Abstract
The sea star Aquilonastra corallicola reproduces asexually by splitting across the central disc, a process known as fission. Although photoperiod and body mass are known to influence the rate of reproduction in fissiparous asteroid species, to our knowledge, the effects of population density have not been experimentally measured in asteroids. We tested the effects of three population densities on the rate of fission in laboratory populations maintained for 4 months at low, medium, and high (n = 5, 15, and 30 per treatment replicate, respectively) population densities. Our results show that population density has a significant, inverse effect upon rate of reproduction in this species. This suggests that density-dependent regulation of asexual reproduction is a significant factor in explaining the observed patterns of population structure in marine clonal invertebrates.
Introduction
Fissiparous sea stars are capable of reproducing asexually by dividing across the central disc, usually resulting in two approximately equal halves that then regenerate into two complete animals (Emson and Wilkie Citation1980). This tendency is distinguished from other asteroids capable of reproducing through ray autotomy where one or more rays can be detached from the animal, sometimes as a defense mechanism, and the individual rays then regenerate into a complete animal (Emson and Wilkie Citation1980).
Fissiparous sea stars are generally thought to be heterogonic, able to reproduce both asexually and sexually (Emson and Wilkie Citation1980). Sexual reproduction seems to appear in favorable habitats and under stable environmental conditions while asexual reproduction appears to be favored in marginal habitat and unstable environmental conditions (Crump and Barker Citation1985; Achituv and Sher Citation1991; Mladenov Citation1996). This pattern of reproduction is expected to generate considerable local population structure, as is now well-documented among clonal marine species exhibiting metapopulations (Okamura et al. Citation2002).
Other factors such as photoperiod, temperature, availability of food, salinity, pH, oxygen, and overall stability of the habitat may influence the ratio of asexual to sexual reproduction in a population of fissiparous sea stars at any point in time (Emson and Wilkie Citation1980; Mladenov Citation1996; Lawrence and Herrera Citation2000). Mladenov's (Citation1996) model illustrated various endogenous and exogenous conditions that may lead to either the sexual or the asexual phase and its associated body form. There is also evidence that similar exogenous and endogenous factors regulate rates of fission.
Rates of fission in the sea star Stephanasterias albula reach their peak in the spring and summer (Mladenov et al. Citation1986) when daylight is longest in the northern hemisphere. Photoperiod, tidal influences, and temperature were all positively correlated with higher rates of fission in Coscinasterias tenuispina (Alves et al. Citation2002), while temperature and photoperiod were positively correlated with higher rates of fission in Allostichaster capensis (Rubilar et al. Citation2005). Higher temperature was also found to be a factor regulating fission in Coscinasterias acutispina (Haramoto et al. Citation2007). Body mass has been shown to affect the rate of fission in Allostichaster insignis and Coscinasterias muricata, with smaller animals having a higher rate of fission (Crump and Barker Citation1985; Barker and Scheibling Citation2008). The availability of food has also been shown to influence relative rates of fission (Crump Citation1971; Skold et al. Citation2002; Haramoto et al. Citation2007; Barker and Scheibling Citation2008).
Another factor that has been mentioned as a potential cause of fluctuations in rates of fission in sea stars is population density (Mladenov Citation1996); although to our knowledge there have been no experimental publications upon this topic in asteroids. Evidence that population density has an effect upon rates of asexual reproduction has been found in other invertebrates such as holothurians (Lee et al. Citation2008), zoanthids (Tanner Citation1999), rotifers (Hagiwara et al. Citation1994), Hydra pseudoligactis (Bell and Wolfe Citation1985), soft corals (Karlson et al. Citation1996), and the polychaete worm Pygiospio elegans (Wilson Citation1983).
The sea star genus Aquilonastra was established by O’Loughlin and Waters's (Citation2004) revision of Asterinidae, who subsequently reorganized Aquilonastra species based on morphological characters resulting in 13 new species and the recognition of several undescribed species (O’Loughlin and Rowe Citation2006). O’Loughlin and Rowe (Citation2006) further state that it is likely that extant species with large geographical ranges could prove to be more than one species. Described species are also known to show great morphological variation across their range (O’Loughlin and Rowe Citation2006). Current taxonomic relationships within the genus Aquilonastra are at best provisional and as no molecular phylogenies have been performed, we will use the species name Aquilonastra corallicola for the animal used in our experiment though we recognize that it may be an undescribed form of Aquilonastra. This species designation was based on morphological characters from published descriptions (Marsh Citation1977; Oguro Citation1983; O’Loughlin and Rowe Citation2006). A. corallicola is a small, cryptic sea star, the longest ray averaging only 4.33 mm in the experimental population which is close to the mean size of 4.50 mm for the holotype and paratypes originally collected at Palau (Marsh Citation1977). Ray number is variable ranging from 3 to –8, with 6-rayed animals being the most common. Their known range extends from the tropical eastern Indian Ocean into the Western Pacific Ocean. Our experiment investigated the effects of different levels of population density upon rate of fission in Aquilonastra sp.
Materials and methods
To examine the effect of population density on the rate of fission in A. corallicola, we used three population densities: low = 5 animals; medium = 15 animals; and high = 30 animals. We established 7 replicates of the low density treatment for a total of 35 animals, 5 replicates of the medium density treatment for a total of 75 animals, and 4 replicates of the high density treatment for a total of 120 animals and 16 replicates.
Each replicate was housed in one of the sixteen 18.93 L aquaria we placed into two incubators, eight aquaria per incubator. Replicates from each treatment were split between the two incubators. We set photoperiod to identical 12-h light/dark cycles and temperature to 25°C. Light was provided by standard 40-W fluorescent fixtures and pH was maintained between 8.2 and 8.3. The buffering capacity of the water was 10–12 dKH (degrees carbonate hardness). We dissolved a commercially available synthetic sea water mix in distilled water to achieve a specific gravity of 1.023. Shelter was provided by artificial rocks made from Portland cement that we poured into molds so that the rocks in each aquarium were identical. Three of these rocks were placed in each aquarium. A small pump was placed in each aquarium to provide water circulation and a 6 cm thick layer of dark gray calcite gravel provided the substrate. The sea stars fed on diatoms and green algae growing on the rocks and glass walls of the aquarium. As the glass walls and rocks had identical surface areas in each aquarium, it was assumed that food resources were identical in each aquarium.
Sea stars were obtained through a retail reef aquarium supplier and the exact location of the source of the animals is unknown. Animals were divided into four size classes (<4 mm, 4–5 mm, 5–6 mm, and >6 mm) and animals from each size class were haphazardly placed into replicates so that the mean size of the animals was roughly the same in each replicate. We did not place the animals into the aquaria until 6 weeks, after we had set them up to allow the growth of diatoms and green algae as a food resource. We did not start collecting data until after the animals had been in the aquaria for 6 more weeks, enabling us to monitor the water conditions within each aquarium and make sure that those conditions were stable. Weekly water quality tests were performed and a 10% water change was performed every other week, both during the initial 6-week stabilization periods and during the experimental phase.
We performed surveys on each aquarium every other week from October 31, 2006 to March 5, 2007 for a total of 10 surveys. During the surveys, all animals in each aquarium were removed and incidents of fission were recorded. When an animal divided, one half was retained in the aquarium it came from and the other was removed. Because fission primarily occurred asynchronously, all individuals were easily identified. Each time an animal reproduced, we first retained the larger half and transplanted the smaller half, and then we reversed this procedure in each successive division through the course of the study. In this way, we were able to maintain the population density in each aquarium and also control for the mean size of the animals in each replicate. When more than one fission event took place within an aquarium, it was possible to match up the correct two halves as each half of an animal would have grown to the same stage of regeneration. In this way, it was possible to ensure that we removed only one half of a sea star.
Data were analyzed using JMP 5.1 (SAS Institute) statistical software and were square root transformed. A test of Mauchly's criterion demonstrated that our data did not meet the assumptions of univariate repeated measures ANOVA. So we used multivariate repeated measures ANOVA (MANOVA) to determine whether the three population density treatments had significantly different rates of fission and if the rate of fission changed significantly over time.
Results
Our MANOVA to identify the effects of treatment and experiment duration on rate of fission was significant for treatment, F [2,13] = 75.23, p ≤ 0.0001, and experiment duration effects: F [9,5] = 6.76, p = 0.024. Four tests are used by JMP to test for interaction and none were significant: Wilk's lambda, F [18,10] = 0.77, p = 0.70; Pillai's trace, F [18,12] = 0.72, p = 0.74; Hotelling–Lawson, F [18,8] = 0.78, p = 0.69; and Roy's maximum, F [9,6] = 2.067, p = 0.19.
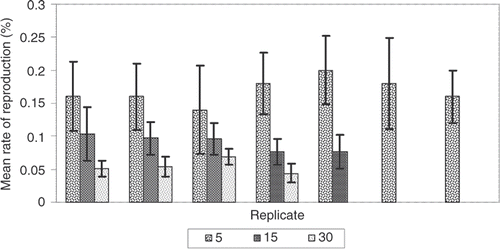
The small treatment (5 animals) had the highest proportion of fission events, while the large treatment (30 animals) had the lowest proportion of fission events. The proportion for the medium treatment (15 animals) was between the low and high treatments. This pattern holds true not only for each treatment overall, but also for each replicate within each treatment ().
Discussion
Our MANOVA shows that the population density treatments did have a significant effect on rate of fission. Experiment duration was also significant, showing that the rate of fission did change through time, but there was no significant interaction between experiment duration and treatment. This indicates that the change in rate of reproduction over time was not different between treatments. In other words, the change in the rate of reproduction through time is the same across treatments even as the rate of reproduction between treatments is different (). Because the treatments and replicates were split up between two incubators, each replicate in its own aquarium, this pattern does not reflect an incubator or aquarium affect.
Figure 2. Rate of reproduction per treatment. The mean proportion of reproduction per treatment is shown for each of 10 surveys with standard error: 5 animals = low density, 15 animals = medium density, and 30 animals = high density.
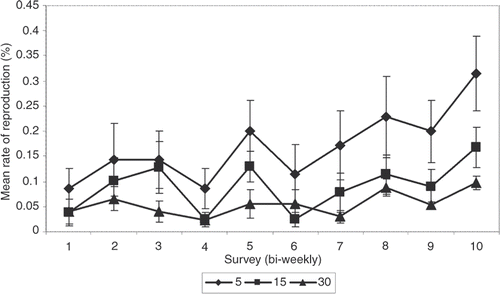
One possible explanation is that the sea stars were experiencing a lingering effect from introduction into a new environment. When fissiparous sea stars are introduced into laboratory aquaria, they frequently undergo fission (Mladenov Citation1996). The experimental population did undergo fission when first introduced into the experimental aquaria and it is possible that when most of the animals reproduced within 2 weeks of each other, their reproductive cycles were synchronized. This view is further supported because the change in reproductive rates over time was not significantly different between treatments. This synchronization effect has been shown to happen in another experimental setting, after our experiment was conducted (Barker and Scheibling Citation2008).
Another possible factor influencing rate of fission in our experiment is food availability, which may have been limiting. We attempted to make food equally available across treatments, but food may have been more rapidly depleted in the higher density treatments. A. corallicola feeds on diatoms and green algae growing on the rock and glass walls of the aquaria. Before animals were added to the aquaria, the aquaria were allowed to sit for 6 weeks to allow the growth of diatoms and algae. During the course of the experiment, the algae were seen to increase rather than decrease after the addition of the sea stars, suggesting that food resources were sufficient to sustain many more animals than were in the aquaria and so allowing this possibility to be discounted. Future experiments could extend the acclimation period before data collection to 12 or even 16 weeks to determine if oscillations in fission rates within and among treatments diminish or disappear.
The results of our experiment suggest that density-dependent chemical cues regulate rate of reproduction in A. corallicola. As stated in the ‘introduction’ section, other environmental factors have been shown to regulate reproduction in marine invertebrates. However, environmental factors such as water temperature, photoperiod, salinity, pH, food resources, and overall stability were controlled for. The best explanation for our observed results is density-dependent regulation of reproduction. This is supported by theory regarding fissiparous echinoderms in general (Mladenov Citation1996), which predicts that lower densities should promote increased asexual reproduction. This was also proposed to be true in fissiparous holothurians (Uthicke Citation2001) and a recent study using the tropical sea cucumber Holothuria atra was found to have higher rates of reproduction at lower densities and lower rates of reproduction at higher densities in a natural mesocosm experiment (Lee et al. Citation2008), though the results were not statistically significant and other factors than population density such as food resources may have influenced the results. As stated in the ‘introduction’ section, population density has been found to be a factor influencing rates of asexual reproduction in other marine invertebrate taxa. This regulating mechanism appears to be common and to have arisen through convergent evolutionary processes. What might be an explanation for this? One answer might be that these taxa, including fissiparous sea stars, fit the classic metapopulation model as applied to clonal invertebrates.
An overview of the metapopulation model as applied to clonal aquatic invertebrates can be found in Okamura et al. (Citation2002). The key point is that there is a premium on being able to rapidly reproduce from one or few founding individuals into a new, viable subpopulation while not exceeding carrying capacity. Density-dependent regulation of reproduction would help accomplish this. There is evidence that populations of fissiparous species are patchy with some species seemingly well-adapted to quickly saturating patchy habitats (Mladenov Citation1996). The asteroid Coscinasterias calamaria was found to have genetically distinct clonal populations that were sometimes only separated by 50 m (Johnson and Threlfall Citation1987).
Once a subpopulation is established, continued asexual reproduction to achieve a required level of population density could then set the stage for successful sexual reproduction (Bell and Wolfe Citation1985). For instance, it has been found in Clypeaster rosaceus (Echinodermata: Echinoidea) that successful fertilization of eggs occurs within specific population densities and population sizes (Levitan and Young Citation1995). Whether or not fissiparous asteroids fit the metapopulation model, successful colonization of patchy habitat would be enhanced by density-dependent regulation of asexual reproduction.
Population density has a significant effect upon rates of reproduction with the highest population density having the slowest reproductive rate and the lowest density having the highest rate. This may be explained as an adaptive advantage for a marine clonal animal utilizing a metapopulation life strategy. We do not suggest that population density is the only factor regulating reproductive rate in A. corallicola. As noted, rates of fission are known to be influenced by several environmental factors. Further investigation of these factors could help to determine the relationship between endogenous and exogenous factors in the regulation and causes of fission in heterogonic echinoderms.
Acknowledgements
We thank the Hooper Undergraduate Research Awards program at Northern Arizona University and Jimmy “JJ” Callan for financial and material support.
References
- Achituv , Y and Sher , E . 1991 . Sexual reproduction and fission in the sea star Asterina burtoni from the Mediterranean coast of Israel . Bulletin of Marine Science , 48 : 670 – 678 .
- Alves , LSS , Pereira , A and Ventura , C . 2002 . Sexual and asexual reproduction of Coscinasterias tenuispina (Echinodermata: Asteroidea) from Rio de Janeiro, Brazil . Marine Biology , 140 : 95 – 101 .
- Barker , MF and Scheibling , RE . 2008 . Rates of fission, somatic growth and gonadal development of a fissiparous sea star, Allostichaster insignis, in New Zealand . Marine Biology , 153 : 815 – 824 .
- Bell , G and Wolfe , LM . 1985 . Sexual and asexual reproduction in a natural population of Hydra pseudoligactis . Canadian Journal of Zoology , 63 : 851 – 856 .
- Crump , RG . 1971 . Annual reproductive cycles in three geographically seperated populations of Patiriella regularis (Verrill), a common New Zealand asteroid . Journal of Experimental Marine Biology and Ecology , 7 : 137 – 162 .
- Crump , RG and Barker , MF . 1985 . Sexual and asexual reproduction in geographically separated populations of the fissiparous asteroid Coscinasteria calamaria (Gray) . Journal of Experimental Marine Biology and Ecology , 88 : 109 – 127 .
- Emson , RH and Wilkie , IC . 1980 . Fission and Autotomy in Echinoderms . Oceanography and Marine Biology: An Annual Review , 18 : 155 – 250 .
- Hagiwara , A , Hamada , K , Hori , S and Hirayama , K . 1994 . Increased sexual reproduction in Brachionus plicatilis (Rotifera) with the addition of bacteria and rotifer extracts . Journal of Experimental Marine Biology and Ecology , 181 : 1 – 8 .
- Haramoto , S , Komatsu , M and Yamazaki , Y . 2007 . Patterns of asexual reproduction in the fissiparous seastar Coscinasterias acutispina (Asteroidea: Echinodermata) in Japan . Zoological Science , 24 : 1075 – 1081 .
- Johnson , MS and Threlfall , TJ . 1987 . Fissiparity and population genetics of Coscinasterias calamaria . Marine Biology , 93 : 517 – 525 .
- Karlson , RH , Hughes , TP and Karlson , SR . 1996 . Density-dependent dynamics of soft coral aggregations: the significance of clonal growth and form . Ecology , 77 : 1592 – 1599 .
- Lawrence , JM and Herrera , J . 2000 . Stress and deviant reproduction in echinoderms . Zoological Studies , 39 : 151 – 171 .
- Lee , J , Byrne , M and Uthicke , S . 2008 . The influence of population density on fission and growth of Holothuria atra in natural mesocosms . Journal of Experimental Marine Biology and Ecology , 365 : 126 – 135 .
- Levitan , DR and Young , CM . 1995 . Reproductive success in large populations: empirical measures and theoretical predictions of fertilization in the sea biscuit Clypeaster rosaceus . Journal of Experimental Marine Biology and Ecology , 190 : 221 – 241 .
- Marsh , LM . 1977 . Coral reef Asteroids of Palau, Caroline Islands . Micronesica , 13 : 251 – 281 .
- Mladenov , PV . 1996 . Environmental factors influencing asexual reproductive processes in echinoderms . Oceanologica Acta , 19 : 227 – 235 .
- Mladenov , PV , Carson , SF and Walker , CW . 1986 . Reproductive ecology of an obligately fissiparous population of the sea star Stephanasterias albula (Stimpson) . Journal of Experimental Marine Biology and Ecology , 96 : 155 – 175 .
- Oguro , C . 1983 . Supplementary notes on the sea stars from the Palau and Yap Islands . Annotationes Zoologicae Japonenses , 56 : 221 – 226 .
- Okamura , B , Freeland , JR and Hatton-Ellis , T . 2002 . “ Clones and metapopulations ” . In Reproductive biology of invertebrates. Vol. XI. Progress in asexual reproduction , Edited by: Hughes , RN , Adiyodi , KG and Adiyodi , RG . 283 – 312 . Chichester, West Sussex, , England : John Wiley & Sons .
- O’Loughlin , PM and Rowe , FWE . 2006 . A systematic revision of the Asterinid genus Aquilonastra O’Loughlin, 2004 (Echinodermata: Asteroidea) . Memoirs of Museum Victoria , 63 : 257 – 287 .
- O’Loughlin , PM and Waters , JM . 2004 . A molecular and morphological revision of genera of Asterinidae (Echinodermata: Asteroidea) . Memoirs of Museum Victoria , 61 : 1 – 40 .
- Rubilar , T , Pastor de Ward , CT and Diaz de Vivar , ME . 2005 . Sexual and asexual reproduction of Allostichaster capensis (Echinodermata: Asteroidea) in Golfo Nuevo . Marine Biology , 146 : 1083 – 1090 .
- Skold , M , Barker , MF and Mladenov , PV . 2002 . Spatial variability in sexual and asexual reproduction of the fissiparous sea star Coscinasterias muricata: the role of food and fluctuating temperature . Marine Ecology Progress Series , 233 : 143 – 155 .
- Tanner , JE . 1999 . Density-dependent population dynamics in clonal organisms: a modeling approach . Journal of Animal Ecology , 68 : 390 – 399 .
- Uthicke , S . 2001 . Influence of asexual reproduction on the structure and dynamics of Holothuria (Halodeima) atra and Stichopus chloronotus populations of the Great Barrier Reef . Marine and Freshwater Research , 52 : 205 – 215 .
- Wilson , HW . 1983 . The role of density dependence in a marine infaunal community . Ecology , 64 : 295 – 306 .