, and were more often on β-blocker treatment than those who did not experience SCD in the follow-up (). The mean value (±SD) for peak TWA levels measured during exercise in precordial leads was 30±21 µV. The Pearson correlation was 0.537 (P <0.001) between maximum heart rate and exercise capacity in METs and −0.099 (P<0.001) between maximum heart rate and TWA magnitude.
Exercise capacity and mortality
In our population of consecutive patients referred for clinical exercise testing, 58.5% had reduced exercise capacity. The mean value (±SD) for exercise capacity in METs for men (1,305) was 7.4±3.0 and for women (739) was 6.7±2.8. For patients with reduced exercise capacity (MET < 8, n=1,195), the unadjusted prevalence of all-cause death was 8.6%, cardiovascular death 4.4%, and SCD 2.3%. For those with preserved exercise capacity (MET ≥ 8, n=849), the prevalence was 2.0%, 0.6%, and 0.2%, respectively.
The adjusted relative risk for SCD for those with poor exercise capacity (MET < 8) was 8.8 (95% confidence interval (CI) 2.0–38.9, P=0.004), was 5.2 (2.0–13.6, P=0.001) for cardiovascular mortality, and was 3.3 (1.9–5.6, P<0.001) for all-cause mortality. Age, sex, body mass index, use of β-blockers, and prior diagnosis of myocardial infarction and coronary heart disease were significant covariates for death from any cause. Age, sex, body mass index, and use of β-blockers were significant covariates for cardiovascular mortality, while only sex was a significant covariate for SCD. MET was a highly sensitive predictor (93.1%) of SCD, with high negative predictive value (NPV) (99.8%) (). Similar predictivity was determined for cardiovascular and total mortality ().
As a continuous variable, increasing MET significantly improved survival in terms of all-cause and cardiovascular mortality and SCD (relative risk 0.77 per 1 MET increase, 95% CI 0.70–0.84, P<0.001 for all-cause mortality; relative risk 0.69 per 1 MET increase, 95% CI 0.60–0.80, P<0.001 for cardiovascular mortality; and relative risk 0.67 per 1 MET increase, 95% CI 0.57–0.83, P<0.001 for SCD).
In the subgroup analyses in women (n=739) increasing MET as a continuous variable significantly improved the survival in terms of all-cause and cardiovascular mortality (relative risk 0.77 per 1 MET increase, 95% CI 0.62–0.95, P=0.016 for all-cause mortality; and relative risk 0.52 per 1 MET increase, 95% CI 0.32–0.83, P=0.006 for cardiovascular mortality). The cut-point of MET < 8 did not reach significance in women, but the cut-point of MET < 5 predicted statistically significantly cardiovascular mortality (relative risk 15.0, 95% CI 2.0–111.8, P=0.008) in women.
In men the results were highly comparable to results of the all participants.
Exercise capacity, TWA, and mortality
The predictive power of TWA (≥65 µV) alone with covariates used in this study remained highly significant in this expanded database, resulting in a relative risk of 2.2 (1.1–4.2, P=0.018) for total mortality, 4.0 (1.9–8.5, P<0.001) for cardiovascular mortality, and 3.9 (1.4–11.5, P=0.011) for SCD.
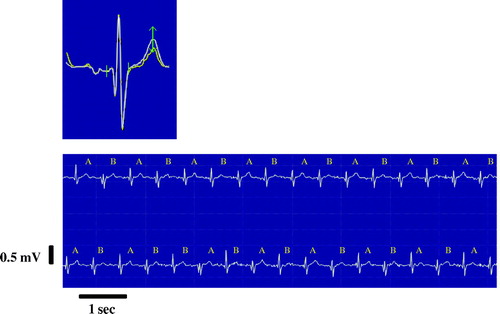
SCD risk was further categorized according to TWA test results. Three groups of patients, MET ≥ 8 and TWA < 65 µV, MET < 8 or TWA ≥ 65 µV, and MET < 8 and TWA ≥ 65 µV, contained 811, 1177, and 56 patients, respectively. The combination of poor exercise capacity and elevated TWA identified patients with the highest prevalence of SCD and of cardiovascular and total mortality (). Survival curves depict events across 4 years of follow-up for the combined analysis of reduced MET < 8 and elevated TWA (≥65 µV) (). The adjusted relative risk for SCD for patients with both reduced exercise capacity (MET < 8) and heightened TWA (≥65 µV) was 36.1 (6.3–206.0, P<0.001), for cardiovascular mortality was 21.1 (6.7–66.2, P<0.001), and for all-cause mortality was 7.8 (3.5–17.4, P<0.001), over patients with neither factor (). For SCD, the only significant covariate was sex. Combined analysis of SCD risk with METs and TWA ≥ 65 µV yielded high specificity (94.0%) while retaining high NPV (99.8%) (). A representative example of exercise-induced TWA is provided ().
Figure 1. Prevalence of all-cause and cardiovascular mortality as well as sudden cardiac death (SCD) among patients according to metabolic equivalents (MET) and T-wave alternans (TWA). The P-values are from chi-square test. The subjects with metabolic equivalents (MET) ≥ 8 and T-wave alternans (TWA) < 65 microvolts (µV) were compared to the subjects with either MET < 8 or TWA ≥ 65 µV and to the subjects with MET < 8 and TWA ≥ 65 µV.
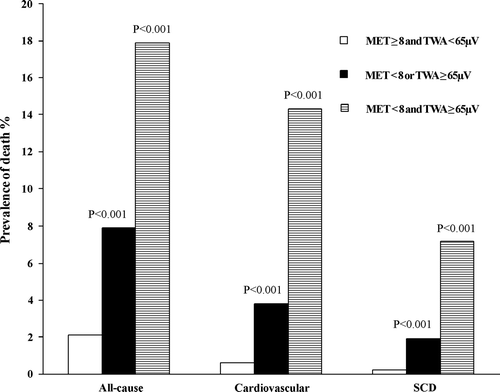
Figure 2. Adjusted survival curves by Cox regression anlysis for subjects with metabolic equivalents (MET) ≥ 8 and T-wave alternans (TWA) < 65 microvolts (µV) (the upper curve in each panel), MET < 8 or TWA ≥ 65 µV (the middle curve), and MET < 8 and TWA ≥ 65 µV (the lower curve) for a) all-cause mortality, b) cardiovascular mortality, and c) sudden cardiac death. Please note that the scale for the y-axis is from 0.75 to 1.00.
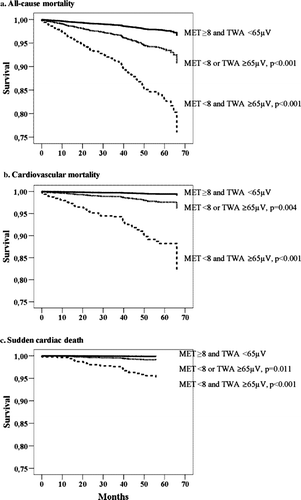
Figure 3. A representative electrocardiogram tracing and superimposed complexes of the lead V5 illustrating exercise-induced T-wave alternans (TWA) of 71 microvolts in a patient who experienced sudden cardiac death caused by an acute myocardial infarction at 3 months following the recording. The superimposed waveforms (upper panel) and rhythm strip (lower panel) are provided. The bidirectional arrow refers to the point of maximum TWA.
The combination of low exercise capacity (MET < 8) and elevated TWA (≥65 µV) did not reach significance in women. In men the results were highly comparable to the results of all participants.
Discussion
Our study is the first to demonstrate that reduced exercise capacity is a risk factor for SCD. It also provides evidence that TWA, an indicator of ventricular electrical instability, adds significantly to the prognostic strength of reduced exercise capacity. This full-cohort examination of risk stratification with TWA enrolled more than 2,000 patients. In half of the patients, left ventricular ejection fraction was measured, and in 90% of these, it was found to be normal. It is probable although not documented that the remaining half of the cohort, in whom ejection fraction was not measured, had even better cardiovascular health, because ejection fraction determination was not indicated. Thus, the present results are relevant to a large group of individuals whose elevated risk for SCD and major cardiovascular events is not disclosed by other contemporary tests.
Previous studies
Exercise capacity is a superior predictor of all-cause and cardiovascular mortality Citation[1–4]. The usefulness of exercise-induced TWA in predicting arrhythmic events and death has been investigated Citation[11], Citation[22–25]. We reported in ∼1,000 FINCAVAS patients that TWA assessed during routine symptom-limited exercise testing is a strong risk marker for SCD and cardiovascular and total mortality in a general population of patients referred for a clinical exercise test Citation[11]. By contrast, most studies of TWA have been performed in populations with high risk of life-threatening arrhythmias Citation[22], Citation[23], Citation[25] or lower-risk patients with prior myocardial infarction Citation[24] and employed spectral analysis of TWA during a target heart rate exercise protocol. These TWA test results are indeterminate in 20%–40% of cases Citation[6] due to patient factors, in the majority to inability to achieve the target heart rate of 105–110 beats/min. Classification of these indeterminate tests as ‘abnormal’ conferred prediction to avoid repetation of capacity although exercise capacity itself was not measured. Thus, the present study, in which exercise capacity was measured, is the first to provide direct evidence of its predictive value for SCD, particularly when combined with TWA.
Current investigation
Our study provides new evidence that in a general population of patients referred for a clinical exercise test, reduced exercise capacity increases risk for SCD as well as for cardiovascular death and total mortality. When heightened TWA, a validated marker of arrhythmia risk, is also present, risk of SCD is further elevated over that of patients with neither factor (). Poor exercise capacity alone was found to be a highly sensitive (93.1%) marker of SCD risk, with specificity of 42.0%, as it detected 27 of 29 cases of SCD (). Combined analysis with elevated TWA greatly improved the specificity of the test, to 94.0%.
The NPV for SCD for patients with both low exercise capacity and high exercise-induced TWA was 99.8% over patients with neither factor (). This finding is similar to our previous results using only exercise-induced TWA as predictor (98.6%) Citation[11] and to results of TWA testing with the spectral method, for which NPV averages 97.2% (95% CI 96.5–97.9) Citation[22]. Reduced exercise capacity alone does not provide high positive predictive value (PPV) (2.3%) for SCD in our low-risk population (). However, when low exercise capacity is combined with TWA ≥ 65 µV, PPV for SCD rose to 7.1% (), which is highly comparable to the 8.0% result achieved with only TWA as a predictor in our previous study Citation[11] and to the 6.0% level (95% CI 4.5–7.4) provided by TWA testing with the spectral method for cardiac arrhythmic events in low-risk patients Citation[22].
With the exception of sex differences, the relative risks linked to traditional cardiovascular risk markers () were lower than those for reduced exercise capacity, with or without TWA. Thus, the combination of depressed exercise capacity and heightened TWA provides a marked prognostic index independent of traditional risk factors.
In the subgroup analyses in women, low exercise capacity (MET < 5) predicted cardiovascular mortality, and increasing MET as continuous variable improved survival for all-cause and cardiovascular mortality. However, the low exercise capacity (MET < 8) alone or in combination with elevated TWA (≥65 µV) did not reach significance as predictor of all-cause or cardiovascular mortality in women. This may be due to the smaller number of events in this subgroup. Thus, further studies are needed to evaluate the prognostic power of combined analysis of low exercise capacity and elevated TWA in women.
SCD in a general population without congestive heart failure most commonly results from ventricular fibrillation triggered by an ischemic event Citation[26]. TWA reflects the presence of abnormal repolarization and electrophysiologic inhomogeneities that underlie vulnerability to ventricular fibrillation during myocardial ischemia Citation[27]. Exercise testing serves to expose latent electrical instability, as indicated by elevated levels of TWA. When analyzed together, exercise capacity and TWA provide supplementary information that strengthens the predictive value of either parameter alone, to 36-fold over risk in the absence of both factors (). The fact that the end-points measure largely different characteristics is likely to underlie the additive effect. Exercise capacity essentially provides a measure of cardiac mechanical function, whereas TWA is an indicator of cardiac electrical instability.
There are some limitations in our study. Establishing definitively that an event is SCD is inherently challenging. Our main criterion was death within 24 hours following onset of symptoms. The majority of deaths that were classified as SCD in this study were caused by acute coronary events, which have been shown to be the triggers for ventricular tachyarrhythmias leading to SCD Citation[26], Citation[28], Citation[29]. There were no signs of pulmonary embolism or pulmonary edema in autopsy information in patients with SCD. The presence of elevated exercise-induced TWA in patients with reduced exercise capacity was a stronger predictor of SCD than of either cardiovascular mortality or total deaths (). The combination of heightened TWA with reduced exercise capacity also improved prediction of all-cause mortality, which is a definite end-point. A second limitation is the low PPV for SCD of exercise capacity alone, which is typical of low-risk groups, and which is improved by combined assessment with TWA. A third limitation is that we do not have information on changes in parameters affecting mortality risk (e.g. smoking, life-style, and medications) during follow-up. As with any observational study, it is not possible to draw causal inferences, and differences in variables that were not adjusted for or residual confounding may exist. Although the data reported in our study are from bicycle ergometer tests, it is likely that the results can be also generalized to populations undergoing a clinically indicated treadmill exercise test.
A broad implication of the present finding is that a mainstay measurement, namely exercise capacity, especially when combined with TWA assessment, is capable of identifying individuals whose risk for SCD is elevated but whose ejection fraction is normal. As exercise capacity was reduced in 58.5% of our population of consecutive patients referred for clinical exercise testing, TWA measurement can provide useful confirmatory information regarding their cardiac status. Because both parameters can be acquired automatically during the course of routine, symptom-limited exercise testing, without a specialized protocol or non-standard electrodes, this test has the potential for screening broad, diverse populations. The population tested was at relatively low risk of events, the group in which the greatest incidence of SCD occurs but in which identification of SCD risk has been elusive Citation[26]. Because combined measurement of mechanical function by exercise capacity and of cardiac electrical instability by TWA provides important insights into a potential basis for patients’ risk for arrhythmia, it could prove helpful in identifying therapeutic targets for SCD reduction.
Acknowledgements
Financial support was received from the Medical Research Fund of Tampere University Hospital, the Finnish Cultural Foundation, the Finnish Foundation for Cardiovascular Research, the Academy of Finland (grant no. 104821), the Emil Aaltonen Foundation, Finland, and the Tampere Tuberculosis Foundation. The authors thank the staff of the Department of Clinical Physiology for collecting the exercise test data. Automated analysis of TWA was performed by Willi Kaiser of GE Healthcare, Freiburg, Germany, who was blinded to patient characteristics and clinical outcomes. Declaration of interest: Dr Richard L. Verrier is co-inventor of patents for T-wave alternans measurement, including by the modified moving average method, which were assigned to Georgetown University and Beth Israel Deaconess Medical Center and licensed to GE Healthcare. The other authors do not have any conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002; 346: 793–801
- Gulati M, Pandey DK, Arnsdorf MF, Lauderdale DS, Thisted RA, Wicklund RH, et al. Exercise capacity and the risk of death in women: the St James Women Take Heart Project. Circulation. 2003; 108: 1554–9
- Gulati M, Black HR, Shaw LJ, Arnsdorf MF, Merz CN, Lauer MS, et al. The prognostic value of a nomogram for exercise capacity in women. N Engl J Med. 2005; 353: 468–75
- Kokkinos P, Myers J, Kokkinos JP, Pittaras A, Narayan P, Manolis A, et al. Exercise capacity and mortality in black and white men. Circulation. 2008; 117: 614–22
- Tapanainen JM, Still AM, Airaksinen KE, Huikuri HV. Prognostic significance of risk stratifiers of mortality, including T wave alternans, after acute myocardial infarction: results of a prospective follow-up study. J Cardiovasc Electrophysiol. 2001; 12: 645–52
- Kaufman ES, Bloomfield DM, Steinman RC, Namerow PB, Costantini O, Cohen RJ, et al. ‘Indeterminate’ microvolt T-wave alternans tests predict high risk of death or sustained ventricular arrhythmias in patients with left ventricular dysfunction. J Am Coll Cardiol. 2006; 48: 1399–404
- Gibbons RJ, Balady GJ, Bricker JT, Chaitman BR, Fletcher GF, Froelicher VF, et al. ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). Circulation. 2002; 106: 1883–92
- Nearing BD, Verrier RL. Modified moving average analysis of T-wave alternans to predict ventricular fibrillation with high accuracy. J Appl Physiol. 2002; 92: 541–9
- Zuckerman, BD. T-wave alternans (TWA) algorithm option. United States Food and Drug Administration; 2002: K023380. www.fda.gov/cdrh/pdf2/k023380.pdf.
- Zuckerman, BD. T-wave alternans (TWA) algorithm option. United States Food and Drug Administration; 2003: K032513. www.fda.gov/cdrh/pdf3/k032513.pdf.
- Nieminen T, Lehtimaki T, Viik J, Lehtinen R, Nikus K, Koobi T, et al. T-wave alternans predicts mortality in a population undergoing a clinically indicated exercise test. Eur Heart J. 2007; 28: 2332–7
- Mason RE, Likar I. A new system of multiple-lead exercise electrocardiography. Am Heart J. 1966; 71: 196–205
- Hostetler B, Xue J, Young B, Kaiser K, Findeis M, Gutterman D. Detect short run of TWA event with time-domain algorithm. Comput Cardiol. 2005; 32: 483–86
- Kaiser W, Findeis M, Young B. Improving T-wave alternans measurement quality by reducing noise and artifacts. Comput Cardiol. 2004; 31: 445–8
- Bloomfield DM, Hohnloser SH, Cohen RJ. Interpretation and classification of microvolt T wave alternans tests. J Cardiovasc Electrophysiol. 2002; 13: 502–12
- Nearing BD, Oesterle SN, Verrier RL. Quantification of ischaemia induced vulnerability by precordial T wave alternans analysis in dog and human. Cardiovasc Res. 1994; 28: 1440–9
- Martinez JP, Olmos S, Wagner G, Laguna P. Characterization of repolarization alternans during ischemia: time-course and spatial analysis. IEEE Trans Biomed Eng. 2006; 53: 701–11
- Pajunen P, Koukkunen H, Ketonen M, Jerkkola T, Immonen-Raiha P, Karja-Koskenkari P, et al. The validity of the Finnish Hospital Discharge Register and Causes of Death Register data on coronary heart disease. Eur J Cardiovasc Prev Rehabil. 2005; 12: 132–7
- Mora S, Redberg RF, Cui Y, Whiteman MK, Flaws JA, Sharrett AR, et al. Ability of exercise testing to predict cardiovascular and all-cause death in asymptomatic women: a 20-year follow-up of the lipid research clinics prevalence study. JAMA. 2003; 290: 1600–7
- Mora S, Redberg RF, Sharrett AR, Blumenthal RS. Enhanced risk assessment in asymptomatic individuals with exercise testing and Framingham risk scores. Circulation. 2005; 112: 1566–72
- Nieminen T, Lehtinen R, Viik J, Lehtimaki T, Niemela K, Nikus K, et al. The Finnish Cardiovascular Study (FINCAVAS): characterising patients with high risk of cardiovascular morbidity and mortality. BMC Cardiovasc Disord. 2006; 6: 9
- Gehi AK, Stein RH, Metz LD, Gomes JA. Microvolt T-wave alternans for the risk stratification of ventricular tachyarrhythmic events: a meta-analysis. J Am Coll Cardiol. 2005; 46: 75–82
- Bloomfield DM, Bigger JT, Steinman RC, Namerow PB, Parides MK, Curtis AB, et al. Microvolt T-wave alternans and the risk of death or sustained ventricular arrhythmias in patients with left ventricular dysfunction. J Am Coll Cardiol. 2006; 47: 456–63
- Ikeda T, Yoshino H, Sugi K, Tanno K, Shimizu H, Watanabe J, et al. Predictive value of microvolt T-wave alternans for sudden cardiac death in patients with preserved cardiac function after acute myocardial infarction: results of a collaborative cohort study. J Am Coll Cardiol. 2006; 48: 2268–74
- Chow T, Kereiakes DJ, Bartone C, Booth T, Schloss EJ, Waller T, et al. Prognostic utility of microvolt T-wave alternans in risk stratification of patients with ischemic cardiomyopathy. J Am Coll Cardiol. 2006; 47: 1820–7
- Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med. 2001; 345: 1473–82
- Narayan SM. T-wave alternans and the susceptibility to ventricular arrhythmias. J Am Coll Cardiol. 2006; 47: 269–81
- Myerburg RJ, Interian A, Jr, Mitrani RM, Kessler KM, Castellanos A. Frequency of sudden cardiac death and profiles of risk. Am J Cardiol. 1997; 80: 10F–19F
- Zipes DP, Wellens HJ. Sudden cardiac death. Circulation. 1998; 98: 2334–51