Abstract
Background and aim. Clinically diagnosed coeliac disease patients carry an increased risk of mortality. As coeliac disease is markedly underdiagnosed, we aimed to quantify the risk of mortality in subjects with unrecognized and thus untreated coeliac disease.
Method. Blood samples from 6,987 Finnish adults were drawn in 1978–80, and sera were tested for immunoglobulin A (IgA)-class tissue transglutaminase antibodies (Eu-tTG) in 2001. Positive sera were further analysed for endomysial (EMA) and tissue transglutaminase antibodies by another test (Celikey tTG). EMA- and Celikey tTG-positive cases were compared to negatives as regards mortality in up to 28 years of surveillance, yielding a total follow-up of 147,646 person years. Dates and causes of death were extracted from the nation-wide database.
Results. Altogether 74 (1.1%) of the participants were EMA- and 204 (2.9%) Celikey tTG-positive. The age- and sex-adjusted relative risk of overall mortality was not increased in either EMA (0.78, 95% CI 0.52–1.18) or Celikey tTG (1.19, 95% CI 0.99–1.42) -positive subjects. However, antibody-positive cases evinced a tendency to die from lymphoma, stroke, and diseases of the respiratory system.
Conclusions. The prognosis of unrecognized coeliac disease was good as regards overall mortality, which does not support screening of asymptomatic coeliac disease cases.
Introduction
Coeliac disease is a chronic autoimmune-like disorder with intestinal and extraintestinal manifestations induced by wheat gluten and related proteins of rye and barley. Conceptions of the epidemiology of coeliac disease have changed substantially over time. It is currently considered a global prevalent disorder increasing over time and affecting up to 1%–2% of the Western population Citation[1–5]. Diarrhoea and malabsorption as clinical manifestations are nowadays more rarely seen, and up to 70%–90% of the coeliac disease population in Western countries remain unrecognized due to the absence or the atypical nature of symptoms Citation[1–5]. Prognostic studies on undiagnosed and thus untreated cases are scant, and debate thus continues as to whether health care professionals should rigorously seek out these cases even by population mass-screening Citation[6–11].
Diagnosed clinically detected coeliac disease cases carry a 1.3–3.8-fold increased risk of mortality mainly attributable to malignancies, and the risk seems to decrease on a gluten-free diet Citation[12–20]. However, there have been only two previous attempts to assess the association between unrecognized coeliac disease and mortality Citation[21], Citation[22]. Due to the relatively short follow-up times and modest number of antibody-positive cases in the studies in question, the results remain inconclusive.
Key messages
The prognosis of undetected coeliac disease is good as regards overall mortality.
Coeliac antibody-positive undetected cases evinced an additional tendency to die from lymphoma, stroke, and diseases of the respiratory system.
We followed a large Finnish population-based adult-representative cohort of 6,987 people collected in 1978–80, focusing on mortality and aiming to establish whether unrecognized coeliac disease individuals carry an increased risk of mortality during a surveillance of up to 28 years. Sequential analysis of sera from the participants by tissue transglutaminase and endomysial antibody tests with reported high validity Citation[23], Citation[24] enabled us to define coeliac autoantibody-positive cases most likely representing unrecognized coeliac disease. We availed ourselves of the causes of death register of Statistics Finland and could compare the overall and cause-specific mortality between antibody-positive and -negative subjects throughout the follow-up period.
Material and methods
Study population
The Mini-Finland Health Survey carried out in 1978–80 provided a basis for the current population-based follow-up study between autoantibody-positive unrecognized coeliac disease and mortality. Detailed information on the design and base-line results of the primary study has been published elsewhere Citation[25], Citation[26]. In brief, a nationally representative sample of 8,000 persons was drawn from the population aged 30–99 years according to a stratified two-stage cluster-sampling model planned by Statistics Finland. The second stage of sampling was implemented in 40 areas in different parts of the country. The participants attended a health examination which included interviews, questionnaires, drawing of blood samples, and a clinical examination by a physician.
Abbreviations
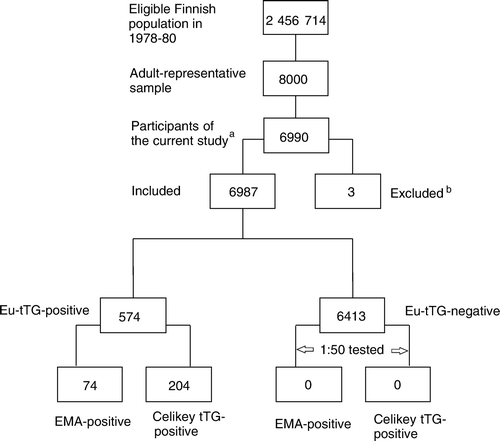
The participation rate was 90% (n =7,217), and sera from 6,990 individuals were still available for the purposes of this study in 2001 (). Three coeliac disease or dermatitis herpetiformis cases previously diagnosed and treated were excluded from the analysis at the beginning of the follow-up, yielding a total of 6,987 participants (3,766 females, mean age 51 years, age range 30–95) for this study (). Information on age, sex, education, body mass index, alcohol consumption, smoking, hypertension, serum cholesterol, low-density lipoprotein, high-density lipoprotein, triglycerides, diabetes, coronary heart disease, stroke, and cancer were extracted for purposes of adjustment. The definitions and measurement of potential confounding and effect-modifying factors have been described in greater detail elsewhere Citation[25], Citation[26]. All participants gave informed consent. The Ethical Committee of Tampere University Hospital approved the study protocol.
Figure 1. Flow chart of the present study. Eu-tTG = IgA-class tissue transglutaminase antibody (Eu-tTG® umana IgA); Celikey tTG = IgA-class tissue transglutaminase antibody (Celikey® Tissue Transglutaminase IgA Antibody Assay); EMA = immunoglobulin A (IgA)-class endomysial antibody; a = Not including non-participants in the primary study (n=783) or participants with no sera available for the purposes of the current study in 2001 (n=227); b = Cases with previous coeliac disease or dermatitis herpetiformis diagnosis (n=3).
Measurement of antibodies
Serum samples from the participants were stored at −20°C, and altogether 6,987 sera were analysed for immunoglobulin A (IgA)-class tissue transglutaminase antibodies in the first stage of screening (Eu-tTG® umana IgA, Eurospital S.p.A., Trieste, Italy; abbreviated as Eu-tTG) in 2001 (). Further, positive sera were analysed parallelly for both IgA endomysial antibodies (abbreviated as EMA) and using another IgA-class tissue transglutaminase antibody kit (Celikey®, Phadia, Freiburg, Germany; abbreviated as Celikey tTG) (). The endomysial antibodies were defined by a standardized and validated indirect immunofluorescence method using human umbilical cord as antigen, and a characteristic staining pattern at a serum dilution 1:≥5 was considered positive Citation[27], Citation[28]. Both commercial tissue transglutaminase antibody kits use human recombinant tissue transglutaminase as antigen, and results are given in arbitrary units (AU). The cut-off point for the Eu-tTG was 7.0 AU/mL and for the Celikey tTG 5.0 AU/mL according to manufacturers’ instructions. These IgA-class tissue transglutaminase and EMA tests have been shown to be valid for coeliac disease, with pooled specificities approaching 100% and sensitivities mainly over 90% in adult populations Citation[24], Citation[29]. In accordance with previous studies Citation[5], Citation[30] the definition of unrecognized coeliac disease was based on a two-stage screening algorithm where cases yielding a positive result for both Eu-tTG and either EMA or Celikey tTG were considered to constitute undetected coeliac disease. The main motivation for the two-stage screening algorithm was to decrease the likelihood of false-positive results and thus the dilution of a real effect. Furthermore, to find a close estimate of the real risk we defined unrecognized coeliac disease by two different antibody tests (Celikey tTG and EMA) in the second stage of screening.
In view of the unexpectedly high number of Eu-tTG-positive sera collected 22 years earlier, we wished to check that the influence of storage on Eu-tTG values was not arbitrary. We therefore also tested 128 (1 in 50) randomly selected Eu-tTG-negative sera for EMA and Celikey tTG (). As none were positive, we could ascertain that the likelihood of coeliac disease in Eu-tTG-negative individuals was low (). We have previously extensively discussed the possible influence of storage of sera on coeliac autoantibodies Citation[5], Citation[30].
Mortality
Date and cause of death were identified by linking the unique personal identification codes with records from the nation-wide database of Statistics Finland Citation[31]. The principal causes of death were coded either according to International Classification of Diseases (ICD)-8, -9, or -10, depending on the time of death. Follow-up commenced the day the blood samples were drawn in 1978–80, and the study subjects were under surveillance until the end of 2005 or death; this yielded a total follow-up of 147,646 person years. The maximum follow-up time was 28 years. The mortality among antibody-positive cases was compared to that of antibody-negative individuals in the same cohort.
Statistical analysis
Adjusted relative risks (RR) and their 95% confidence intervals (95% CI) for mortality comparing antibody-positive with antibody-negative participants were estimated based on a Cox regression model (Cox 1972). Statistical significance of heterogeneity was tested using the likelihood ratio test based on the model and expressed by P-value. The possible confounding effects of age, sex, education, body mass index, alcohol consumption, smoking, hypertension, serum cholesterol, high-density lipoprotein and triglycerides, diabetes, coronary heart disease, stroke, and cancer were assessed using a series of multivariate models. Stratified analyses were conducted to assess mortality risk also according to the level of antibodies (titres 1:<500 and 1:≥500 in EMA, and <6.4 and ≥6.4 AU/mL in Celikey tTG, divided by medians of positive values) and length of follow-up (1–10 years and >10 years). In addition, potential effect-modifying factors such as age, sex, education, body mass index, alcohol consumption, smoking, hypertension, and cholesterol values were entered into the models. The analyses were performed using SAS 9.1 (SAS Institute, Cary, NC, USA).
Results
Altogether 574 out of the 6,987 analysed serum samples proved Eu-tTG-positive (). Seventy-four (53 females, mean age 49 years) out of the Eu-tTG-positive had positive EMA, and 204 (125 females, mean age 59 years) positive Celikey tTG. Thus, 1.1% of the 6,987 analysed samples were EMA-positive and, correspondingly, 2.9% Celikey tTG-positive.
As to the personal characteristics of the screened population, subjects positive in either EMA or Celikey tTG had better cholesterol profiles compared to antibody-negative participants (). In addition, Celikey tTG-positive cases were older, consumed more alcohol, and more likely suffered from diabetes than antibody-negative subjects. Otherwise, no statistically significant differences were detected across antibody status. A total of 3,069 (43.9%) participants out of the 6,987 died during the surveillance.
Table I. Association of personal characteristics with endomysial (EMA) and tissue transglutaminase (Celikey tTG) antibodies: age- and sex-adjusted distributions or mean values with standard deviations (SD) are shown.
No increased age- and sex-adjusted relative risk of overall mortality could be detected among EMA-positive cases, but there was border-line significant modestly elevated risk of overall mortality in Celikey tTG-positive individuals (). The risk levels remained virtually the same after further adjustment for body mass index, smoking, education, alcohol consumption, hypertension, serum cholesterol profile, diabetes, coronary disease, stroke, and cancer. Nor was the risk statistically significantly different in the first 10 years of follow-up (EMA-positive 0.36 (95% CI 0.12–1.11, P=0.03); Celikey tTG-positive 1.17 (95% CI 0.89–1.54, P=0.26)) or thereafter (EMA-positive 0.95 (95% CI 0.61–1.47, P=0.81); Celikey tTG-positive 1.23 (95% CI 0.98–1.56, P=0.09)). Furthermore, high EMA titre had likewise no influence on risk level (0.74, 95% CI 0.42–1.30, P=0.45), but individuals with high Celikey tTG levels had a border-line significantly elevated risk of overall mortality (1.32, 95% CI 1.00–1.72, P=0.12). No statistically significant interactions between any of the potentially effect-modifying factors and antibody status were noted in the prediction of all-cause mortality (data not shown).
Table II. Age-, sex-, and multivariate-adjusted relative risks of all-cause mortality between persons with positive and negative endomysial (EMA) and tissue transglutaminase (Celikey tTG) antibodies.
Diseases of the circulatory system and malignant neoplasm were leading causes of death among both the antibody-positive and -negative population, comprising 72.0% of all deaths. Antibody-positive cases ran an increased risk of death from lymphoma (). Equally, the risk estimates for stroke and diseases of the respiratory system were increased in both EMA- and Celikey tTG-positive subjects. The risk estimate for dementia as a cause of death was increased only in Celikey tTG-positive cases ().
Table III. Age- and sex-adjusted relative risks of cause-specific mortality between persons with positive and negative endomysial (EMA) and tissue transglutaminase (Celikey tTG) antibodies.
Discussion
No statistically significantly increased risk of all-cause mortality was detected among coeliac antibody-positive unrecognized coeliac disease cases. This is in contrast to findings in earlier studies in apparently symptomatic clinically detected coeliac disease patients, which have shown an increased risk of overall mortality Citation[12–20]. The association between unrecognized coeliac disease and mortality has previously been approached in only two separate studies, with discrepant results Citation[21], Citation[22]. Metzger and associates could show a 2.5-fold increased risk of overall mortality among tissue transglutaminase antibody-positive cases Citation[22]. Their cases were mostly men, whereas in coeliac disease in general females predominate Citation[32]. Comparable to our findings again, Johnston and colleagues Citation[21] found no excess risk, but their cohort consisted of only few EMA- or antireticulin antibody-positive cases. However, it remains unclear whether a border-line statistically significant 19% increased risk of overall mortality among Celikey tTG-positive cases of our study could reach statistical significance in still larger settings. We hypothesize that any possible difference in risk of mortality between endomysial and tissue transglutaminase antibody-positive individuals might be due to border-line positive tissue transglutaminase antibodies outside celiac disease or reflect different subtypes of coeliac disease.
Relating to cause-specific mortality, malignancies at any site were not in general overrepresented in unrecognized coeliac disease. However, albeit based on few cases, the present study indicated that non-Hodgkin's lymphoma as cause of death was overrepresented in undetected coeliac disease. The association is strongly supported by the previous literature, where a connection between diagnosed coeliac disease and non-Hodgkin's lymphoma has repeatedly been reported Citation[15], Citation[16], Citation[18], Citation[20], Citation[33]. As to diseases of the circulatory system, the association with coeliac disease has remained inconclusive Citation[16], Citation[18], Citation[20], Citation[34], Citation[35]. We could show lower cholesterol levels in antibody-positive compared to antibody-negative individuals. Even though the mechanisms for the phenomenon should be evaluated in further studies, we suggest that it might be due to impaired absorption in the intestine. A favourable cardiovascular risk profile has also previously been connected with undetected coeliac disease Citation[3], thus possibly reducing the risk of diseases of the circulatory system. On the other hand, malabsorption of folic acid followed by hyperhomocysteinaemia might increase the risk of these diseases Citation[36–38] and explain the increased risk of stroke in unrecognized coeliac disease cases in the current study. Furthermore, an excess risk of diseases of the respiratory system in general could be demonstrated in our study, as has previously been reported in diagnosed cases Citation[15], Citation[16]. According to the previous literature, specific illnesses such as tuberculosis Citation[39], other lung cavities Citation[40], and sarcoidosis Citation[41] might be in the background.
We are confident regarding the main results of the present study, as we used an adult-representative population-based cohort with a high participation rate (90%) and a substantial number (147,646) of person years, and further tested all sera from the participants by a two-stage screening-algorithm. In addition, with a cohort study design with historical components we could avoid the possible ethical concern associated with a fully prospective design, i.e. following up diagnosed coeliac disease cases without treatment for decades. Nor would a fully prospective study have yielded results in reasonable time. By our population-based screening we were able to avoid confining participants to the most serious cases as is commonly the case in studies with clinically diagnosed coeliac disease. In addition, we compared antibody-positive and -negative individuals of the same cohort and could thus adjust for several potential confounding factors Citation[42]. However, imperfect sensitivity due to IgA deficiency could slightly dilute the real effect Citation[43]. As to the outcome variable, the causes of death register has included all deaths in Finland since 1936, and good validity and coverage of the data in the register have been reported Citation[31], Citation[44]. In brief, details of our study design strengthen the validity of the present results on the prognosis of the unrecognized section of the coeliac population.
Since it might be asked whether good prognosis as regards overall mortality is due to the diagnosis and treatment of unrecognized cases during surveillance, we evaluated risk estimates in the first 10 years of follow-up and thereafter. We found no increased risk of mortality in the first period of follow-up, where the likelihood of coeliac disease diagnosis most probably remained low, or later on. However, individuals with higher levels of tissue transglutaminase antibodies carried a modestly increased risk of mortality, this possibly being explained by more severe disease in these cases Citation[15], Citation[45–47].
The good prognosis found here as regards overall mortality would suggest that a search for asymptomatic coeliac disease by screening programmes is not warranted even though the mortality risks of specific diseases were increased. Other outcome variables such as fractures and quality of life in undetected disease should still be evaluated in future studies to obtain an overall picture of the entity of undetected coeliac disease.
Acknowledgements
The Coeliac Disease Study Group is supported by grants from the Competitive Research Funding of the Pirkanmaa Hospital District, the Emil Aaltonen Foundation, the Foundation for Paediatric Research, the Yrjö Jahnsson Foundation, the Finnish Coeliac Society, and the Academy of Finland, Research Council for Health. The present study was also funded by the Commission of the European Communities in the form of the Research and Technology Development programme ‘Quality of Life and Management of Living Resources’ (QLRT-1999-00037), ‘Evaluation of the Prevalence of Coeliac Disease and its Genetic Components in the European Population’. The study does not necessarily reflect the current views or future policies of the Commission of European Communities. The authors’ work was independent of the funders. Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Fasano A, Berti I, Gerarduzzi T, Not T, Colletti RB, Drago S, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003; 163: 286–92
- Mäki M, Mustalahti K, Kokkonen J, Kulmala P, Haapalahti M, Karttunen T, et al. Prevalence of celiac disease among children in Finland. N Engl J Med. 2003; 348: 2517–24
- West J, Logan RF, Hill PG, Lloyd A, Lewis S, Hubbard R, et al. Seroprevalence, correlates, and characteristics of undetected coeliac disease in England. Gut. 2003; 52: 960–5
- Bingley PJ, Williams AJ, Norcross AJ, Unsworth DJ, Lock RJ, Ness AR, et al. Undiagnosed coeliac disease at age seven: population based prospective birth cohort study. BMJ. 2004; 328: 322–3
- Lohi S, Mustalahti K, Kaukinen K, Laurila K, Collin P, Rissanen H, et al. Increasing prevalence of coeliac disease over time. Aliment Pharmacol Ther. 2007; 26: 1217–25
- Logan RF. Screening for coeliac disease–-has the time come for mass screening?. Acta Paediatr Suppl. 1996; 412: 15–9
- Kumar PJ. European and North American populations should be screened for coeliac disease. Gut. 2003; 52: 170–1
- Fasano A. European and North American populations should be screened for coeliac disease. Gut. 2003; 52: 168–9
- Young EH, Wareham NJ. Screening for coeliac disease: what evidence is required before population programmes could be considered?. Arch Dis Child. 2004; 89: 499–500
- Hoffenberg EJ. Should all children be screened for celiac disease?. Gastroenterology. 2005; 128: S98–103
- Collin P. Should adults be screened for celiac disease? What are the benefits and harms of screening?. Gastroenterology. 2005; 128: S104–8
- Nielsen OH, Jacobsen O, Pedersen ER, Rasmussen SN, Petri M, Laulund S, et al. Non-tropical sprue. Malignant diseases and mortality rate. Scand J Gastroenterol. 1985; 20: 13–8
- Logan RF, Rifkind EA, Turner ID, Ferguson A. Mortality in celiac disease. Gastroenterology. 1989; 97: 265–71
- Cottone M, Termini A, Oliva L, Magliocco A, Marrone C, Orlando A, et al. Mortality and causes of death in celiac disease in a Mediterranean area. Dig Dis Sci. 1999; 44: 2538–41
- Corrao G, Corazza GR, Bagnardi V, Brusco G, Ciacci C, Cottone M, et al. Mortality in patients with coeliac disease and their relatives: a cohort study. Lancet. 2001; 358: 356–61
- Peters U, Askling J, Gridley G, Ekbom A, Linet M. Causes of death in patients with celiac disease in a population-based Swedish cohort. Arch Intern Med. 2003; 163: 1566–72
- West J, Logan RF, Smith CJ, Hubbard RB, Card TR. Malignancy and mortality in people with coeliac disease: population based cohort study. BMJ. 2004; 329: 716–9
- Viljamaa M, Kaukinen K, Pukkala E, Hervonen K, Reunala T, Collin P. Malignancies and mortality in patients with coeliac disease and dermatitis herpetiformis: 30-year population-based study. Dig Liver Dis. 2006; 38: 374–80
- Anderson LA, McMillan SA, Watson RG, Monaghan P, Gavin AT, Fox C, et al. Malignancy and mortality in a population-based cohort of patients with coeliac disease or ‘gluten sensitivity’. World J Gastroenterol. 2007; 13: 146–51
- Solaymani-Dodaran M, West J, Logan RF. Long-term mortality in people with celiac disease diagnosed in childhood compared with adulthood: a population-based cohort study. Am J Gastroenterol. 2007; 102: 864–70
- Johnston SD, Watson RG, McMillan SA, Sloan J, Love AH. Coeliac disease detected by screening is not silent—simply unrecognized. QJM. 1998; 91: 853–60
- Metzger MH, Heier M, Mäki M, Bravi E, Schneider A, Lowel H, et al. Mortality excess in individuals with elevated IgA anti-transglutaminase antibodies: the KORA/MONICA Augsburg cohort study 1989–1998. Eur J Epidemiol. 2006; 21: 359–65
- Aro P, Ronkainen J, Storskrubb T, Bolling-Sternevald E, Carlsson R, Johansson SE, et al. Valid symptom reporting at upper endoscopy in a random sample of the Swedish adult general population: the Kalixanda study. Scand J Gastroenterol. 2004; 39: 1280–8
- Rostom A, Dube C, Cranney A, Saloojee N, Sy R, Garritty C, et al. The diagnostic accuracy of serologic tests for celiac disease: a systematic review. Gastroenterology. 2005; 128: S38–46
- Aromaa, A, Heliövaara, M, Impivaara, O, Knekt, P, Maatela, J. The execution of the Mini-Finland Health Survey. Part 1: Aims, methods and study population. Helsinki and Turku: the Social Insurance Institution; 1989, (in Finnish with English summary).
- Lehtonen, R, Kuusela, V. The execution of the Mini-Finland Health Survey. Part 5: Statistical efficiency of the Mini-Finland Health Survey's sampling design. Helsinki and Turku: the Social Insurance Institution; 1986, (in Finnish with English summary).
- Sulkanen S, Halttunen T, Laurila K, Kolho KL, Korponay-Szabo IR, Sarnesto A, et al. Tissue transglutaminase autoantibody enzyme-linked immunosorbent assay in detecting celiac disease. Gastroenterology. 1998; 115: 1322–8
- Stern M. Working Group on Serologic Screening for Celiac Disease. Comparative evaluation of serologic tests for celiac disease: a European initiative toward standardization. J Pediatr Gastroenterol Nutr. 2000; 31: 513–9
- Hill ID. What are the sensitivity and specificity of serologic tests for celiac disease? Do sensitivity and specificity vary in different populations?. Gastroenterology. 2005; 128: S25–32
- Lohi S, Maki M, Montonen J, Knekt P, Pukkala E, Reunanen A, et al. Malignancies in cases with screening-identified evidence of coeliac disease: a long-term population-based cohort study. Gut. 2009; 58: 643–7
- Causes of death, description of statistics. Available at: http://www.tilastokeskus.fi/meta/til/ksyyt_en.html, , (accessed 20 January 2008).
- Green PH, Jabri B. Coeliac disease. Lancet. 2003; 362: 383–91
- Gao Y, Kristinsson SY, Goldin LR, Bjorkholm M, Caporaso NE, Landgren O. Increased risk for non-Hodgkin lymphoma in individuals with celiac disease and a potential familial association. Gastroenterology. 2009; 136: 91–8
- West J, Logan RF, Card TR, Smith C, Hubbard R. Risk of vascular disease in adults with diagnosed coeliac disease: a population-based study. Aliment Pharmacol Ther. 2004; 20: 73–9
- Wei L, Spiers E, Reynolds N, Walsh S, Fahey T, MacDonald TM. The association between coeliac disease and cardiovascular disease. Aliment Pharmacol Ther. 2008; 27: 514–9
- Homocysteine Studies Collaboration. Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA. 2002; 288: 2015–22
- Dickey W, Ward M, Whittle CR, Kelly MT, Pentieva K, Horigan G, et al. Homocysteine and related B-vitamin status in coeliac disease: Effects of gluten exclusion and histological recovery. Scand J Gastroenterol. 2008; 43: 682–8
- Milani RV, Lavie CJ. Homocysteine: the Rubik's cube of cardiovascular risk factors. Mayo Clin Proc. 2008; 83: 1200–2
- Ludvigsson JF, Wahlstrom J, Grunewald J, Ekbom A, Montgomery SM. Coeliac disease and risk of tuberculosis: a population based cohort study. Thorax. 2007; 62: 23–8
- Stevens FM, Connolly CE, Murray JP, McCarthy CF. Lung cavities in patients with coeliac disease. Digestion. 1990; 46: 72–80
- Douglas JG, Gillon J, Logan RF, Grant IW, Crompton GK. Sarcoidosis and coeliac disease: an association?. Lancet. 1984; 2: 13–5
- Card TR, Solaymani-Dodaran M, Hubbard R, Logan RF, West J. Is an internal comparison better than using national data when estimating mortality in longitudinal studies?. J Epidemiol Community Health. 2006; 60: 819–21
- Cataldo F, Marino V, Ventura A, Bottaro G, Corazza GR. Prevalence and clinical features of selective immunoglobulin A deficiency in coeliac disease: an Italian multicentre study. Italian Society of Paediatric Gastroenterology and Hepatology (SIGEP) and ‘Club del Tenue’ Working Groups on Coeliac Disease. Gut. 1998; 42: 362–5
- Gissler M, Haukka J. Finnish health and social welfare registers in epidemiological research. Norsk Epidemiologi. 2004; 14: 113–20
- Fabiani E, Catassi C. International Working Group. The serum IgA class anti-tissue transglutaminase antibodies in the diagnosis and follow up of coeliac disease. Results of an international multi-centre study. International Working Group on Eu-tTG. Eur J Gastroenterol Hepatol. 2001; 13: 659–65
- Abrams JA, Diamond B, Rotterdam H, Green PH. Seronegative celiac disease: increased prevalence with lesser degrees of villous atrophy. Dig Dis Sci. 2004; 49: 546–50
- West J, Logan RF, Hill PG, Khaw KT. The iceberg of celiac disease: what is below the waterline?. Clin Gastroenterol Hepatol. 2007; 5: 59–62