Abstract
Background and aims. The aim of this study was to evaluate the long-term effects of the titanium-nitride-oxide-coated (TITANOX) stent and the paclitaxel-eluting stent (PES) in patients who had undergone a percutaneous coronary intervention for acute myocardial infarction (MI).
Methods and results. The TITAX-AMI trial randomly assigned 425 patients with MI to receive either a TITANOX stent or a PES. The primary end-point was a composite of MI, target lesion revascularization, or death from cardiac causes. At 12 months, there was no significant difference between patients receiving TITANOX stent or PES in the rate of primary end-point (10.3% versus 12.8%, P=0.5). After 2 years of follow-up, a significantly lower rate of primary end-point was observed in the TITANOX stent group compared with the PES group (11.2% versus 21.8%, HR 2.2, 95% confidence interval (CI) 1.3–3.8, P=0.004). This difference was driven by a reduced rate of MI (5.1% versus 15.6%, P<0.001) and cardiac death (0.9% versus 4.7%, P=0.02) in favour of the TITANOX stent. Definite stent thrombosis occurred in 0.5% and 6.2% of the patients (P=0.001), respectively.
Conclusions. The implantation of a TITANOX stent resulted in better clinical outcome compared with a PES during 2 years of follow-up among patients treated for acute MI.
Introduction
Drug-eluting stents (DES), including the paclitaxel-eluting stent (PES), have been shown to improve both early and late outcomes, as compared with bare-metal stents (BMS), predominantly as a result of a reduction in target lesion revascularization (TLR) Citation[1], Citation[2]. However, most randomized DES trials have excluded patients with acute myocardial infarction (MI), though invasive approach is currently the preferred method for treatment of acute MI Citation[3–5]. Previous trials and meta-analyses demonstrated that the use of DES in patients with acute ST-elevation myocardial infarction (STEMI) is safe and improves clinical outcomes mainly by decreasing the risk of reintervention compared with BMS Citation[6–13]. Non-ST-elevation MI (NSTEMI) and STEMI are usually considered to be different entities, but recent reports suggested that the prognosis of either subgroup of MI is similar despite different management strategies Citation[14], Citation[15].
The 1-year follow-up of the titanium-nitride-oxide-coated (TITANOX) stents versus paclitaxel-eluting stents (PES) in acute MI trial (TITAX-AMI) showed no significant superiority of PES compared to TITANOX stents in MI Citation[16]. The TITAX-AMI trial indicated a higher rate of stent thrombosis (ST) in patients receiving PES. The present analysis was performed to evaluate whether clinical outcomes of TITANOX stents and PES will differ at 2 years after stent implantation for MI.
Key messages
The implantation of a titanium-nitride-oxide-coated (TITANOX) stent resulted in better clinical outcome compared with a paclitaxel-eluting stent (PES) during 2 years of follow-up among patients treated for acute myocardial infarction (MI).
The overall rate of stent thrombosis (ST) was significantly higher in PES-treated patients.
In multivariable analyses, MI, cardiac death, definite ST, and major adverse cardiac events (MACE) were predicted by the use of PES.
The occurrence of serious adverse events caused by very late ST was the other principal interest in this analysis. There have been concerns about the safety of DES, e.g. most notably late ST. For on-label use, identical rates of ST were observed in both selected DES and BMS patients after up to 4 years according to pooled analyses of randomized DES trials Citation[17], Citation[18]. It has been postulated that delayed endothelialization and DES malapposition may lead to late ST resulting in MI or death Citation[19]. In addition, vessel healing at the culprit site in MI patients treated with DES is substantially delayed compared with the lesion site in patients receiving DES for stable angina, emphasizing the importance of the arterial response to DES Citation[20]. On the other hand, new strategies with BMS technology have also been aimed at enhanced vascular healing. The TITANOX stent seems to decrease acute surface thrombogenicity Citation[21–24] and reduce in-stent restenosis when compared with conventional stainless steel stents Citation[21].
We designed a randomized trial to determine whether TITANOX stents are safe compared to PES in the setting of acute MI as measured by major adverse cardiac events (MACE) at 2-year follow-up.
Material and methods
Study design and patient population
The design of the original study has been previously reported Citation[16]. Briefly, the TITAX-AMI (Titanium-Nitride-Oxide-Coated Stents versus Paclitaxel-Eluting Stents in Acute Myocardial Infarction) trial is a prospective, randomized, and multicentre trial conducted from December 2005 to November 2006 in six Finnish hospitals. The study was initiated by the investigators and conducted according to the declaration of Helsinki, and written informed consent was obtained from all patients. The study protocol was approved by the Ethics Committees of the co-ordinating centre, Satakunta Central Hospital, and the participating hospitals. The study has been registered in www.clinicaltrials.gov, number NCT00495664.
Abbreviations
All patients >18 years of age presenting with acute MI were eligible for this trial. Diagnostic criteria for NSTEMI included symptoms and signs of myocardial ischaemia, dynamic ECG changes, and detection of rise and/or fall of cardiac biomarkers (troponin) with at least one value above the 99th percentile of upper reference limit. STEMI was diagnosed if the patient had chest pain at rest >20 minutes and persistent ≥1 mm of ST-segment elevation in at least two contiguous limb leads or ≥2 mm in two contiguous precordial leads. Exclusion criteria included unprotected left main disease, ostial or restenotic lesions, contraindication to aspirin, clopidogrel, or heparins, life expectancy of less than 12 months, and need for a stent longer than 28 mm. According to the trial protocol, randomization was performed after visualization of the culprit lesion or a totally occluded infarct-related vessel during coronary angiography. Patients were randomly assigned to the study groups in a 1:1 fashion.
Procedures and clinical follow-up
Lesions were treated according to current interventional techniques, with the final strategy left entirely up to the operator's discretion. Angiographic success was defined as a residual stenosis <30% by visual analysis in the presence of thrombolysis in myocardial infarction (TIMI) flow grade 3. If more than one stent was needed, stents of the same type as the assigned stent were recommended. The study protocol recommended premedication with aspirin (dose 100–500 mg) or a loading dose of intravenous aspirin (250–500 mg), and clopidogrel before the procedure. If clopidogrel was not utilized before the procedure, a loading dose of 300–600 mg of clopidogrel was administered immediately after the index procedure. Administration of intravenous heparin, low-molecular-weight heparin, bivalirudin, and glycoprotein IIb/IIIa receptor inhibitors were left to the investigator's discretion.
The TITANOX stent (Titan-2®, Hexacath, Paris, France) is a thin strut balloon expandable stent made of stainless steel and coated by plasma-enhanced vapour deposition of titanium in a prespecified gas mixture of nitrogen and oxygen in a vacuum chamber Citation[22–24]. The PES (Taxus-Liberte®, Boston Scientific, Natick, Massachusetts, USA) is comprised of a stainless steel stent platform, a polyolefin polymer derivative, and a microtubular stabilizing agent paclitaxel, with two-phase 30-day polymeric release kinetics that provide antiproliferative effect Citation[2]. Paclitaxel release is completed within 30 days of implantation, although a substantial portion (>90%) of the paclitaxel remains within the polymer indefinitely.
At discharge, 100 mg of aspirin daily indefinitely and 75 mg of clopidogrel daily for at least 6 months were prescribed for all patients. We recorded adverse events during hospitalization, and clinical follow-up was performed at 12 and 24 months.
Primary and secondary end-points
The primary end-point was the first occurrence of MACE within 1 year defined as the composite of TLR, recurrent MI, or death from cardiac causes. The 2-year analysis was prespecified per the protocol (follow-up data were planned to be collected yearly up to 5 years after randomization). TLR was defined as a repeat percutaneous intervention of the target lesion to treat a stenosis (>50%) within the stent or in the segments 5 mm distal or proximal to the stent, driven by clinical symptoms and/or signs of myocardial ischaemia, or by-pass surgery of the target vessel due to in-stent restenosis or other complications of the target lesion. Myocardial reinfarction during the follow-up was diagnosed when a rise in the myocardial injury marker level (troponin I or T) above the upper reference limit was detected together with symptoms suggestive of acute myocardial ischaemia. For the diagnosis of myocardial reinfarction during the index hospitalization, a new rise >50% above the previously measured injury marker level was required. Cardiac death was defined as any death due to cardiac causes, unwitnessed death, or death of unknown causes.
The secondary end-points of the trial included all-cause mortality, composite of cardiac death or reinfarction and ST. According to the protocol, ST was diagnosed in the presence of an acute coronary syndrome with angiographic evidence of either vessel occlusion or thrombus within the study stent, or in autopsy. ST was categorized as acute (<24 hours after the stenting), subacute (1–30 days after the stenting), or late (>30 days after the stenting). Additionally, we agreed to use the definition of ST according to the Academic Research Consortium (ARC) classification as definite, probable, or possible Citation[25]. Blinded outcome assessment was performed by the independent clinical event committee.
Statistical analysis
The overall sample size calculation for this clinical trial has been previously reported Citation[16]. Briefly, because of the exploratory nature of the study and the consequent lack of prior knowledge about the effect size, the sample size calculation was based on analysis of the results of published registry data of TITANOX stents and PES in the real world clinical practice Citation[22]. In this registry, the incidence of MACE at 12 months in patients presenting with acute MI was ~7% in the TITANOX stent group and ~15% in the PES group. Given the above assumption, we estimated that a total of 200 patients would be required in each group to provide 80% power at the 5% level of significance to detect this difference of 8% in MACE between the study groups.
Continuous variables are presented as means (SD), and study groups were compared by Student's unpaired t test. Categorical variables are presented as counts and percentages and were compared by the chi-square or Fisher's exact test. Univariate and multivariable logistic regression analyses were performed to identify independent predictors for MACE, TLR, cardiac death, and ST during the 2 years of follow-up. Because of multiple testing only variables with a two-sided P < 0.05 in the univariate analysis were entered into multivariable models. A two-sided P-value <0.05 was required for statistical significance. The rates of MACE- and MI-free survival during the 2-year follow-up period were estimated with the Kaplan-Meier method. The significance of differences between treatment groups was assessed by the log-rank test. All data were analysed with the use of SPSS version 11 Citation[26].
All analyses were done on the basis of intention-to-treat principle. The authors had full access to the data and take responsibility for its integrity. All authors have read and agreed to the manuscript as written.
Results
Baseline and procedural characteristics
During the study period, 840 eligible patients (368 STEMI and 472 NSTEMI patients) were screened for participation in the trial. A total of 425 patients (51%) fulfilled the inclusion criteria and were randomized to the two treatment groups (214 to the TITANOX group and 211 to the PES group). Baseline characteristics of both study groups were well matched, except by a higher incidence of previous percutaneous coronary interventions (PCIs) in the TITANOX group (). The baseline angiographic variables and procedural characteristics are presented in . Procedural success was achieved in 99.5% of patients in the TITANOX group and in 98.1% in the PES group.
Table I. Baseline clinical characteristics in TITANOX group versus PES group.
Table II. Baseline angiographic variables and procedural characteristics.
Clinical outcome
Complete clinical follow-up data at 2 years was obtained for all patients 95% (HR 2.5, 95% CI). The cumulative incidence of MACE was 11.2% in the TITANOX group and 21.8% in the PES group (hazard ratio (HR) 2.2, 95% confidence interval (CI) 1.3–3.8, P = 0.004) (). This difference in MACE was driven by a reduced rate of MI (5.1% versus 15.6%, P < 0.001) and cardiac death (0.9% versus 4.7%, P = 0.02) in favour of the TITANOX stent. Definite ST occurred in 0.5% of patients in the TITANOX group and in 6.2% of patients in the PES group (P = 0.001). When using ARC classification, we observed a significantly lower rate of ST in the TITANOX group (0.9% versus 8.5%, respectively, P < 0.001). In 8 patients, clopidogrel was prematurely discontinued before the event of ST, and all of these patients were in the PES group (). Four patients out of 14 (29%) who suffered ST died.
Figure 1. Antiplatelet utilization at the time of the outcome event of definite stent thrombosis (ST). A: Antiplatelet therapy in 14 patients suffering definite ST. B: Time of the event and the utilization of clopidogrel during the definite ST. aDual antiplatelet therapy with aspirin and clopidogrel. Dark colour indicates the duration of clopidogrel withdrawal.
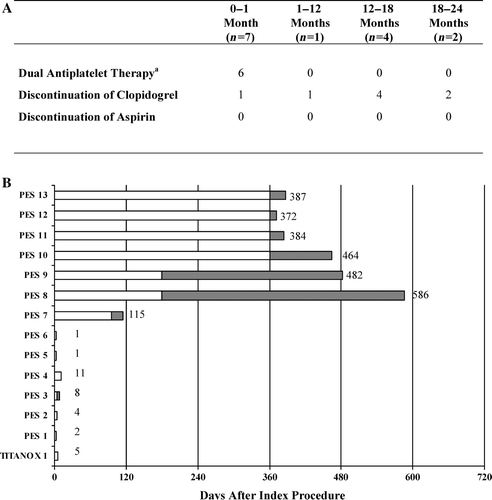
Table III. Clinical events during 2 years of follow-up.
A total of 245 patients presented with NSTEMI before the index procedure (131 patients in the TITANOX group versus 114 in the PES group). The rate of MACE (16.7% in NSTEMI patients versus 16.1% in STEMI patients, P = 0.9), MI, cardiac death, and TLR were similar in these two subgroups of patients during the 2 years of follow-up. Furthermore, the rate of ST was comparable in these two subgroups of patients (2.0% versus 5.0%, P = 0.1, respectively).
Clopidogrel was prescribed at discharge for a mean period of 7.6 months in the TITANOX group and of 10.0 months in the PES group (P < 0.001). A total of 67 patients (31%) in the TITANOX group and 138 patients (65%) in the PES group were receiving dual antiplatelet therapy with aspirin and clopidogrel at the time of the 12-month follow-up (P < 0.001). In the present trial, there were no extended clopidogrel treatments beyond 12 months in either study groups.
In multivariable analysis, the use of PES was an independent predictor of recurrent MI (HR 4.0, 95% CI 1.9–8.4, P < 0.001), as well as of cardiac death (HR 5.9, 95% CI 1.3–28.0, P = 0.02), definite ST (HR 14.5, 95% CI 1.9–114.4, P = 0.01), and MACE (HR 2.5, 95% CI 1.4–4.4, P = 0.002) after 2 years of follow-up ().
Table IV. Independent predictors of clinical outcomes.
Discussion
Main study findings
To our knowledge, the TITAX-AMI trial is the first head-to-head randomized comparison of the TITANOX stent and PES among patients presenting with acute MI. The main finding of this trial is a significantly higher rate of MI, cardiac death, and overall MACE in patients who received PES. Secondly, the overall rate of ST was significantly higher in PES-treated patients. In multivariable analyses, MI, cardiac death, definite ST, and MACE were predicted by the use of PES.
Previous studies
The STRATEGY, TYPHOON, and SESAMI trials compared sirolimus-eluting stent (SES) to BMS in the clinical setting of acute STEMI. The 1-year follow-up of these trials indicated a significant benefit of SES over BMS Citation[6–8]. However, longer-term follow-up data from the TYPHOON and SESAMI trials are lacking. In the STRATEGY trial, the cumulative incidence of MI, death, and target vessel revascularization at 2 years was 24.2% in patients who received SES Citation[27], which is comparable to our findings when using PES in acute MI. As for the use of PES, the PASSION trial showed no significant superiority of PES compared to BMS after 2 years of follow-up, although there was a trend towards a lower rate of TLR in the PES group Citation[28]. In the present trial, the cumulative incidence of primary end-points in the PES group was 21.8%, which is in contrast to the results from the PASSION trial (11.2%).
There are various salient differences between the TITAX-AMI trial and previous MI trials (TYPHOON/PASSION). The present trial included patients with NSTEMI and STEMI, whereas previous studies included only patients with STEMI. Therefore it may be difficult to compare the present study with the STEMI studies. However, recent reports suggest that the prognosis of NSTEMI and STEMI are similar despite different management strategies Citation[14], Citation[15].
The BASKET-LATE trial reported a trend towards a higher rate of death or recurrent MI in patients treated with DES after thienopyridine discontinuation as compared with BMS Citation[29]. This observation is in line with the findings of the present trial, since after the clopidogrel withdrawal we observed a significantly higher incidence of thrombotic events in the PES group compared to the TITANOX group (i.e. MI, definite ST, and MACE).
Stent thrombosis
In the present study, the incidence of definite ST was 6.2% in the PES group, which is higher than in the PASSION trial (2.1%) Citation[28]. On the other hand, after the 2 years of follow-up, the incidence of definite ST in the TITANOX group was low (0.5%), especially considering the thrombotic environment at the time of the stent deployment and the inclusion of patients with thrombolysis therapy in the present trial. Recently, a rate of infarct-related artery ST (3.2%) in STEMI patients at 2 years was reported Citation[30]. This analysis also demonstrated a remarkably high incidence of ST (8.2%) in patients with a large thrombus burden before the stent implantation.
A higher rate of late ST was observed in MI patients than in stable patients in post-mortem analysis of patients who died after DES implantation Citation[20]. This analysis suggests that the culprit lesion morphology influences local vascular healing after DES placement. Greater delay in arterial healing as manifested by poor endothelialization and persistent peristrut fibrin deposition may extend the risk of ST far beyond 30 days after DES implantation. Late ST is potentially due to a mismatch between the stent and the vessel and may be related to stent malapposition, overlapping stent placement, penetration of necrotic core, excessive stent length, bifurcation lesions, hypersensitivity to drug or polymer, or thrombogenic surface Citation[19], Citation[31]. Premature antiplatelet therapy discontinuation has been the most important predisposing factor for late ST Citation[29], which was also seen in the present trial. Previously, late ST was reported to occur at an annual rate of ~0.6% up to 4 years after DES implantation Citation[32], Citation[33], while acute coronary syndrome and PES implantation were associated with late ST Citation[32].
New strategies with BMS technology have also been aimed at enhanced vascular healing. Titanium features better biocompatibility when compared with stainless steel, gold, or other surface coatings Citation[34]. In vitro titanium-nitride-oxide showed diminished platelet adhesion and fibrinogen binding in comparison to stainless steel. In addition, metallic sheaths coated with titanium-nitride or titanium-oxide exhibited higher cell density values on their surface compared to those without coating, supporting the view that deployment of stents with these coatings may achieve earlier complete endothelial coverage Citation[35]. These findings reinforce our present report demonstrating a lower incidence of MI and late ST in TITANOX stent-treated patients compared with PES-treated patients.
Study limitations
The sample size was based on a small real-life cohort, and therefore the present trial is underpowered to reveal potential small differences in primary and individual end-points, although we chose the setting of acute MI known to predispose to clinical complications. The design of our study did not include angiographic follow-up or routine non-invasive testing for myocardial ischaemia, and therefore we probably underestimated the incidence of silent or angiographic restenosis. On the other hand, by relying on clinical follow-up only, the chance of unnecessary TLR due to the ‘oculostenotic reflex’ or patient's unjustified anxiety was avoided. In addition, the stenting was performed in patients with relatively large infarct-related arteries with low risk of in-stent restenosis.
Conclusions
In conclusion, the implantation of TITANOX stents resulted in better clinical outcome compared with PES during 2 years of follow-up among patients treated for acute MI.
Acknowledgements
The authors thank Tuija Vasankari, RN, Eija Niemelä, RN, and Marja-Liisa Sutinen, RN, for their support in the conduct of this study. Funding sources: This work (Dr Airaksinen) was supported by grants from the Finnish Foundation for Cardiovascular Research, Helsinki, Finland. Clinical trial registration information: URL: http://www.clinicaltrials.gov. Unique identifier: NCT00495664. Declaration of interest: All authors state that no conflict of interest exists.
References
- Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O'Shaughnessy C, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003; 349: 1315–23
- Stone GW, Ellis SG, Cox DA, Hermiller J, O'Shaughnessy C, Mann JT, et al. One-year clinical results with the slow-release, polymer-based, paclitaxel-eluting TAXUS stent: the TAXUS-IV trial. Circulation. 2004; 109: 1942–7
- Silber S, Albertson P, Aviles FF, Camici PG, Colombo A, Hamm C, et al. Guidelines for percutaneous coronary interventions. The task force for percutaneous coronary interventions of the European society of cardiology. Eur Heart J. 2005; 26: 804–47
- Mehta SR, Cannon CP, Fox KA, Wallentin L, Boden WE, Spacek R, et al. Routine vs selective invasive strategies in patients with acute coronary syndromes: a collaborative meta-analysis of randomized trials. JAMA. 2005; 293: 2908–17
- Boersma, E ; The Primary Coronary Angioplasty vs. Thrombolysis (PCAT)-2 Trialist’ Collaborative Group. Does time matter? A pooled analysis of randomized clinical trials comparing primary percutaneous coronary intervention and in-hospital fibrinolysis in acute myocardial infarction patients. Eur Heart J. 2006; 27:779–88
- Valgimigli M, Percoco G, Malagutti P, Campo G, Ferrari F, Barbieri D, et al. Tirofiban and sirolimus-eluting stents vs abciximab and bare-metal stent for acute myocardial infarction. JAMA. 2005; 293: 2109–17
- Spaulding C, Henry P, Teiger E, Beatt K, Bramucci E, Carrie D, et al. Sirolimus-eluting versus uncoated stents in acute myocardial infarction. N Engl J Med. 2006; 355: 1093–104
- Menichelli M, Parma A, Pucci E, Fiorilli R, De Felice F, Nazzaro M, et al. Randomized trial of sirolimus-eluting stent versus bare-metal stent in acute myocardial infarction (SESAMI). J Am Coll Cardiol. 2007; 49: 1924–30
- Laarman GJ, Suttorp MJ, Dirksen MT, van Heerebeek L, Kiemeneij F, Slagboom T, et al. Paclitaxel-eluting versus uncoated stents in primary percutaneous coronary intervention. N Engl J Med. 2006; 355: 1105–13
- Tanimoto S, Tsuchida K, Daemen J, Boersma E. Drug-eluting stent implantation in acute myocardial infarction. Do we need another randomised trial?. EuroIntervention. 2006; 2: 23–7
- Pasceri V, Patti G, Speciale G, Pristipino C, Richichi G, Di Sciascio G. Meta-analysis of clinical trials on use of drug-eluting stents for treatment of acute myocardial infarction. Am Heart J. 2007; 153: 749–54
- Moreno R, Spaulding C, Laarman GJ, Tierala I, Kaiser CA, Lopez-Sendon J-L. Effectiveness and safety of paclitaxel-eluting stents in patients with ST-segment elevation acute myocardial infarction. EuroIntervention. 2007; 3: 386–91
- Kastrati A, Dibra A, Spaulding C, Laarman GJ, Menichelli M, Valgimigli M, et al. Meta-analysis of randomized trials on drug-eluting stents vs. bare-metal stents in patients with acute myocardial infarction. Eur Heart J. 2007; 28: 2706–13
- Terkelsen CJ, Lassen JF, Norgaard BL, Gerdes JC, Jensen T, Gotzsche L, et al. Mortality rates in patients with ST-elevation vs. non-ST-elevation acute myocardial infarction: observations from an unselected cohort. Eur Heart J. 2005; 26: 18–26
- Montalescot G, Dallongeville J, van Belle E, Rouanet S, Baulac C, Degrandsart A, et al. STEMI and NSTEMI: are they so different? 1 year outcomes in acute myocardial infarction as defined by the ESC/ACC definition (the OPERA registry). Eur Heart J. 2007; 28: 1409–17
- Karjalainen P, Ylitalo A, Niemelä M, Kervinen K, Mäkikallio T, Pietilä M, et al. Titanium-nitride-oxide coated stents versus paclitaxel-eluting stents in acute myocardial infarction: A 12-month follow-up report from the TITAX AMI trial. EuroIntervention. 2008; 4: 232–41
- Stone GW, Moses JW, Ellis SG, Schofer J, Dawkins KD, Morice M-C, et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med. 2007; 356: 998–1008
- Mauri L, Hsieh W, Massaro JM, Ho K, D'Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med. 2007; 356: 1020–9
- Finn AV, Nakazawa G, Joner M, Kolodgie FD, Mont EK, Gold HK, et al. Vascular responses to drug eluting stents. Importance of delayed healing. Arterioscler Thromb Vasc Biol. 2007; 27: 1500–10
- Nakazawa G, Finn AV, Joner M, Ladich E, Kutys R, Mont EK, et al. Delayed arterial healing and increased late stent thrombosis at culprit sites after drug-eluting stent placement for acute myocardial infarction patients. Circulation. 2008; 118: 1138–45
- Windecker S, Simon R, Lins M, Klauss V, Eberli FR, Roffi M, et al. Randomized comparison of a titanium-nitride oxide-coated stent with a stainless steel stent for coronary revascularization. The TINOX trial. Circulation. 2005; 111: 2617–22
- Karjalainen PP, Ylitalo A, Airaksinen KEJ. Titanium and nitride oxide coated stents and paclitaxel eluting stents for coronary revascularization in an unselected population. J Invasive Cardiol. 2006; 18: 462–8
- Karjalainen P, Ylitalo A, Airaksinen KEJ. Real world experience with TITAN® stent: A 9-month follow-up report from the Titan PORI Registry. EuroIntervention. 2006; 2: 187–91
- Mosseri M, Miller H, Tamari I, Plich M, Hasin Y, Brizines M, et al. The titanium-NO stent: results of a multicenter registry. EuroIntervention. 2006; 2: 192–6
- Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es G-A, et al. Clinical end points in coronary stent trials. A case for standardized definitions. Circulation. 2007; 115: 2344–51
- Buehl A, Zoefel P. SPSS 11: Introduction in modern data analysis8th ed. Addison-Wesley, MunichGermany 2002
- Valgimigli M, Campo G, Arcozzi C, Malagutti P, Carletti R, Ferrari F, et al. Two-years clinical follow-up after sirolimus-eluting versus bare-metal stent implantation assisted by systematic glycoprotein IIb/IIIa inhibitor infusion in patients with myocardial infarction. Results from the STRATEGY study. J Am Coll Cardiol. 2007; 50: 138–45
- Dirksen MT, Vink MA, Suttorp MJ, Tijssen JGP, Patterson MS, Slagboom T, et al. Two year follow-up after primary PCI with a paclitaxel-eluting stent versus a bare-metal stent for acute ST-elevation myocardial infarction (the PASSION trial): a follow-up study. EuroIntervention. 2008; 4: 64–70
- Pfisterer M, Brunner-La Rocca HP, Buser PT, Rickenbacher P, Hunziker P, Mueller C, et al. Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents. An observational study of drug-eluting versus bare-metal stents (BASKET-LATE). J Am Coll Cardiol. 2006; 48: 2584–91
- Sianos G, Papafaklis MI, Daemen J, Vaina S, van Mieghem CA, van Domburg RT, et al. Angiographic stent thrombosis after routine use of drug-eluting stents in ST-elevation myocardial infarction. J Am Coll Cardiol. 2007; 50: 573–83
- Virmani R, Guagliumi G, Farb A, Musumeci G, Grieco N, Motta T, et al. Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent: should we be cautious?. Circulation. 2004; 109: 701–5
- Wenaweser P, Daemen J, Zwahlen M, van Domburg R, Juni P, Vaina S, et al. Incidence and correlates of drug-eluting stent thrombosis in routine clinical practice. J Am Coll Cardiol. 2008; 52: 1134–40
- Daemen J, Wenaweser P, Tsuchida K, Abrecht L, Vaina S, Morger C, et al. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet. 2007; 369: 667–78
- Giraud-Sauveur Y. Titan 2 bio-active stent (BAS) with titanium-NO. EuroIntervention. 2007; 3: 526–8
- Yeh HI, Lu SK, Tian TY, Hong RC, Lee WH, Tsai CH. Comparison of endothelial cells grown on different stent materials. J Biomed Mater Res. 2006; 76: 835–41