Abstract
The aim of the study was to assess coronary flow reserve (CFR) in tako-tsubo cardiomyopathy (TC).
Methods and results. Thirty consecutive patients (5 males; age 68±12 years) meeting diagnostic criteria for TC were evaluated with transthoracic dipyridamole (0.84 mg/kg over 6 min) stress echo and pulsed Doppler CFR assessment on mid-distal left anterior descending (LAD) and posterior descending of right coronary artery (PD). Wall motion score index (WMSI) was evaluated at base-line and during stress. All patients were followed up clinically and—on day 1, day 7 (±2 days), and at 6 months—by repeat stress echo. Thirty gender- matched controls were also studied. CFR was obtained in all patients on LAD and in 25 on PD. All showed a transient apical ballooning in the acute phase (day 1 of admission), with progressive recovery of function at follow-up (WMSI, day 1 = 1.7±0.2; day 7 = 1.4±0.14; 6 months = 1.0±0.1; P<0.001 versus day 1 and versus day 7). When compared to controls (3.1±0.5), CFR on LAD was reduced on day 1 (1.8±0.24, P<0.001) (upon admission), and it showed early recovery in the subacute (pre-discharge) assessment on day 7. CFR values remained stable at 6-month follow-up (2.6±0.3).
Conclusion. TC is characterized by a profound, diffuse coronary microcirculatory disturbance in the acute phase, with early reversal to near-normal values within a few days, paralleling the functional recovery in regional wall motion.
Stress cardiomyopathy, first reported in Japan as ‘tako-tsubo’, is a recently described clinical entity characterized by acute but rapidly reversible left ventricle (LV) systolic dysfunction in the absence of atherosclerotic coronary artery disease, triggered by profound psychological or physiologic stress Citation[1]. This distinctive form of ventricular stunning typically affects older women and usually involves the distal portion of the LV chamber (‘apical ballooning’), with the hypercontractile basal LV. In 2006, the American Heart Association incorporated ‘apical ballooning’ syndrome into its classification of cardiomyopathies as a primary acquired cardiomyopathy Citation[2]. The pathophysiology of stress cardiomyopathy is not very well understood. Several mechanisms for this reversible cardiomyopathy have been proposed, including catecholamine-induced myocardial stunning, ischemia-mediated stunning due to multivessel epicardial or microvascular spasm, and myocarditis. In a number of studies, single-photon emission computed tomography using thallium and sestamibi tracers and positron emission tomography using 13N-ammonia have shown impaired perfusion in the regions of the wall motion abnormality Citation[3], Citation[4], but whether myocardial hypoperfusion is the underlying mechanism remains to be established. Coronary flow reserve (CFR) evaluated by pulsed Doppler echocardiography associated with vasodilatory stress has recently entered the stress echo lab Citation[5–8]. In patients with dilated non-ischemic cardiomyopathy, Doppler-derived CFR evaluated by pulsed Doppler echocardiography is reduced and is not related to LV inotropic reserve during dipyridamole stress Citation[9]. In its turn, the presence of an abnormal CFR during dipyridamole infusion identifies a subgroup of patients with LV dysfunction at high risk of developing progressive ventricular deterioration and heart failure Citation[10], Citation[11]. Therefore the aim of the present study was to determine the behavior of Doppler echocardiographic-derived CFR in patients with TC.
Key messages
Stress cardiomyopathy is an increasingly recognized clinical syndrome characterized by acute reversible apical ventricular dysfunction.
Tako-tsubo cardiomyopathy is characterized by a profound, diffuse coronary microcirculatory disturbance in the acute phase, with early reversal to near-normal values within a few days, paralleling the functional recovery in regional wall motion.
Methods
Patients
From April 2003 to June 2007, we studied 30 patients (5 males; mean age 68±12 years), admitted to two Italian cardiology institutions (Mestre and Vallo della Lucania), with tako-tsubo-like LV dysfunction, who underwent emergency angiography within 24 h after onset of symptoms. Tako-tsubo-like LV dysfunction was defined according to the Mayo Clinic criteria Citation[12]. All patients at time of admission had symptoms and ST segment modifications consistent with acute myocardial infarction, and urgent coronary angiography was recommended. Exclusion criteria were: idiopathic cardiomyopathy, history of myocardial infarction, recent significant head trauma, intracranial bleeding, myocarditis, and hypertrophic cardiomyopathy. All patients had absence of obstructive coronary disease or angiographic evidence of acute plaque rupture. All patients were evaluated with transthoracic dipyridamole (0.84 mg/kg over 6 h) stress echo and pulsed Doppler CFR assessment on mid-distal left anterior descending (LAD) and posterior descending of right coronary artery (PD). Wall motion score index (WMSI) was evaluated at base-line and during stress. All patients were followed up clinically and—on day 7 (±2 days) and at 6 months—by repeat stress echo. The control group (n=30; 8 females; mean age 56±14 years) comprised patients who sought cardiology counseling and experienced chest pain of unknown origin. All of them had normal coronary arteries and no co-morbidities such as diabetes, hypertension/hypertrophy, significant heart valve diseases, and/or previous myocardial infarction (all conditions that may reduce CFR), and a normal LV function (ejection fraction (EF) 62±3) and normal coronary flow reserve (CFR) (3.2±0.3). All stress echocardiograms were performed within 24 h from coronary angiography, while the patient was still in the intensive care unit (ICU). Coronary angiography and dipyridamole echocardiography test (DET) were separately and independently performed and analyzed by cardiologists unaware of the results of the other tests. Stress echo data were collected and analyzed by stress echocardiographers not involved in patient care.
Abbreviations
Data were entered in the data bank at the time of testing on the same day of test performance. The study was approved by the institution's review board. All patients gave their written informed consent when they underwent stress echocardiography and coronary angiography.
Resting and stress echocardiography
Transthoracic stress echocardiographic studies were performed with commercially available ultrasound machines (Sonos 5500-7500 Philips Ultrasound, Andover, Mass.; Sequoia C256 Acuson Siemens Mountain View, Calif.; and Vivid System 7, GE/Vingmed, Milwaukee, Wis.) equipped with multifrequency phased-array sector scan probe (S3-S8 or V3-V7) and with second harmonic technology. All standard echocardiographic views were obtained when possible: parasternal long and short axis, apical two-, three-, and four-chamber, and substernal views. Besides the classic projections for stress echocardiography testing, specific projection for LAD coronary artery imaging is integrated into the cardiac imaging sequence. Two-dimensional echocardiography and 12-lead electrocardiographic (ECG) monitoring were performed in combination with high-dose dipyridamole (up to 0.84 mg over 6 min) in accordance with state-of-the-art protocol Citation[13]. During the procedure, blood pressure and ECG were recorded every minute. The left ventricle was divided into 17 segments as suggested by the American Heart Association and American Society of Echocardiography Citation[14], Citation[15]. Segmental wall motion was graded as follows: normal = 1; hypokinetic = 2; akinetic = 3; and dyskinetic = 4. Wall motion score index (WMSI) was derived by dividing the sum of individual visualized segments scores by the number of visualized segments Citation[13].
CFR was performed during the standard stress echo examination by a semi-simultaneous imaging of both wall motion and LAD flow Citation[13]. Coronary flow in the mid-distal portion of LAD and PD was searched in the low parasternal long axis cross-section under the guidance of color Doppler flow mapping Citation[16]. All studies were digitally stored to simplify off-line reviewing and measurements. Coronary flow parameters were analyzed off-line using the built-in calculation package of the ultrasound unit. Flow velocities were measured at least twice for each study: at base-line and at peak stress (before aminophylline injection). At each time point, three optimal profiles of peak diastolic Doppler flow velocities were measured, and the results were averaged. Coronary blood flow velocity reserve was defined as the ratio between hyperemic and basal peak diastolic coronary flow. CFR was considered normal when it was >2 Citation[9]. All the digitally stored images were independently read and reviewed by a core laboratory for wall motion (Institute of Clinical Physiology, Pisa; author RS) and a core laboratory for CFR (Cardiology Division, Umberto I Hospital, Mestre-Venice; author FR). All investigators were instructed to obtain all the standard stress echo projections both for wall motion assessment and coronary flow reserve (see above); images were digitally stored at rest, at peak dipyridamole stress test, and after aminophylline infusion Citation[13]. The digital images obtained were sent to the core laboratory by web along with the investigator's stress echo report and measurements of CFR. Quality control of stress echo performance and reading in enrolled centers was previously described in depth Citation[13]. Briefly, the reader from each recruiting center met the predefined criteria for stress echo reading. At that point the center could start recruiting patients, and stress echo readings from the recruiting center were then sent directly to the core laboratories.
Coronary angiography
Coronary angiography in multiple views was performed according to standard Judkins or Sones technique. At least five views (including two orthogonal views) were acquired for the left and at least two orthogonal views for the right coronary arteries, respectively. Additional appropriate projections were obtained in case of superimposition of side branches or foreshortening of the segment of interest. All angiograms were visually evaluated by two independent observers who identified the stenotic segments and scored control arteries as smooth.
Statistical analysis
The statistical analyses included descriptive statistics (frequency and percentage of categorical variables and mean and standard deviation of continuous variables). ANOVA was used to compare the three sequential values of CFR and WMSI during follow-up (SPSS statistical software, SPSS Inc., Chicago, Illinois and S-plus 6.1). Correlation analysis was performed using Pearson coefficient for the continuous variables. The chi-square test and Fisher's exact test were used to compare categorical data. All tests were two-sided and a P-value < 0.05 was considered to be statistically significant.
Results
The clinical and echocardiographic characteristics in the 30 patients with TC are summarized in . Rest ejection fraction was 37%±5% at admission, improved to 48%±5% at day 7, and normalized at 6 months of follow-up to 54%±4% (< 0.001 versus day 1 and versus day 7). All the echo parameter changes are reported in . According to individual need and physician's choice, nine patients were evaluated during anti-anginal treatment (nitrates and/or calcium antagonists and/or beta-blockers and/or ace-inhibitors); 1 (11%) was on calcium channel blocker therapy, 7 (78%) were on beta-blocking agents, 2 (22%) were on long-acting nitrates, and 7 (78%) on ace-inhibitors or on various combinations of the four drugs. CFR on LAD was not significantly affected by concomitant medical therapy at time of testing (1.7±0.1 off therapy versus 1.8±02, P = 0.06) and CFR on right coronary artery (RCA) (1.8±0.1 off therapy versus 1.9±0.2, P=0.2). Four patients had minor coronary abnormalities (< 30% at quantitative angiography), two in the LAD and two in the RCA.
Table I. Rest and stress findings in the study population and in control group.
Table II. Rest echo changes over time.
Stress echocardiographic findings
No limiting side-effects occurred during dipyridamole stress testing at each time point.
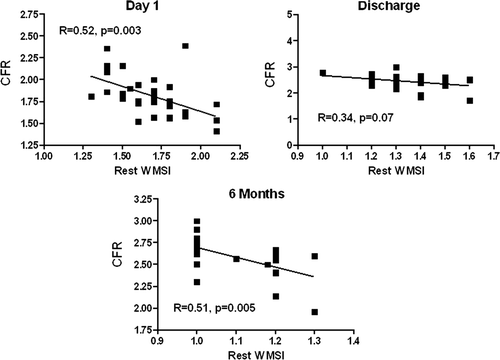
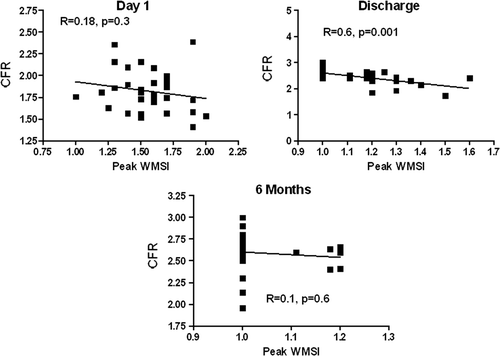
CFR was obtained in all patients on LAD and in 25 on PD. At individual patient analysis 23 patients had abnormal results (CFR on LAD < 2.0) at admission, in only 3 patients CFR on LAD was abnormal at discharge, and in 1 patient at 6-month follow-up. In the single patient that did not recover CFR at follow-up, there was no recovery of regional function as expressed by WMSI. The same behavior can be observed in the CFR on RCA. All patients showed a transient apical ballooning in the acute phase (day 1 of admission), with progressive recovery of function at follow-up (WMSI, day 1 = 1.7±0.2; day 7 = 1.4±0.14; 6 months = 1.0±0.1; P<0.001 between all groups). A sample case with wall motion and Doppler assessment on LAD and PD is depicted in . When compared to controls, CFR on LAD and PD was reduced on day 1 (upon admission), and it showed early recovery in the subacute (pre-discharge) assessment on day 7. CFR on LAD and PD values remained stable at 6-month follow-up (). A significant albeit weak correlation was found between CFR on LAD and rest WMSI at day 1 and at 6 months (), and between CFR on LAD and peak WMSI at discharge (). No correlation was observed between resting EF and CFR on LAD and PD at the different time points.
Figure 1. Transthoracic echocardiography: time course of left ventricular four-chamber view (top) and Doppler-derived coronary flow reserve (CFR) on left anterior descending coronary artery (middle) and on right coronary artery (bottom) at day 1 (acute phase), at discharge, and 6 months after discharge. Top: end-systolic frame showing a large akinetic apex at admission (rest WMSI) and improving at discharge (rest WMSI), with complete recovery of regional function after 6 months (rest WMSI). Middle panel: coronary flow velocity profile at base-line and after dipyridamole on LAD at each time point as for wall motion score index (WMSI). Bottom panel: coronary flow velocity profile at base-line and after dipyridamole on right coronary artery at each time point as for WMSI.
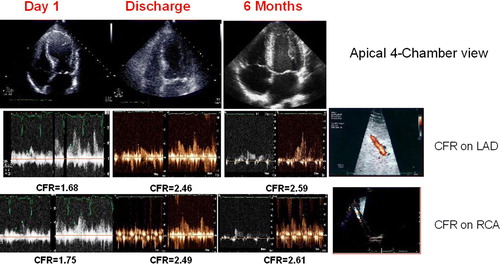
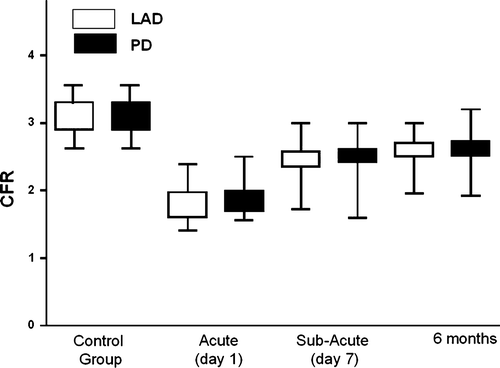
Figure 2. Coronary flow reverse (CFR) in the left anterior descending artery (LAD) area (white boxes) and in the right coronary artery (RCA) area (black boxes) in control subjects, at admission (acute), at discharge (subacute), and at 6-month follow-up (values are expressed as means±SD).
Discussion
Tako-tsubo cardiomyopathy is characterized by a profound, diffuse coronary microcirculatory disturbance in the acute phase, with early reversal to near-normal values within a few days, paralleling the functional recovery in regional wall motion. Although the improvement of CFR parallels the improvement of regional LV function, this does not occur at the same rate. In fact, CFR reached normal values at time of discharge in almost all patients, whereas WMSI kept on improving over time with complete normalization at 6-month follow-up as shown in . The improvement in WMSI is mimicked at peak stress, but this correlation is lost at 6 months when virtually all segments are normalized. The reversible coronary microcirculatory abnormality can also be observed in the area of the right coronary artery, perfusing left ventricular segments largely spared by the acute wall motion disturbances.
Comparison with previous studies
Transthoracic Doppler-derived coronary flow reserve has been previously assessed in one study on a patient population of only 12 subjects Citation[17]. The authors showed that serial non-invasive CFR measurements suggest a transient microcirculatory impairment during the acute phase of the syndrome. The wall motion improvement parallels the improvement of CFR, suggesting a role for coronary microcirculatory damage in the pathogenesis of acute and transient wall motion abnormalities in TC. Reversible perfusion abnormalities in the left ventricular apex in patients presenting with the syndrome have been documented Citation[4], Citation[18], Citation[19]. In addition, patients with the syndrome exhibit a pronounced abnormality in apical myocardial fatty acid Citation[4] or glucose metabolism Citation[19] that is out of proportion to apical perfusion abnormalities. Coronary microvascular function has been shown to be diffusely abnormal when assessed immediately after presentation by using invasive measurements of coronary flow reserve and TIMI frame count techniques Citation[20], Citation[21]. The series reported that an abnormal TIMI frame count in all three major epicardial coronary arteries during the acute phase of the syndrome was observed, suggesting a wide-spread coronary microvascular dysfunction Citation[20], Citation[21]. Still it is not clear whether coronary microcirculatory dysfunction is the underlying mechanism or just a secondary phenomenon.
The present study has several strengths: 1) This is the largest population studied so far, showing a high feasibility and a safety profile of the method (the test was performed on day of admission in this set of patients); 2) Two vascular territories have been assessed (anterior and posterior), showing that the left ventricle is globally affected by a microvascular impairment; 3) The long follow-up (up to 6 months with serial assessment of both wall motion and CFR showing that recovery was obtained early and is sustained); and 4) Regional flow and function parameters were simultaneously obtained during stress, allowing serial assessment of flow-function match.
Pathophysiologic mechanisms
The pathophysiology of TC has not yet been clearly elucidated. However, in the present study we found evidence of diffuse reduction of coronary flow reserve occurring in spite of angiographically normal coronary arteries, suggesting that TC may be another condition of coronary microvascular dysfunction, which characterizes several forms of coronary artery disease and cardiomyopathy Citation[22–28]. Also in idiopathic dilated cardiomyopathy the mechanisms of the progressive deterioration of cardiac function are largely unknown, but both myocardial hypoperfusion and myocardial ischemia at the microvascular level are most probably involved Citation[29]. Nonetheless, it is unclear whether coronary microvascular dysfunction is the primary mechanism involved in the pathogenesis of the syndrome, or whether it is simply an associated secondary phenomenon. It is likely that in this specific clinical entity, catecholamine stimulation, provoked by emotional or physiologic stress, may be the trigger Citation[12]. In fact, patients presenting with the syndrome appear to have abnormalities of cardiac sympathetic innervation with evidence of sympathetic hyperactivity at the cardiac apex Citation[30]. The distribution of apical wall motion abnormalities in the syndrome is similar to the distribution reported with catecholamine-induced cardiomyopathy Citation[31]. It has been recently hypothesized that stress cardiomyopathy is a form of myocardial stunning, but with different cellular mechanisms from those seen during transient episodes of ischemia secondary to coronary stenoses Citation[32].
Study limitations
One the main limitations of Doppler echocardiographic derived CFR on two coronaries is related to sub-optimal feasibility in the RCA territory. Accordingly, we found 100% feasibility for CFR on LAD and 83% for CFR on RCA. As test feasibility is heavily affected by the skill of the operator and advances in technology, it is likely that it may improve over time. Doppler technique needs a maximal vasodilation in order to compare rest and peak diastolic velocities, whereas the rest velocities may exert a prognostically significant meaning only when measured in a quantitative way as it happens with PET Citation[33]. In the absence of coronary artery disease, coronary flow reserve can be reduced in microvascular disease (e.g. in syndrome X, arterial hypertension, diabetes, and/or left ventricular hypertrophy Citation[34–36], which may per se reduce coronary flow reserve independently of microvascular damage Citation[37–40].
Conclusions
In patients with TC, CFR is impaired but it recovers early after hospital admission. The recovery of CFR is paralleled by wall motion improvement over time. Both vascular territories, anterior and posterior, are similarly affected showing a global LV microvascular dysfunction. The microvascular damage appears to be profound, global, reversible, and sustained. Larger populations are needed in order to assess the prognostic meaning of these parameters in patients with TC Citation[33].
Acknowledgements
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Sharkey SW, Lesser JR, Zenovich AG, Maron MS, Lindberg J, Longe TF, et al. Acute and reversible cardiomyopathy provoked by stress in women from the United States. Circulation. 2005; 111: 472–9
- Maron, BJ, Towbin, JA, Thiene, G, Antzelevitch, C, Corrado, D, Arnett, D, ; American Heart Association, et al; Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; Council on Epidemiology and Prevention. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113:1807–16.
- Bybee KA, Murphy J, Prasad A, Wright RS, Lerman A, Rihal CS, et al. Acute impairment of regional myocardial glucose uptake in the apical ballooning (takotsubo) syndrome. J Nucl Cardiol. 2006; 13: 244–50
- Kurisu S, Inoue I, Kawagoe T, Ishihara M, Shimatani Y, Nishioka K, et al. Myocardial perfusion and fatty acid metabolism in patients with tako-tsubo-like left ventricular dysfunction. J Am Coll Cardiol. 2003; 41: 743–8
- Hozumi T, Yoshida K, Akasaka T, Asami Y, Ogata Y, Takagi T, et al. Noninvasive assessment of coronary flow velocity and coronary flow velocity reserve in the left anterior descending coronary artery by Doppler echocardiography: comparison with invasive technique. J Am Coll Cardiol. 1998; 32: 1251–9
- Caiati C, Montaldo C, Zedda N, Bina A, Iliceto S. New noninvasive method for coronary flow reserve assessment: contrast-enhanced transthoracic second harmonic echo Doppler. Circulation. 1999; 99: 771–8
- Daimon M, Watanabe H, Yamagishi H, Muro T, Akioka K, Hirata K, et al. Physiologic assessment of coronary artery stenosis by coronary flow reserve measurements with transthoracic Doppler echocardiography: comparison with exercise thallium-201 single piston emission computed tomography. J Am Coll Cardiol. 2001; 37: 1310–5
- Saraste M, Koskenvuo J, Knuuti J, Toikka J, Laine H, Niemi P, et al. Coronary flow reserve: measurement with transthoracic Doppler echocardiography is reproducible and comparable with positron emission tomography. Clin Physiol. 2001; 21: 114–22
- Santagata P, Rigo F, Gherardi S, Pratali L, Drosdz J, Varga A, et al. Clinical and functional determinants of coronary flow reserve in non-ischemic dilated cardiomyopathy. An echocardiographic study. Int J Cardiol. 2005; 105: 46–52
- Rigo F, Gherardi S, Galderisi M, Pratali L, Cortigiani L, Sicari R, et al. The prognostic impact of coronary flow reserve assessed by Doppler echocardiography in non-ischemic dilated cardiomyopathy. Eur Heart J. 2006; 27: 1319–23
- Rigo F, Gherardi S, Galderisi M, Sicari R, Picano E. The independent prognostic value of contractile and coronary flow reserve determined by dipyridamole stress echocardiography in patients with idiopathic dilated cardiomyopathy. Am J Cardiol. 2007; 99: 1154–8
- Bybee KA, Kara T, Prasad A, Lerman A, Barsness GW, Wright RS, et al. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann Intern Med. 2004; 141: 858–65
- Sicari R, Nihoyannopoulos P, Evangelista A, Kasprzak J, Lancellotti P, Poldermans D, et al. Stress echocardiography expert consensus statement from the European Association of Echocardiography. Eur J Echocardiogr. 2008; 9: 415–37
- Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002; 105: 539–42
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka EA, et al. Chamber Quantification Writing Group; American Society of Echocardiography's Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005; 18: 1440–63
- Rigo F, Murer B, Ossena G, Favaretto E. Transthoracic echocardiographic imaging of coronary arteries: tips, traps, and pitfalls. Cardiovasc Ultrasound. 2008; 6: 7–12
- Meimoun P, Malaquin D, Sayah S, Benali T, Luycx-Bore A, Levy F, et al. The coronary flow reserve is transiently impaired in tako-tsubo cardiomyopathy: a prospective study using serial Doppler transthoracic echocardiography. J Am Soc Echocardiogr. 2008; 21: 72–7
- Abe Y, Kondo M, Matsuoka R, Araki M, Dohyama K, Tanio H. Assessment of clinical features in transient left ventricular apical ballooning. J Am Coll Cardiol. 2003; 41: 737–42
- Yoshida T, Hibino T, Kako N, Murai S, Oguri M, Kato K, et al. A pathophysiologic study of tako-tsubo cardiomyopathy with F-18 fluorodeoxyglucose positron emission tomography. Eur Heart J. 2007; 28: 2598–604
- Kume T, Akasaka T, Kawamoto T, Yoshitani H, Watanabe N, Neishi Y, et al. Assessment of coronary microcirculation in patients with takotsubo-like left ventricular dysfunction. Circ J. 2005; 69: 934–9
- Bybee KA, Prasad A, Barsness GW, Lerman A, Jaffe AS, Murphy JG, et al. Clinical characteristics and thrombolysis in myocardial infarction frame counts in women with transient left ventricular apical ballooning syndrome. Am J Cardiol. 2004; 94: 343–6
- Treasure CB, Vita JA, Cox DA, Fish RD, Gordon JB, Mudge GH, et al. Endothelium-dependent dilation of the coronary microvasculature is impaired in dilated cardiomyopathy. Circulation. 1990; 81: 772–9
- Inoue T, Sakai Y, Morooka S, Hayashi T, Takayanagi K, Yamaguchi H, et al. Vasodilatory capacity of coronary resistance vessels in dilated cardiomyopathy. Am J Heart. 1994; 127: 376–81
- Cannon III RO, Cunnion RE, Parillo JE, Palmeri ST, Tucker EE, Schenke WH, et al. Dynamic limitation of coronary vasodilator reserve in patients with dilated cardiomyopathy. J Am Coll Cardiol. 1987; 10: 1190–200
- Neglia D, Parodi O, Gallopin M, Sambuceti G, Giorgetti A, Pratali L, et al. Myocardial blood flow response to pacing tachycardia and to dipyridamole infusion in patients with dilated cardiomyopathy without overt heart failure: a quantitative assessment by positron emission tomography. Circulation. 1995; 92: 796–804
- Chen JW, Ting CT, Chen YH, Wu TC, Hsu NW, Lin SJ, et al. Differential coronary microvascular function in patients with left ventricular dysfunction of unknown cause—implication for possible mechanism of myocardial ischemia in early stage of cardiomyopathy. Int J Cardiol. 1999; 69: 251–61
- Cecchi F, Olivotto I, Gistri R, Lorenzoni R, Chiriatti G, Camici PG. Coronary microvascular dysfunction and prognosis in hypertrophic cardiomyopathy. N Engl J Med. 2003; 349: 1027–35
- Cortigiani L, Rigo F, Gherardi S, Galderisi M, Sicari R, Picano E. Prognostic implications of coronary flow reserve on left anterior descending coronary artery in hypertrophic cardiomyopathy. Am J Cardiol. 2008; 102: 1718–23
- Neglia, D, L'abbate, A. Coronary microvascular dysfunction and idiopathic dilated cardiomyopathy. Pharmacol Rep. 2005;57, Suppl:151–5.
- Akashi YJ, Nakazawa K, Sakakibara M, Miyake F, Musha H, Sasaka K. 123I-MIBG myocardial scintigraphy in patients with ‘takotsubo’ cardiomyopathy. J Nucl Med. 2004; 45: 1121–7
- Scott IU, Gutterman DD. Pheochromocytoma with reversible focal cardiac dysfunction. Am Heart J. 1995; 130: 909–11
- Lyon AR, Rees PS, Prasad S, Poole-Wilson PA, Harding SE. Stress (Takotsubo) cardiomyopathy—a novel pathophysiological hypothesis to explain catecholamine-induced acute myocardial stunning. Nat Clin Pract Cardiovasc Med. 2008; 5: 22–9
- Uren NG, Melin JA, De Bruyne B, Wijns W, Baudhuin T, Camici PG. Relation between myocardial blood flow and the severity of coronary-artery stenosis. N Engl J Med. 1994; 330: 1782–8
- Palinkas A, Toth E, Amyot R, Rigo F, Venneri L, Picano E. The value of ECG and echocardiography during stress testing for identifying systemic endothelial dysfunction and epicardial artery stenosis. Eur Heart J. 2002; 23: 1587–95
- Rigo F, Pratali L, Pálinkás A, Picano E, Cutaia V, Venneri L. Coronary flow reserve and brachial artery reactivity in patients with chest pain and ‘false positive’ exercise-induced ST-segment depression. Am J Cardiol. 2002; 89: 1141–4
- Galderisi M, de Simone G, Cicala S, De Simone L, D'Errico A, Caso P, et al. Coronary flow reserve in hypertensive patients with appropriate or inappropriate left ventricular mass. J Hypertens. 2003; 21: 2183–8
- Zeiher AM, Krause T, Schächinger V, Minners J, Moser E. Impaired endothelium-dependent vasodilation of coronary resistance vessels is associated with exercise-induced myocardial ischemia. Circulation. 1995; 91: 2345–52
- Panting JR, Gatehouse PD, Yang GZ, Grothues F, Firmin DN, Collins P, et al. Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. N Engl J Med. 2002; 346: 1948–53
- Lanza GA, Buffon A, Sestito A, Natale L, Sgueglia GA, Galiuto L, et al. Relation between stress-induced myocardial perfusion defects on cardiovascular magnetic resonance and coronary microvascular dysfunction in patients with cardiac syndrome X. J Am Coll Cardiol. 2008; 51: 466–72
- Moonen, M, Senechal, M, Cosyns, B, Melon, P, Nellessen, E, Pierard, L, , et al. Impact of contractile reserve on acute response to cardiac resynchronization therapy. Cardiovasc Ultrasound. 2008;1;6:65.