Abstract
Background. Surfactant protein (SP) C has been shown to be expressed also outside pulmonary alveoli. Certain SP-C gene (SFTPC) polymorphisms associate with lung diseases and very preterm birth.
Aims. We investigated the association of SFTPC single nucleotide polymorphism (SNP) rs4715 with factors affecting spontaneous preterm birth and characterized the SP-C expression in human and mouse gestational tissues.
Methods. The SFTPC SNP rs4715 polymorphism was genotyped in a homogeneous northern European population of mothers and infants in spontaneous preterm birth and term controls. The expression and protein of SP-C in gestational tissues was analyzed.
Results. SFTPC SNP rs4715 did not associate with spontaneous preterm birth. However, fetuses with short interval (<72 hours) between preterm premature rupture of fetal membranes (PPROM) and preterm birth had significant over-representation of the minor allele A, whereas in fetuses with prolonged PPROM (≥72 hours) the frequency was decreased. Maternal SFTPC did not associate with the duration of PPROM. SP-C mRNA and proprotein were detected in fetal membranes, placenta, and pregnant uterus.
Conclusion. SFTPC SNP rs4715 associates with the duration of PPROM, and SP-C is expressed in gestational tissues. We propose that fetal SFTPC moderates the inflammatory activation within the fetal extra-embryonic compartment.
Introduction
Pulmonary surfactant is a phospholipid-rich complex that is secreted from alveolar cells into alveoli and small airways. Some 5%–10% of the surfactant components are proteins, including surfactant proteins A, B, C, and D. While surfactant reduces the alveolar surface tension, thereby preventing collapse of alveoli and small airways at low lung volume, the deficiency of surfactant causes respiratory distress syndrome (RDS) in preterm infants Citation[1]. Surfactant proteins were originally found in the lung; however, at present, the extrapulmonary expression of these proteins is evident Citation[2], Citation[3]. In addition, surfactant proteins have important roles in innate immunity Citation[4], Citation[5].
Key messages
Surfactant protein C (SP-C) has functions in host defense even before birth. Fetal SFTPC associates with the duration of PPROM whereas maternal SFTPC alleles or genotypes showed no association.
Besides the lung, SP-C is expressed in placenta, fetal membranes, and pregnant uterus, and proforms of SP-C protein are observed in these tissues.
Surfactant protein C (SP-C) is a highly hydrophobic proteolipid that promotes the surface adsorption of phospholipids and the stability of surfactant lipid film Citation[6], Citation[7]. The synthesis, intracellular processing, and secretion of SP-C have been considered to be unique in type 2 alveolar cells. Recently, evidence of the expression of SFTPC and the synthesis of the SP-C proprotein (proSP-C) was described in ocular tissue Citation[8], skin Citation[9], and kidney Citation[10]. In type 2 alveolar cells SP-C is produced as 21-kDa proSP-C from which mature protein is generated via multiple proteolytic cleavages as proSP-C is trafficked through the regulated secretory pathway. Covalent attachment of two palmitoyl chains to proSP-C takes place in Golgi apparatus. The first and second cleavages take place in multivesicular bodies and include the removal of C-flanking domain leading to 16- and 7-kDa proSP-C intermediates. The first N-terminal cleavage, producing a 6-kDa intermediate, occurs before packaging into the lamellar bodies, and the 3.7-kDa mature SP-C is generated in lamellar bodies (for review, see Citation[11], Citation[12]).
Mutations causing SP-C deficiency do not commonly associate with failure of respiratory adaptation at birth. Instead, affected humans and animals develop severe abnormalities in lung function and pathology consistent with interstitial lung disease with several categories and variable penetrance Citation[13–19]. In addition to the well characterized functions of SP-A and SP-D in innate defense, also SP-C has been considered to possess an immunomodulatory role Citation[12], Citation[20]. SP-C has been shown to directly bind lipopolysaccharide (LPS), a bacterial endotoxin from Gram-negative bacteria Citation[21], and to interact with CD14, having a role in the recognition of LPS Citation[22]. SFTPC SNP rs4715 (C/A, Thr138Asn) has been shown to associate with the susceptibility to RDS in premature infants, and, according to post hoc analysis, the same single nucleotide polymorphism (SNP) associates with very preterm birth in humans Citation[23].
Abbreviations
Spontaneous preterm labor progressing to preterm birth is a common and serious problem associated with major life-threatening, chronic morbidity in the new-born Citation[24], Citation[25]. In 40%–50% of cases of spontaneous preterm births, the presenting symptom is PPROM. Within one hour to several weeks after PPROM, regular uterine contractions appear as a sign of labor Citation[25], Citation[26]. The activation of spontaneous preterm labor is multifactorial. An important pathway leading to labor is inflammatory decidual activation that in part takes place by the fetal-decidual paracrine system. Bacterial endotoxins and excessive inflammatory cytokine response in placental and fetal tissues have been proposed roles as inducing mediators that are responsible for regular uterine contractions and cervical changes taking place in preterm labor and delivery Citation[27], Citation[28]. Currently, SP-A secreted from the fetal lung to the amniotic fluid has been suggested to be involved in the regulation of the onset of labor Citation[29].
The aim of the present project was to investigate the possible involvement of SP-C in the process of preterm delivery and premature birth. According to epidemiological evidence, both the mother and the fetus influence the susceptibility to preterm birth in humans Citation[30]. Therefore both maternal and fetal SFTPC genotypes were used for a candidate gene study. Our previous observation on the association between SFTPC SNP rs4715 alleles and very preterm birth Citation[23] was extended by submitting a homogeneous northern European population of mothers and infants for a detailed study of spontaneous preterm labor and preterm birth. In addition, we characterized the expression of SP-C in mouse and human gestational tissues and investigated the LPS responsiveness in fetal and maternal compartments by using our previously established mouse model of LPS-induced preterm birth of live-born pups Citation[31].
Materials and methods
Study population and collection of human DNA specimens
This study was approved by the Ethics Committee of Oulu University Hospital, and written informed consent was obtained from the mothers. The population of mothers with spontaneous preterm birth (gestational age < 36 wk) was selected retrospectively from birth diaries of Oulu University Hospital between the years 1973 and 2003, and prospectively from 2003 to 2005. The length of the pregnancy was determined by ultrasound examination at 16–18 weeks of pregnancy. Since the measurement is not quite accurate, the preterm births at 36 weeks were excluded. Only singleton pregnancies and families of Finnish origin were studied. Mothers with one or more than one spontaneous preterm birth were studied. However, only one of the at least two mothers who were first-, second-, or third-degree relatives was included. Pregnancies with known risk factors of preterm birth (pre-existing chronic disease of the mother, narcotic or heavy alcohol abuse, pre-eclampsia, polyhydramnios, accidents, septic infection of the mother, uterine malformation, or fetuses with severe disease or malformation syndrome) were excluded.
For the primary analysis a single preterm infant from each family was included. In the families with more than one preterm delivery, the infant was prospectively selected according to the following low-risk criteria: 1) the age of the mother between 20 and 35 years at the time of the birth of the preterm infant, 2) preference of a girl over a boy, and 3) no deliveries within the preceding 2 years or the longest time interval from the previous delivery. The characteristics of the study population are presented in . The control population consists of mothers and their infants (202 mothers, 199 infants). Controls were prospectively recruited from Oulu University Hospital in the years 2004–2005 among mothers with at least three exclusively term deliveries without any pregnancy or labor-associated complications.
Table I. Characteristics of the selected population involved in spontaneous preterm birth.
The pregnancies with PPROM were defined as preterm births with leakage of amniotic fluid as the presenting symptom before the onset of contractions. When indicated, the amniotic origin of the fluid was ascertained using the nitrazine or insulin-like growth factor binding protein-1 test. Prolonged PPROM was defined as duration of PPROM equaling or exceeding 72 h, as originally described Citation[32]. For the post hoc study on the duration of PPROM, only mother–fetus pairs with complete sets of SFTPC genotypes were included (137 pairs). Altogether 127 pairs with PPROM were identified from the primary population. In order to increase the power of the study, 10 additional pairs were selected from families with two or more spontaneous preterm births. In these pairs, the analyzed PPROM fetus was a sibling of the low-risk preterm infant included in the primary analysis. None of these 137 mothers had more than one pregnancy with PPROM.
SNP selection criteria, DNA sample preparation, and genotyping
The two common non-synonymous SFTPC polymorphisms rs4715 (C/A Thr138Asn) and rs1124 (G/A Ser186Asn) are in strong linkage disequilibrium, resulting in a nearly 100% combined frequency of the SFTPC haplotypes 138Asn-186Asn and 138Thr-186Ser Citation[23]. Therefore, SFTPC rs4715 was genotyped as a candidate SNP to study the relevance of SFTPC in the clinical setting.
Puregene DNA Isolation Kit (Gentra Systems, Minneapolis, MN) and UltraClean DNA Blood Isolation Kit (MO BIO Laboratories, Inc., Carlsbad, CA) were used to extract genomic DNA from whole-blood specimens (n=604). Chelex 100 (Bio-Rad) was used to extract genomic DNA from buccal cells (n=405).
AcycloPrime II SNP Detection Kit (Perkin Elmer Life Sciences, Boston, USA) was used for genotyping of the SNP rs4715 indicated in the dbSNP database (http://ncbi.nih.gov/SNP/) by template-directed dye terminator incorporation with fluorescence polarization detection Citation[33]. Polymerase chain reaction (PCR) primers forward 5′-GCT GCT GAT CGC CTA CAA G-3′ and reverse 5′-AGG GAG ACA GCC CAC TCT TT-3′ and SNP primer 5′-ATC CCC AGT CTT GAG GCT CTC A-3′ were used (GenBank accession no. NT_023666). For the samples with inadequate DNA concentration, 1 µL of the PCR product was reamplified using nested primers 5′-CTG CTG ATC GCC TAC AAG C-3 (forward) and 5′-GGG AGA CAG CCC ACT CTT TT-3′ (reverse) before the genotyping reaction.
Collection of mouse samples
Animal studies were approved by the Finnish Animal Ethics Committee. Timed-pregnant C57BL/6 mice were used. Gestational age (±12 hours) was determined by the presence of vaginal plug and designated as day 0 of pregnancy. Mouse tissues were collected as previously described Citation[31]. Briefly, the mice were intraperitoneally injected at 17 days post coitum (dpc) with either phosphate-buffered saline (PBS) or 25 µg/mouse of Escherichia coli LPS (serotype 0111:B4, Sigma, St. Louis, MO) dissolved in PBS. The dams were anesthetized 3 or 8 hours after the PBS or LPS injections and killed with cervical dislocation. In each group, 5–10 dams or their litters were examined, and a total of 2–4 pups from every litter were analyzed individually. Fetal lung, fetal membranes (amnion and yolk sac), placenta, uterus (myometrium and endometrium), and maternal lung were harvested. In addition, uteri were collected from nulliparous mice, aged 8–9 weeks. The tissues were frozen in liquid nitrogen or fixed in 4% formaldehyde.
Collection of human tissues for mRNA and protein analysis
The study was approved by the Ethics Committee of Oulu University Hospital, and permission was obtained from the donors. Human placenta, amnion, and chorion were obtained from normal term delivery. A sample of adult lung was obtained during surgical procedure. The tissues were frozen in liquid nitrogen for further analysis.
Preparation of RNA
Total RNA from the harvested tissues was isolated using Tri-reagent (Sigma) according to the manufacturer's instructions. For RNase protection assay (RPA) and Northern blot the RNA was vacuum-dried and dissolved in RNA sample buffer. For reverse transcriptase polymerase chain reaction (RT-PCR) the extracted RNA was further purified using RNeasy Micro Kit (Qiagen, Hamburg, Germany) according to the manufacturer's protocol.
RNase protection assay
The mouse custom-designed multi-probe RPA system (BD Biosciences, San Diego, CA) was used to quantitatively determine the SP-C mRNA and housekeeping gene L32 mRNA levels in mouse tissues (fetal and maternal lung, uterus, placenta, and fetal membranes) according to the manufacturer's protocol. Briefly, a radio-labeled antisense RNA probe was hybridized overnight with 10 µg of total RNA. Single-stranded RNA and free probe were digested with RNases, and the protected RNA fragments were resolved on denaturing polyacrylamide gel and quantified by phosphor imaging (Quantity one, version 4.6.0, Bio-Rad, Hercules, CA). The expression levels of SP-C were normalized to the expression levels of L32.
Reverse transcriptase PCR
Two micrograms of purified RNA were transcribed to cDNA using Moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI) according to the manufacturer's instructions. The RNA used in reverse transcriptase polymerase chain reaction (RT-PCR) was purified from several mouse (fetal and maternal lung, uterus, placenta, and fetal membranes) and human tissues (lung, placenta, amnion, and chorion).
RT-PCR was performed using primers specific for mouse or human SP-C mRNA. For mouse Sftpc the forward primer was 5′-TGC ACC TCA AAC GCC TTC TC-3′ and the reverse primer 5′-TTC ATG ATG TAG CAG TAG GTT C-3′, leading to a 280-bp PCR product (GenBank accession no. NM_011359). For human samples the forward primer was 5′-CTG CAG CAA GAT GGA TGT GG-3′ and reverse 5′-GTC CTA GAT GTA GAG CG-3′, giving rise to a 570-bp fragment (GenBank accession no. NM_003018). The primer pairs were located in different exons to exclude the amplification of similar-sized genomic DNA. The PCR cycles used were 15, 20, and 25 for mouse and 30 for human cDNAs. Results of 25 cycles for RNA isolated from mouse tissues and 30 cycles for RNA isolated from human tissues are shown. The PCR products were separated by electrophoresis in agarose gel and visualized or used for cloning.
Three different specific primer pairs were used in the RT-PCR reactions. The size of SP-C PCR products from reproductive tissues was compared to corresponding PCR products from lung mRNA. The PCR products were further verified by sequencing and by comparing them to mouse SP-C sequence (GenBank accession no. NM_011359).
Northern blot
The cDNA fragments obtained by RT-PCR from mouse and human lungs were purified, ligated to pGEM-T Easy vector (Promega), and sequenced. The cloned cDNAs were used as probes in Northern blot analysis. Different amounts of total RNA from several tissues were used (mouse lung 10 µg, other mouse tissues 30 µg, human lung 5 µg, other human tissues 15 µg). RNAs were separated in agarose-formaldehyde gels and blotted onto nylon membranes (Millipore Corporation, MA). For hybridization, mouse or human SP-C cDNA probes were radioactively labeled with Rediprime II Random Prime Labelling System kit according to the manufacturer's instructions (GE Healthcare, Little Chalfont, UK).
Western blot
Protein homogenates from tissue samples were prepared for the analysis of proSP-C. Briefly, the tissue samples were lysed in homogenization buffer (10 mM Tris-HCl, pH 7.5, 0.25 M sucrose, 1 mM EDTA, 5 mM benzamidine, 2 mM phenylmethanesulphonyl fluoride, 10 µg/mL pepstatin A, 10 µg/mL aprotinin, 10 µg/mL antipain A, 10 µg/mL leupeptin, 10 µg/mL chymostatin). After centrifugation, the protein content of the supernatant was quantified by Bio-Rad DC Protein Assay. For Western analysis, different amounts of protein from several mouse tissues were used (maternal lung 15 µg, fetal lung 20 µg, uterus, fetal membranes, and placenta 50 µg). Proteins were separated in 12% Bis-Tris gel (NuPAGE Novex, Invitrogen, Carlsbad, CA) and electrotransferred onto a Protran BA85 (Schleicher & Schuell, Dassel, Germany) nitrocellulose filter. After overnight blocking, antibody incubations and detections were performed. Anti-human proSP-C antibody (FL-197, Santa Cruz Biotechnology, Santa Cruz, CA) was used as primary antibody and visualized using an ECL-Plus Detection kit (GeHealthcare). The Western blot analysis was repeated using WRAB-SP-C antibody (Seven Hills Bioreagents, Cincinnati, OH).
Immunohistochemistry
Tissue sections from wild-type C57BL/6 and Sftpc −/− mice Citation[13] embedded in paraffin were cut into 5 µm sections. Deparaffinized sections were heated in Tris-EDTA buffer (pH 9) and incubated in Peroxidase-Blocking Solution (Dako, Glostrup, Denmark). After the inhibition of endogenous peroxidase, the sections were blocked with donkey serum in PBS-Tween. The sections were then incubated with anti-human proSP-C antibody (WRAB-SP-C). Biotinylated secondary antibodies (Dako) were detected with AB complex (Dako) and stained with DAB substrate (Zymed Laboratories, San Francisco, CA). The sections were counterstained with hematoxylin. Immunohistochemistry was repeated using FL-197 proSP-C antibody.
Statistical analysis
The statistical tests for association of the SFTPC SNP rs4715 with preterm birth in mothers and infants, including comparisons of allele and genotype frequencies by 2×2 and 2×3 contingency tables, respectively, and calculation of Hardy-Weinberg equilibrium, were performed using SPSS (SPSS Inc. version 14.0.2) and Arcus QuickStat software. Bonferroni correction was applied to control for multiple testing by multiplying the original P-value by the number of tests performed. The duration of PPROM was analyzed as a continuous variable, and the differences were calculated using the non-parametric Kruskal-Wallis H test or the Mann-Whitney U test. Power calculations were performed for a case-control study using the online Genetic Power Calculator at http://pngu.mgh.harvard.edu/~purcell/gpcCitation[34]. The disease prevalence was set as 3.0% for spontaneous preterm birth and as 0.7% for short duration of PPROM. The polymorphism was assumed to be causal, allele frequency 0.3, and type I error rate 0.05. The power of our study is estimated to be 80% to detect an allelic association with a small to moderate genetic effect on the risk of spontaneous preterm birth (genotypic relative risk of 1.18 for heterozygotes and 2.36 for homozygotes under an additive risk model). Considering the low number of individuals with spontaneous preterm birth and short duration of PPROM and estimation of the power of 80%, moderate to large genotypic relative risks could be detected (1.66 for the heterozygous and 3.32 for the high-risk homozygous genotypes).
The mRNA values quantified by RPA were analyzed using Origin 7.0 and SPSS (SPSS Inc., version 14.0.2). The data were tested for normal distribution, and statistical significances of the differences between the controls and the LPS groups were analyzed using either Student's t test or Mann-Whitney U test.
Results
SFTPC rs4715 polymorphism does not associate with spontaneous preterm birth
Previously, an association between SFTPC and very preterm birth has been reported Citation[23]. In the present study, we analyzed whether a similar association of the SFTPC SNP rs4715 (C/A Thr138Asn) with preterm birth after spontaneous onset of labor would be detectable (). Contrary to the previous results, SFTPC SNP rs4715 alleles and genotypes were not associated with either preterm birth of the fetus or spontaneous preterm delivery by the mother (). Additional stratification by gestational age, smoking status of the mother, number of spontaneous preterm births in family, or gender did not reveal any differences in the distribution of the alleles and genotypes (data not shown). The genotypes of both the mothers and the infants involved in either term or preterm births were in Hardy-Weinberg equilibrium.
Table II. Allele and genotype frequencies of the SFTPC SNP rs4715 in preterm (gestational age <36 wk) and term (gestational age ≥ 37 wk) infants, in mothers with preterm deliveries and in mothers with exclusively term deliveries.
SP-C is expressed in mouse gestational tissues
Despite the lack of an association of SFTPC rs4715 polymorphism with spontaneous preterm birth, our analysis of the mouse model of LPS-induced preterm birth Citation[31] had suggested a role for surfactant proteins in the regulation of pregnancy. Therefore, the expression of SP-C in gestational tissues was further characterized. Besides the strong expression in both maternal and fetal lung, weak but clearly detectable mRNA for SP-C was observed in fetal membranes, placenta, and uterus. SP-C expression was determined quantitatively in several tissues of control and LPS-treated mice by RPA (). In fetal lung LPS challenge caused a minor but statistically significant decrease at 8 hours. No significant changes in the mRNA level of SP-C were detected in other tissues studied (A–E). SP-C mRNA was not evident by RPA in the uterus of 8–9-week-old nulliparous females.
Figure 1. SP-C expression in mouse gestational tissues is not affected by LPS. A–E: The changes in the mRNA levels of SP-C in mouse tissues as a response to maternal LPS were analyzed by RPA in maternal lung (A), fetal lung (B), uterus (C), fetal membranes (D), and placenta (E) and normalized to the expression level of the housekeeping gene L32. The mRNAs levels are shown as fold increases compared to PBS controls. Representative gel electrophoresis of SP-C and L32 is shown for each tissue. Tissues were collected at 17 dpc 3 or 8 hours after PBS or LPS injection for RNA isolation. Analyses of 3–7 dams or their litters are shown as means±SEM. F: RT-PCR (25 cycles) was performed to confirm the SP-C expression in mouse gestational tissues harvested from PBS-treated animals. LPS: lipopolysaccharide, RPA: ribonuclease protection assay, RT-PCR: reverse transcriptase polymerase chain reaction, SP-C: surfactant protein C, PBS: phosphate-buffered saline.
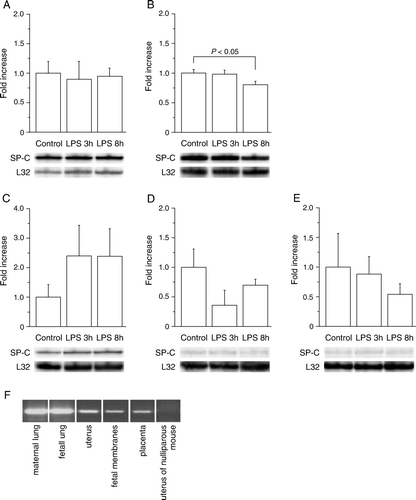
RT-PCR was used to confirm the observed expression of SP-C in gestational tissues (F). SP-C expression was detected in maternal and fetal lung after 15 PCR cycles. The PCR product was obtained after 20 cycles for uterine mRNA and after 25 cycles for placental and fetal membrane mRNAs (data not shown). The uterus of nulliparous mouse was used as negative control in RT-PCR.
The processing of SP-C transcript is similar in lung and gestational tissues
The size of SP-C mRNA in extrapulmonary tissues was studied using Northern blot analysis. As the expression in other mouse tissues was low compared to that seen in the lung, the amount of RNA from placenta, fetal membranes, and uterus was 3-fold compared to the RNA from lung. Even so, by Northern blot the expression was detected only in uterus and lung, and the mRNA level in placenta and fetal membranes was below the detection limit (A). The size of SP-C mRNA for lung and uterus was similar.
Figure 2. SP-C transcript is similar in mouse lung and gestational tissues. A: For Northern blot the mouse tissues were harvested from PBS-treated mice at 17 dpc. The amount of total RNA used was 10 µg for mouse lung and 30 µg for other mouse tissues. B: In schematic representation of mouse SP-C cDNA the exons are indicated as numbered boxes, and start and stop codons are shown. The locations of primers (F1, F2, F3, R1, and R2) used in the validation of mouse extrapulmonary SP-C cDNA and lengths of the PCR products of SP-C cDNA are indicated. The PCR products were electrophoresed and further verified by sequencing. PBS: phosphate-buffered saline, SP-C: surfactant protein C, PCR: polymerase chain reaction.
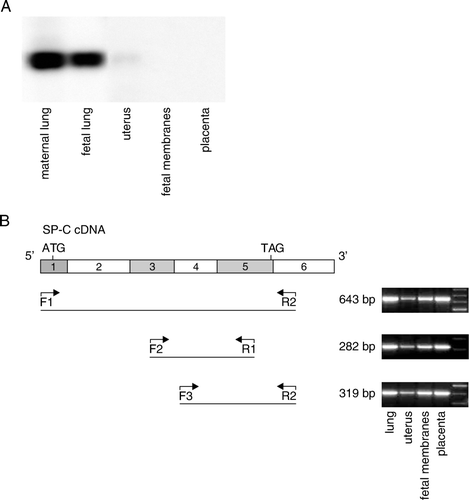
To verify the validity of the amplified SP-C cDNA and to detect possible alternative splicing of SP-C mRNA in uterus, fetal membranes, and placenta, several regions of cDNA were amplified. The sizes and the sequences of PCR products were compared to PCR products obtained with the same primers from lung cDNA. The sizes of all PCR fragments were identical in gestational tissues and lung, indicating that the processing of SP-C mRNA is similar in all tissues (expected PCR product sizes of 643 bp, 282 bp, 319 bp, B). The sequences were compared to SP-C mRNA sequence (GenBank accession no. NM_011359) and confirmed to be identical to lung SP-C mRNA.
ProSP-C is detected in mouse gestational tissues
To investigate whether the extrapulmonary SP-C mRNA is translated to protein and how proSP-C is processed, proteins isolated from gestational tissues were analyzed by Western analysis. The 21-kDa doublet and 16-kDa proforms of SP-C were detected in mouse uterus, fetal membranes, and placenta in addition to maternal and fetal lung (A). The result was confirmed by using two different proSP-C-specific antibodies. Both antibodies recognized the same sized SP-C proproteins in adult lung, an about 21-kDa doublet and 16-kDa protein band, but with different intensities (data not shown). Compared to maternal lung, proteins in fetal lung and reproductive tissues were similar but not identical in size. The proSP-C levels in uterus, placenta, and fetal membranes were lower than in maternal and fetal lung. There were no detectable changes in the protein levels after LPS challenge (data not shown).
Figure 3. SP-C proprotein is present in mouse gestational tissues. A: In Western analysis different amounts of protein from PBS-treated mice were used (maternal lung 10 µg, fetal lung 20 µg, and uterus, fetal membranes, and placenta 50 µg collected at 17 dpc). The proteins were detected using proSP-C antibody FL-197. B–F: Immunohistological localization of proSP-C in fetal membranes (B–E) and maternal lung at 17 dpc (F); tissue sections from either PBS treated wild-type (B, C, and F) or Sftpc −/− (D, E) mouse stained with proSP-C antibody (WRAB-SP-C). Original magnifications in panels B, D, and F are×20 and in panels C and E×40. Positive staining in epithelial yolk sac cells (B and C) and in alveolar type II cells (F) is indicated by arrows. SP-C: surfactant protein C, PBS: phosphate-buffered saline, SFTPC, SP-C gene.
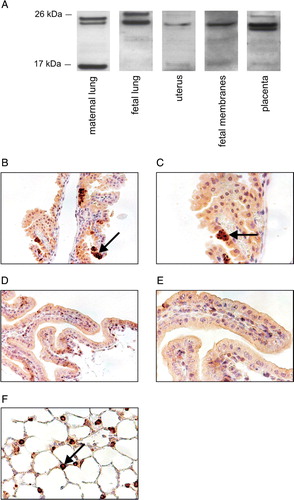
ProSP-C was detected in mouse maternal and fetal lung and fetal membranes by both antibodies used in immunohistology (B–F, data not shown). Specific subsets of inverted epithelial yolk sac cells showed specific granular staining for proSP-C that was lacking in Sftpc −/− mouse tissues. We were not able to detect the protein in uterus or placenta due to the high background staining.
SP-C is expressed in human gestational tissues
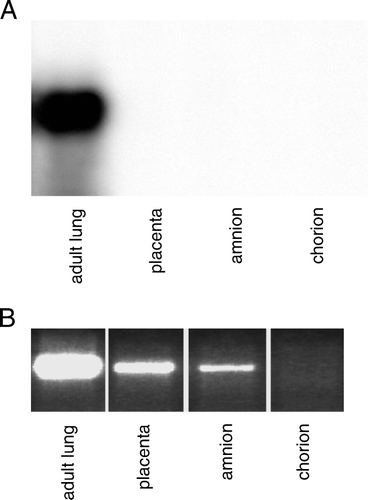
To extend our observations based on the mouse model, the expression of SP-C was analyzed in human gestational tissues. To this end, Northern analysis and RT-PCR were used to study SP-C expression in human lung, placenta, and fetal membranes (A and B). By Northern analysis, expression was observed in the lung but not in the placenta or fetal membranes. Using RT-PCR, SP-C mRNA was detected in all these tissues, and, as expected, the lung showed the strongest expression. Low expression was evident in reproductive tissues except for the chorion, where no SP-C mRNA was detectable after 30 cycles of PCR.
Figure 4. SP-C is expressed in human placenta and fetal membranes. A: The amount of total RNA used in Northern blot: 5 µg for lung and 15 µg for other tissues. B: RT-PCR (30 cycles) was performed to study the SP-C expression in human tissues. Tissues were obtained from normal term delivery or during surgical procedure. RT-PCR: reverse transcriptase polymerase chain reaction, SP-C surfactant protein C gene.
SFTPC rs4715 polymorphism associates with the duration of PPROM
The localization of proSP-C and expression of SP-C in human and mouse tissues suggested a function for SP-C in fetal membranes. Therefore the association of SFTPC SNP rs4715 and PPROM was studied. The allele and genotype distributions of rs4715 were compared between the groups with or without PPROM. As judged by similar allele and genotype distributions, rs4715 did not associate with PPROM as a whole in the fetuses or the mothers (A and C). However, when the fetuses and mothers involved in PPROM were further stratified into two groups on the basis of the interval between PPROM and preterm birth (short (<72 hours) and long duration (≥72 hours)), SNP rs4715 associated with the duration of PPROM in the fetuses but not in the mothers. The allele and genotype distributions differed between fetuses with prolonged PPROM compared to those with short duration (P<0.0001) (B). The association between the SNP and the duration of PPROM remained highly significant even after stringent adjustment for multiple testing (P<0.0006 for both the alleles and the genotypes after Bonferroni correction). The minor allele A was over-represented in preterm fetuses with short duration of PPROM and under-represented in fetuses with long duration. There was a large excess of CC genotypes in the prolonged PPROM group compared to the group with short duration. There was a similar non-significant trend in the distribution of maternal genotypes (D).
Figure 5. Fetal SFTPC SNP rs4715 associates with the duration of PPROM. Frequencies of the SFTPC rs4715 minor allele and genotypes in (A) fetuses born prematurely with (n=131) or without PPROM (n=176), and in (C) mothers with (n=139) or without PPROM (n=162). The fetuses (B) and mothers (D) with PPROM were divided into two groups based on the interval between of PPROM and delivery: short PPROM (duration <72 hours: 51 fetuses, 52 mothers) and prolonged PPROM (duration ≥ 72 hours; 80 fetuses and 87 mothers). SFTPC: surfactant protein C gene, SNP: single nucleotide polymorphism, PPROM: preterm premature rupture of fetal membranes.
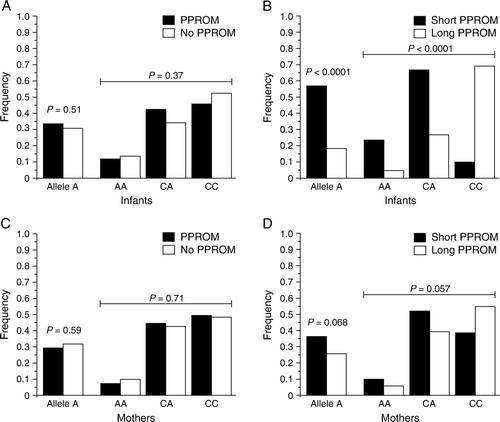
To evaluate further the association of fetal and maternal SFTPC SNP rs4715 on the duration of PPROM, 137 pairs of mothers and their fetuses were studied. The duration associated with the fetal but not with the maternal SFTPC genotypes (). Fetuses with CC genotype had a longer duration of PPROM than those with AA or CA genotypes (P<0.0001). The maternal genotypes neither associated with the duration of PPROM nor influenced the duration together with the fetal genotype ().
Table III. Duration of PPROM in days according to the fetal or maternal SFTPC rs4715 genotype in 137 mother–fetus pairs.
Administration of antibiotics in PPROM increases the duration of pregnancy compared to expectant management Citation[26]. The use of prophylactic antibiotics after PPROM did not interfere with the association of fetal SFTPC polymorphism and the duration of PPROM (Supplementary . Only available in the online version of the journal. Please find this material with the following direct link to the article: http:www.informaworld.com/1080/17482960903358857). The cases managed without prophylactic antibiotics had a shorter duration of PPROM than those treated with antibiotics. Fetuses with CC genotype had longer duration of PPROM than those with AA or CA genotypes, both in the groups managed with prophylactic antibiotics and in the group with expectant management.
Discussion
All surfactant proteins were originally described as components of alveolar surfactant. Since then, extrapulmonary expression of SP-A, SP-B, and SP-D has been described Citation[3], Citation[35], Citation[36], and more recently SP-C has also been detected in extrapulmonary tissues Citation[8–10]. SP-A and SP-D are expressed in the genital tract, placenta, and uterus Citation[31], Citation[37–39]. In the present study of mouse and human tissues, SP-C mRNA and proprotein were detected in pregnant uterus, placenta, and fetal membranes, although in notably lower quantities than in the lung.
In this human study population, the SFTPC rs4715 alleles or genotypes did not associate with spontaneous preterm birth or the risk of PPROM as a whole. This result is not in line with the previous study associating the same SNP with a tendency of extremely preterm birth Citation[23]. This discrepancy may actually originate from evaluation of distinct phenotypic outcomes and therefore be due to non-parallel observations on differently selected study populations as both the length of gestation (<36 versus <28 weeks) and the cause of preterm birth (spontaneous labor versus all causes) were defined discordantly, complicating comparison of the results. While the present study was estimated to have an adequate power to detect an association with a mild or moderate effect, it remains unclear whether the previous study result was indicative of a true association between a subgroup of preterm infants or may be due to type I error. In the end, in the present study we found a strong association between fetal SFTPC genotype and the duration of PPROM. Lack of a similar association with the maternal genotype suggests that the fetus itself has an important role in modifying the preterm labor process in the ascending infection commonly associated with PPROM.
Surfactant proteins A and B are induced in the lung during antenatal differentiation, and they are secreted into the amniotic fluid. A role for amniotic fluid SP-A in signaling the initiation of parturition has also been proposed Citation[29]. Pulmonary SP-C expression increases less than SP-A or SP-B during gestation, and it is already present in the fetal lung during early pregnancy Citation[40]. We show that besides the lung SP-C is expressed in gestational tissues as well. Interestingly, uteri from pregnant mice contained SP-C mRNA measurable by quantitative RPA, whereas it was undetectable in the uteri of nulliparous mice, suggesting a hormonal regulation for SP-C expression. There was no evidence of multiple transcripts or alternative splicing in any of the tissues studied. The LPS-induced suppression of SP-C in fetal lung was more modest than the suppression of collectins SP-A and SP-D in fetal lung Citation[31].
Of the mouse gestational tissues studied, proSP-C was most clearly visualized in the yolk sac membrane. Alveolar type 2 cells secrete surfactant complex into alveoli, including mature SP-C that is secreted exclusively as a 3.7-kDa proteolipid Citation[12], Citation[41]. Since the available antibodies do not recognize the mature form of SP-C, it was not possible to study how the protein was processed in gestational tissues. However, the presence of 21-kDa and 16-kDa proforms indicates that the initial steps of SP-C processing proceed similarly in all tissues studied. It is likely that the further processing or post-translational modification is different in reproductive tissues, possibly leading to secretion of a different form of SP-C with a function separate from that of mature SP-C in the lung. Alternatively, the proforms of SP-C observed in this study may represent intracellular SP-C.
The frequency of minor SFTPC rs4715 allele A was decreased in fetuses delivered after prolonged PPROM (≥72 hours), whereas in fetuses with a short duration of PPROM (<72 hours) the frequency of the minor allele A was increased. In contrast, the maternal SFTPC alleles or genotypes did not associate with the duration of PPROM. The presenting sign in 30%–35% of all premature births is PPROM Citation[25], Citation[26]. However, the duration of PPROM is a subject of considerable variation. Intra-uterine infection and intra-uterine inflammatory response syndrome that result in the activation of the preterm labor process are likely the major factors promoting preterm birth in PPROM Citation[27]. Prophylactic antibiotics significantly increase the duration of PPROM Citation[26]. However, despite prophylactic antibiotics, the association between SFTPC polymorphism and the duration of PPROM was significant. The present data are not in disagreement with the proposed role of ascending infection as a major cause of PPROM. However, we propose that after PPROM fetal SFTPC may influence the activation of preterm labor.
According to animal model data, the deletion of Sftpc results in susceptibility to infection in postnatal animals Citation[13–15]. In addition, mutations affecting SFTPC predispose the patients to interstitial lung disease with recurrent infections Citation[12], Citation[17], Citation[19], Citation[42], Citation[43]. The mutations in the BRICHOS domain within Phe94-Ile197 of proSP-C include cases of pneumonitis and interstitial lung disease with several histological categories and variable penetrance Citation[19]. Interestingly, an interstitial lung disease-associated mutation Leu188Gln is in the vicinity of rs1124 at amino acid 186 which is nearly completely tagged by the tested SNP rs4715 at amino acid 138. This mutation has been shown to interfere with intracellular processing of proSP-C, resulting in the accumulation of intracellular proSP-C aggregates Citation[44]. Although there are currently no data indicating that either of the two SNPs at amino acids 138 or 186 would have phenotypic consequences, the latter of these is located close to a functionally relevant amino acid of the BRICHOS domain. The possible influence of these SNPs on the function of BRICHOS domain during inflammatory challenge remains unknown. We propose that the fetal SFTPC is involved in protection against infection or inflammatory response after PPROM. The short duration of PPROM may additionally increase the risk of RDS as it does not allow sufficient time for differentiation of the pulmonary surfactant system.
Fetal polymorphisms of genes encoding matrix metalloproteinase (MMP) 1 and MMP8 have previously been associated with the risk of PPROM Citation[45–47] consistently with the proposed roles of proteases and protease inhibitors in labor. The investigation on MMP1 and MMP8 raised the hypothesis that instead of the maternal genes the genotype of the extra-embryonic tissues represents the primary genetic determinant of the risk of PPROM Citation[47]. In the present study, the fetal SFTPC genotype associated with the duration of PPROM and SP-C was expressed in extra-embryonic tissue, whereas maternal genotype had no detectable association. These data are consistent with epidemiological evidence suggesting that besides the mother the fetal genes influence the duration of pregnancy Citation[30].
SFTPC may be one of several genes that strengthen the intra-uterine host defense against microbes or moderate labor-inducing innate immune responses. Defining the genotypes and gene–environment interactions may contribute to individualized treatment strategies aimed at delaying or preventing spontaneous preterm birth.
Acknowledgements
The authors thank Maarit Haarala, Mirkka Ovaska, Riitta Sivula, and Riitta Vuento for technical assistance. This work was supported by grants from the Sigrid Jusélius Foundation (M.H.), the Finnish Academy (M.H. and R.H.), the University of Oulu Faculty of Medicine (R.H.) and the Foundation for Pediatric Research (A.S., A.L., and R.H.). Declaration of interest: The authors have no declared conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- Hallman M, Haataja R. Genetic basis of respiratory distress syndrome. Front Biosci. 2007; 12: 2670–82
- Kishore U, Greenhough TJ, Waters P, Shrive AK, Ghai R, Kamran MF, et al. Surfactant proteins SP-A and SP-D: structure, function and receptors. Mol Immunol. 2006; 43: 1293–315
- Paananen R, Glumoff V, Sormunen R, Voorhout W, Hallman M. Expression and localization of lung surfactant protein B in Eustachian tube epithelium. Am J Physiol Lung Cell Mol Physiol. 2001; 280: L214–20
- Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol. 2005; 5: 58–68
- Kingma PS, Whitsett JA. In defense of the lung: surfactant protein A and surfactant protein D. Curr Opin Pharmacol. 2006; 6: 277–83
- Horowitz AD, Elledge B, Whitsett JA, Baatz JE. Effects of lung surfactant proteolipid SP-C on the organization of model membrane lipids: a fluorescence study. Biochim Biophys Acta. 1992; 1107: 44–54
- Johansson J, Szyperski T, Wuthrich K. Pulmonary surfactant-associated polypeptide SP-C in lipid micelles: CD studies of intact SP-C and NMR secondary structure determination of depalmitoyl-SP-C(1–17). FEBS Lett. 1995; 362: 261–5
- Brauer L, Johl M, Borgermann J, Pleyer U, Tsokos M, Paulsen FP. Detection and localization of the hydrophobic surfactant proteins B and C in human tear fluid and the human lacrimal system. Curr Eye Res. 2007; 32: 931–8
- Mo YK, Kankavi O, Masci PP, Mellick GD, Whitehouse MW, Boyle GM, et al. Surfactant protein expression in human skin: evidence and implications. J Invest Dermatol. 2007; 127: 381–6
- Eikmans M, Roos-van Groningen MC, Sijpkens YW, Ehrchen J, Roth J, Baelde HJ, et al. Expression of surfactant protein-C, S100A8, S100A9, and B cell markers in renal allografts: investigation of the prognostic value. J Am Soc Nephrol. 2005; 16: 3771–86
- ten Brinke A, van Golde LM, Batenburg JJ. Palmitoylation and processing of the lipopeptide surfactant protein C. Biochim Biophys Acta. 2002; 1583: 253–65
- Mulugeta S, Beers MF. Surfactant protein C: its unique properties and emerging immunomodulatory role in the lung. Microbes Infect. 2006; 8: 2317–23
- Glasser SW, Burhans MS, Korfhagen TR, Na CL, Sly PD, Ross GF, et al. Altered stability of pulmonary surfactant in SP-C-deficient mice. Proc Natl Acad Sci U S A. 2001; 98: 6366–71
- Glasser SW, Detmer EA, Ikegami M, Na CL, Stahlman MT, Whitsett JA. Pneumonitis and emphysema in sp-C gene targeted mice. J Biol Chem. 2003; 278: 14291–8
- Glasser SW, Senft AP, Whitsett JA, Maxfield MD, Ross GF, Richardson TR, et al. Macrophage dysfunction and susceptibility to pulmonary Pseudomonas aeruginosa infection in surfactant protein C-deficient mice. J Immunol. 2008; 181: 621–8
- Danlois F, Zaltash S, Johansson J, Robertson B, Haagsman HP, van Eijk M, et al. Very low surfactant protein C contents in newborn Belgian White and Blue calves with respiratory distress syndrome. Biochem J 2000; 351 Pt 3: 779–87
- Hamvas A, Nogee LM, White FV, Schuler P, Hackett BP, Huddleston CB, et al. Progressive lung disease and surfactant dysfunction with a deletion in surfactant protein C gene. Am J Respir Cell Mol Biol. 2004; 30: 771–6
- Nogee LM. Alterations in SP-B and SP-C expression in neonatal lung disease. Annu Rev Physiol. 2004; 66: 601–23
- Beers MF, Mulugeta S. Surfactant protein C biosynthesis and its emerging role in conformational lung disease. Annu Rev Physiol. 2005; 67: 663–96
- Pastva AM, Wright JR, Williams KL. Immunomodulatory roles of surfactant proteins A and D: implications in lung disease. Proc Am Thorac Soc. 2007; 4: 252–7
- Augusto L, Le Blay K, Auger G, Blanot D, Chaby R. Interaction of bacterial lipopolysaccharide with mouse surfactant protein C inserted into lipid vesicles. Am J Physiol Lung Cell Mol Physiol. 2001; 281: L776–85
- Augusto LA, Synguelakis M, Johansson J, Pedron T, Girard R, Chaby R. Interaction of pulmonary surfactant protein C with CD14 and lipopolysaccharide. Infect Immun. 2003; 71: 61–7
- Lahti M, Marttila R, Hallman M. Surfactant protein C gene variation in the Finnish population—association with perinatal respiratory disease. Eur J Hum Genet. 2004; 12: 312–20
- Lopez Bernal A. Overview. Preterm labour: mechanisms and management. BMC Pregnancy Childbirth 2007; 7 Suppl 1: S2
- Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008; 371: 75–84
- Garite TJ. Premature rupture of the membranes. Maternal-fetal medicine, RK Creasy, R Resnik. W. B. Saunders Company, Philadelphia 2004; 723–39
- Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, et al. The preterm parturition syndrome. BJOG 2006; 113(Suppl 3)17–42
- Elovitz MA. Anti-inflammatory interventions in pregnancy: now and the future. Semin Fetal Neonatal Med. 2006; 11: 327–32
- Condon JC, Jeyasuria P, Faust JM, Mendelson CR. Surfactant protein secreted by the maturing mouse fetal lung acts as a hormone that signals the initiation of parturition. Proc Natl Acad Sci U S A. 2004; 101: 4978–83
- Plunkett J, Muglia LJ. Genetic contributions to preterm birth: implications from epidemiological and genetic association studies. Ann Med. 2008; 40: 167–95
- Salminen A, Paananen R, Vuolteenaho R, Metsola J, Ojaniemi M, Autio-Harmainen H, et al. Maternal endotoxin-induced preterm birth in mice: fetal responses in toll-like receptors, collectins, and cytokines. Pediatr Res. 2008; 63: 280–6
- Richardson CJ, Pomerance JJ, Cunningham MD, Gluck L. Acceleration of fetal lung maturation following prolonged rupture of the membranes. Am J Obstet Gynecol. 1974; 118: 1115–8
- Chen X, Kwok PY. Homogeneous genotyping assays for single nucleotide polymorphisms with fluorescence resonance energy transfer detection. Genet Anal. 1999; 14: 157–63
- Purcell S, Cherny SS, Sham PC. Genetic power calculator: design of linkage and association genetic mapping studies of complex traits. Bioinformatics. 2003; 19: 149–50
- Akiyama J, Hoffman A, Brown C, Allen L, Edmondson J, Poulain F, et al. Tissue distribution of surfactant proteins A and D in the mouse. J Histochem Cytochem. 2002; 50: 993–6
- Madsen J, Tornoe I, Nielsen O, Koch C, Steinhilber W, Holmskov U. Expression and localization of lung surfactant protein A in human tissues. Am J Respir Cell Mol Biol. 2003; 29: 591–7
- Leth-Larsen R, Floridon C, Nielsen O, Holmskov U. Surfactant protein D in the female genital tract. Mol Hum Reprod. 2004; 10: 149–54
- Kankavi O, Ata A, Gungor O. Surfactant proteins A and D in the genital tract of mares. Anim Reprod Sci. 2007; 98: 259–70
- MacNeill C, Umstead TM, Phelps DS, Lin Z, Floros J, Shearer DA, et al. Surfactant protein A, an innate immune factor, is expressed in the vaginal mucosa and is present in vaginal lavage fluid. Immunology. 2004; 111: 91–9
- Wert SE, Glasser SW, Korfhagen TR, Whitsett JA. Transcriptional elements from the human SP-C gene direct expression in the primordial respiratory epithelium of transgenic mice. Dev Biol. 1993; 156: 426–43
- Weaver TE, Conkright JJ. Function of surfactant proteins B and C. Annu Rev Physiol. 2001; 63: 555–78
- Thomas AQ, Lane K, Phillips J 3rd, Prince M, Markin C, Speer M, et al. Heterozygosity for a surfactant protein C gene mutation associated with usual interstitial pneumonitis and cellular nonspecific interstitial pneumonitis in one kindred. Am J Respir Crit Care Med. 2002; 165: 1322–8
- Brasch F, Griese M, Tredano M, Johnen G, Ochs M, Rieger C, et al. Interstitial lung disease in a baby with a de novo mutation in the SFTPC gene. Eur Respir J. 2004; 24: 30–9
- Johansson H, Nordling K, Weaver TE, Johansson J. The Brichos domain-containing C-terminal part of pro-surfactant protein C binds to an unfolded poly-val transmembrane segment. J Biol Chem. 2006; 281: 21032–9
- Fujimoto T, Parry S, Urbanek M, Sammel M, Macones G, Kuivaniemi H, et al. A single nucleotide polymorphism in the matrix metalloproteinase-1 (MMP-1) promoter influences amnion cell MMP-1 expression and risk for preterm premature rupture of the fetal membranes. J Biol Chem. 2002; 277: 6296–302
- Wang H, Ogawa M, Wood JR, Bartolomei MS, Sammel MD, Kusanovic JP, et al. Genetic and epigenetic mechanisms combine to control MMP1 expression and its association with preterm premature rupture of membranes. Hum Mol Genet. 2008; 17: 1087–96
- Wang H, Parry S, Macones G, Sammel MD, Ferrand PE, Kuivaniemi H, et al. Functionally significant SNP MMP8 promoter haplotypes and preterm premature rupture of membranes (PPROM). Hum Mol Genet. 2004; 13: 2659–69
Supplemtary Table I. Relationship of management practice on the duration of PPROM; analysis according to fetal SFTPC genotype.