Abstract
The population biology of Nematopalaemon schmitti in the Ubatuba region on the northern coast of the state of São Paulo was characterized through analyses of the length-frequency distribution, sex ratio, reproductive period, and juvenile recruitment. The specimens were caught monthly from January 1998 to December 1999, from a shrimp boat equipped with double-rig trawl nets. A total of 1073 specimens were analyzed, and the sex and carapace length (0.1 mm) of each individual were noted. The analyzed specimens consisted of 152 juveniles, 437 adult males, 296 adult females, and 188 ovigerous females (OFs). The monthly distribution of N. schmitti by size classes revealed a stable population structure, with both juveniles and adults continuously present. This population showed a unimodal distribution; however, bimodality was identified in June 1998 and 1999 for males, and June 1998 and July 1998 and 1999 for females, probably related to recruitment pulses of juveniles. Sexual dimorphism was indicated by the presence of females in the larger size classes, probably because of their growth rate. The proportion between males and females differed from 1 : 1 in certain size classes and seasons of the year (χ 2, p < 0.05); in some situations, the females were more abundant than the males, or vice versa. No significant relationship was detected between the seasonal mean temperatures of the bottom water and the percentages of OFs and young, or for the relationship between these two groups (Spearman, p > 0.05). The continuous presence of OFs and young in the population suggests a pattern of continuous reproduction for N. schmitti in the Ubatuba region.
Introduction
Characterization of a population structure can lead to a better understanding of the processes that influence intraspecies interactions in space and time. This understanding aids in the evaluation of the vulnerability of a particular population to fragmentation, which can result from natural or human-induced disturbances (Ricklefs and Miller Citation1999). In addition, knowledge of the reproductive biology of organisms impacted by various types of fishing gear is an important tool for administering a well-regulated and sustainable fishery, aiding in the preservation not only of economically important species, but also of species that are part of the accompanying fauna (bycatch) and that are fundamental components in trophic webs and structuring benthic habitats.
The overexploitation of the marine ecosystem has resulted in a steady increase in ecological studies of population dynamics, for the purpose of understanding, maintaining, and preserving the natural stocks. Decapod crustaceans are among the groups that are most targeted in these investigations, principally species belonging to the infraorders Penaeidea (Crocos and van der Velde Citation1995; Nakagaki and Negreiros-Fransozo Citation1998; Costa and Fransozo Citation2004; Castro et al. Citation2005; Castilho et al. Citation2007a, Citation2007b, Citation2008a, Citation2008b; Yamada et al. Citation2007), Caridea (Oh et al. Citation1999; Bauer Citation2002; Chilari et al. Citation2005; Kim Citation2005; Mossolin et al. Citation2006; Pralon and Negreiros-Fransozo Citation2006; Viegas et al. Citation2007; Bilgin et al. Citation2009), Brachyura (Mantelatto et al. Citation1995; Negreiros-Fransozo et al. Citation1999; Costa and Negreiros-Fransozo Citation2003; Litulo Citation2006; Benetti et al. Citation2007; Doi et al. Citation2008; Teixeira et al. Citation2009), and Anomura (Fransozo and Mantelatto Citation1998; Fransozo and Bertini Citation2001; Martinelli et al. Citation2002; Fernandes-Goés et al. Citation2005).
On the southeast coast of Brazil, few studies have examined the caridean shrimps. Notable among these are the studies on the population structure of Alpheus armillatus H. Milne Edwards, 1837 by Mossolin et al. (Citation2006), and on Exhippolysmata oplophoroides (Holthuis 1948), including aspects related to fecundity (Chacur and Negreiros-Fransozo Citation1998), first larval stage (Negreiros-Fransozo et al. Citation2002), spatial and temporal distribution, population structure, reproductive biology (Fransozo et al. Citation2005; Braga Citation2006), and evidence of hermaphroditism (Braga et al. Citation2009). However, there are few investigations on the caridean shrimp Nematopalaemon schmitti (Holthuis 1950). Only two studies have been carried out on the species, both focused on its spatial and temporal distribution and the influences of environmental factors on its abundance, and both of them were carried out in the Ubatuba region at different times (Almeida Citation2008; Fransozo et al. Citation2009).
On the Brazilian coast, N. schmitti occurs from Amapá to São Paulo (Ramos-Porto and Coelho Citation1998). Because it has no economic value, investigation of this species has been neglected, but it may play an important ecological role within the trophic web of soft bottom environments to which it pertains. In addition, N. shcmitti belongs to the carcinofauna accompanying the fishery targeting species of commercial interest on the northern São Paulo coast, among them Xiphopenaeus kroyeri (Heller 1862), Farfantepenaeus paulensis (Perez-Farfante 1967), Farfantepenaeus brasiliensis (Latreille 1817), Litopenaeus schmitti (Burkenroad 1936), Artemesia longinaris Bate 1888, and Pleoticus muelleri (Bate 1888). Therefore, the objective of this study was to characterize the population biology of N. schmitti, with emphasis on the length-frequency distribution (LFD), sex ratio, reproductive period, and juvenile recruitment.
Materials and method
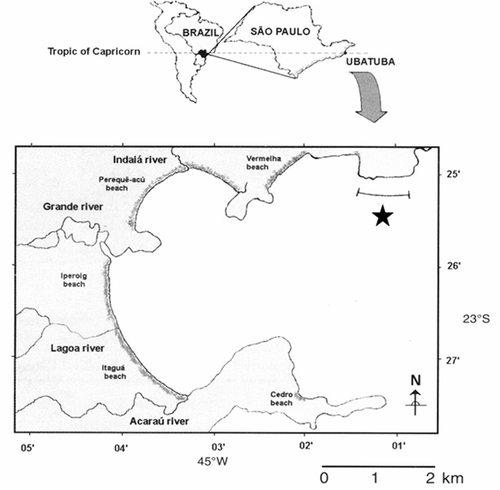
Shrimps were collected monthly from January 1998 to December 1999, in a transect, locally denominated as “Ponta do Respingador,” located at the northernmost part of the outer Ubatuba Bay, northern coast of São Paulo, Brazil ().
The shrimp boat used for trawling was equipped with two double-rig nets (mesh size 20 and 15 mm in the cod end). The transect (2 km) was trawled over a 30 min period, covering 0.018 km2. The average depth in the sampled local was 9.0 ± 1.5 m. Specimens of N. schmitti were labeled, fixed in formalin, and preserved in alcohol 70% until the analysis. Bottom-water samples were also collected monthly with a Nansen bottle for the measurement of temperature using a mercury thermometer.
Individuals were measured to nearest 0.1 mm. Carapace length (CL), corresponding to the distance from the orbital angle to the posterior margin of the carapace, was chosen as the size dimension. The distinction of sex was based on the presence (male) or absence (female) of the appendix masculina on the second pair of pleopods, with the aid of a stereomicroscope. The OFs were identified by the presence of eggs attached to pleopods.
The data were separated into five demographic groups: juvenile male (JM), adult male (AM), juvenile female (JF), adult female (AF), and OF. JM individuals were classified according to methodology adapted from Bauer (Citation1989), defining the juvenile individuals as minor specimens corresponding to 25% of all size classes observed, which correspond to the first classes. JF individuals were classified according to the size of the smallest OF obtained throughout the sampled period.
Monthly LFDs were constructed using 0.5-mm CL size intervals to each demographic group of N. schmitti in order to analyze the structure population. The Kolmogorov–Smirnov (two independent samples, α = 0.05) method was used to compare differences between size classes of males and females.
Bhattacharya's (Citation1967) method was used for the identification of cohorts included in the LFD. Bhattacharya's method assumes normal distributions of the components in a composite LFD. This method, calculated in the FISAT software package, identifies and separates one or more cohorts included in polymodal LFDs (Bhattacharya Citation1967). The separation index between different cohorts was estimated, whose values of less than two indicate a large overlap between cohorts and were considered statistically unacceptable (Sparre and Venema Citation1998). The differences in sex ratio were tested for significant divergence from the expected 1 : 1 ratio using a Chi-squared (χ 2, α = 0.05) test (Sokal and Rohlf Citation1995).
The reproductive period and recruitment of juvenile of N. schmitti were determined seasonally (summer: January–March, autumn: April–June; winter: July–September, and spring: October–December) by the proportion of OFs according to the total number of AFs collected and the proportion of juveniles in relation to the total number of individuals sampled in the population, respectively. The relationship between seasonal mean temperature of bottom water and the percentages of OFs (a) and juveniles (b), and the relationship between (a) and (b) was tested by Spearman's correlation coefficient (α = 0.05).
Results
A total of 1073 specimens of N. schmitti were analyzed in this study. The smallest individuals for which the sex could be determined measured 6.8 (male) and 6.9 mm CL (female).
According to the methodology described for the classification of JM individuals, all the specimens with CL ≤ 8.4 mm were considered JMs. The size of the smallest OF obtained during the sampling period was 9.7 mm CL. Consequently, individuals with CL ≤ 9.7 mm were considered JFs. The analyzed specimens consisted of 43 JMs, 437 AMs, 109 JFs, 296 AFs, and 188 OFs. The ranges of the size and the mean and SD of the CL of the individuals measured are given in .
Table 1. Nematopalaemon schmitti. Size (mm) of individuals based on CL.
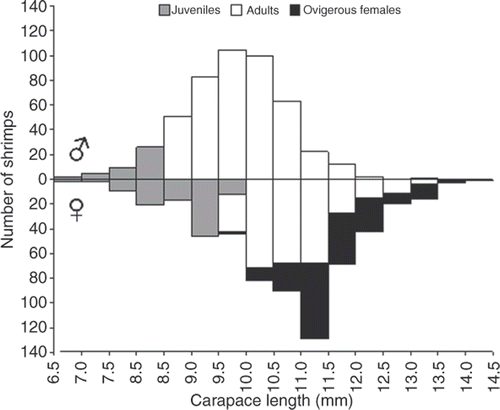
The LFD of N. schmitti for males and females separately for the whole study period is shown in . The distribution of males and females in size classes was statistically different (Kolmogorov–Smirnov, p < 0.05). Males were more than females from the second to the eighth size class, whereas females predominated in the last eight classes. The mean size of the males was 9.7 ± 0.9 mm CL and of the females was 10.7 ± 1.2 mm CL.
Figure 2. Nematopalaemon schmitti. LFD of all shrimps sampled at Ubatuba bay. (Males, superior graphic and Females, inferior graphic; Gray, JM and JF; White, AM and AF; and Black, OF).
Monthly LFDs of males and females, which were apparently unimodal, are shown in . However, from the results of the Bhattacharya's method, bimodality was identified for males in June 1999. With respect to females, three bimodalities were observed in July 1998 and 1999 and June 1999. One possible additional cohort was detected for males and females ().
Figure 3. Nematopalaemon schmitti. Monthly LFDs of males and females. (Males, superior graphic and Females, inferior graphic; Grey, JM and JF; White, AM and AF; and Black, OF).
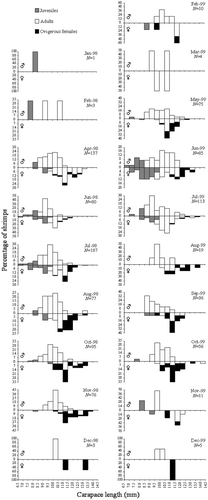
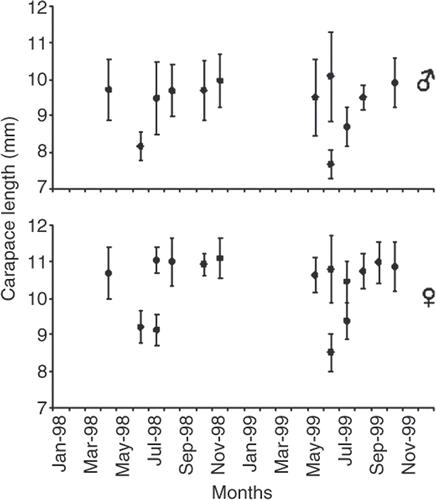
Figure 4. Nematopalaemon schmitti. Calculated cohorts of males and females from Bhattacharya's method during the samples (•, mean of CL; ⊤ ┴, SD of CL).
The proportion of males to females differed in some size classes (χ 2, p < 0.05); it favored males in the classes of 8.5 ≥ 9.0–9.5 ≥ 10.0 mm CL, and favored females in the classes from 11.0 ≥ 11.5–12.0 ≥ 12.5 mm and from 13.0 ≥ 13.5 mm CL (). The sex ratio in winter 1998 and 1999 was in favor of females and in spring 1998 was in favor of males (χ 2, p < 0.05; ).
Figure 5. Nematopalaemon schmitti. Sex ratio as percentage of males in relation to size, with indication of those values showing statistically significant difference from 1:1 ratio (*), nonsignificant (•), or only one sex (◯).
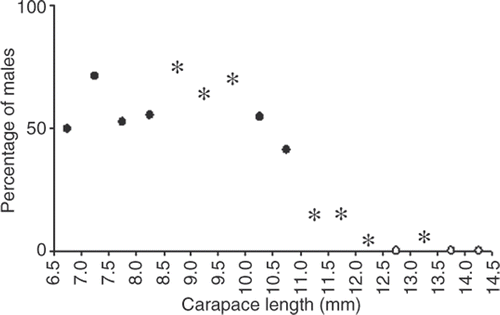
Table 2. Nematopalaemon schmitti. Sexual proportion for season.
OFs were present during the entire sampling period, except in summer 1998. The highest percentages were recorded in spring 1998 and 1999. However, in the first year the percentage of OFs increased steadily through the seasons, whereas in the second year, this percentage increased in summer and autumn, declined in winter, and increased again in spring ().
Figure 6. Nematopalaemon schmitti. Variation in the percentage of OF in relation to mature females obtained, variation in the percentage of juveniles in relation to the individuals sampled in the population and, seasonal mean temperature of bottom water in the study region (BT, water bottom temperature; OF, ovigerous female; J, juveniles; Sum, summer; Aut, autumn; Win, winter; and Spr, spring).
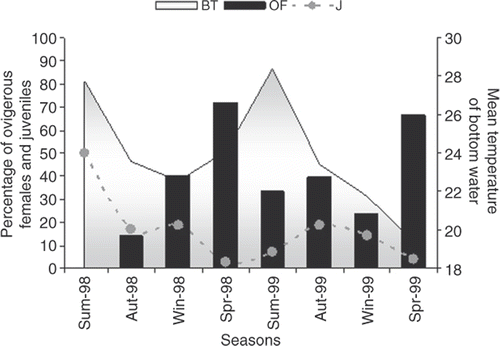
The estimated recruitment of juveniles for N. schmitti showed no obvious pattern. Young shrimps were caught during the entire sampling period. The highest percentages of young individuals were recorded in summer 1998 and in autumn and winter in both years (). However, in summer 1998 very few specimens were analyzed, only two young specimens of the total of four individuals were obtained; consequently, this value is not sufficiently representative to characterize the population in this period.
The seasonal mean temperature of the bottom water ranged from 19.4 ± 1.9°C to 28.4 ± 0.4°C. No relationship of bottom-water temperature with the monthly variation in OFs and juveniles was observed (Spearman, p > 0.05; ). Also, there was no significant relationship between the monthly variation in OFs and the monthly variation of juveniles (Spearman, p > 0.05).
Discussion
The LFD of N. schmitti can be considered relatively stable in view of the presence of both young and adult individuals during almost the whole study period. The bimodality identified in June 1999 for males, and in July 1998 and 1999 and June 1999 for females suggested different age groups present in the population, probably related to the pulses of recruitment and of reproductive adults. Similar results were obtained by Enin et al. (Citation1996) for the caridean shrimp N. hastatus (Aurivillius 1898), a congener of N. schmitti, and by Castilho et al. (Citation2007a) for the penaeid shrimp A. longinaris. According to Díaz and Conde (Citation1989), the unimodality in a population generally reflects continuous recruitment, without interruption of classes and with constant mortality rates, while the bimodality or polymodality reflects differential mortality or catastrophic and/or a differential behavior. The pulses of recruitment observed for N. schmitti can be considered as a differential behavior, suggesting the arrival of juveniles from recruitment areas.
The sexual dimorphism in carapace size observed for N. schmitti, with females reaching larger sizes than males, concords with observations from studies of other species of caridean shrimps, for example Palaemonetes pugio Holthuis 1949 by Bauer and Abdalla (Citation2001); Potimirim glabra (Kingsley 1878); P. potimirim (Müller 1881) by Lima et al. (Citation2006); A. armillatus by Mossolin et al. (Citation2006); and Palaemon (Palaeander) northropi Rankin 1898 by Pralon and Negreiros-Fransozo (Citation2006). This pattern has also been confirmed for the penaeid shrimps (Bauer Citation1991), including Rimapenaeus constrictus (Stimpson 1874), X. kroyeri, Trachysalambria curvirostris (Stimpson 1860), A. longinaris, P. muelleri, and Sicyonia dorsalis Kingsley 1878 (Costa and Fransozo, Citation2004; Castro et al. Citation2005; Castilho et al. Citation2007a, Citation2008a, Citation2008b; Yamada et al. Citation2007). According to Bauer (Citation2004) and Yamada et al. (Citation2007), the larger size attained by the female is related to the egg-producing capacity, i.e., the larger the female, the more eggs she will produce.
In contrast, in some species of caridean shrimps the males are larger than the females, as observed by Galvão and Bueno (Citation2000), Mossolin and Bueno (Citation2002), and Fransozo et al. (Citation2004) for Atya scabra (Leach 1815), Macrobrachium olfersi (Wiegmann 1836), and M. iheringi (Ortmann 1897). Correa and Thiel (Citation2003) and Bauer (Citation2004) attributed this characteristic to the sexual system exhibited by some species: in these cases, the male needs to attract the female or even separate her from another male, in order to assure successful mating and reproduction. Under these circumstances, the populations are characterized by low density, and the males show a relatively high level of aggressiveness as well as a dimorphism in the ornamentation of the cheliped (Bauer Citation2004). These characteristics can be observed in Macrobrachium rosenbergii (De Man 1879) and Rhynchocinetes typus Milne Edwards, 1837, studied, respectively, by Ra’anan and Sagi (Citation1985) and Correa et al. (Citation2000).
In this study, male-to-female ratio differed from 1 : 1; in some situations the females were more abundant than the males, and vice versa. Deviations from 1 : 1 in the sex ratio of marine crustaceans are apparently common (Wenner Citation1972). According to Fisher (Citation1958), natural selection favors the 1 : 1 proportion, but certain factors can cause an imbalance in this expected sex ratio, among them longevity, sex reversal, and differences between the sexes in migration and mortality rates (Wenner Citation1972), and also types of mating and sexual selection (Willson and Pianka Citation1963). For N. schmitti, the differences in the proportion of sexes in favor of the females are probably related to the reproductive aspects and to the growth rate of the females because they reach sexual maturity at larger sizes than the males, in addition to being more abundant in the larger size classes, probably resulting in the differences of growth rate between males and females. Hypotheses about the predominance of females in a population have been suggested for some species of caridean shrimps. Kim (Citation2005) attributed the greater abundance of females in a population of Palaemon gravieri (Yu 1930) to the high mortality of the males soon after mating. Ramirez-Llodra et al. (Citation2007) established that the higher proportion of females found in a population of Pasiphaea multidentata Esmark, 1866 was related to the differences in habitat preferences between the sexes. Wenner (Citation1979) suggested that the observed sex ratio with a predominance of females in the larger size classes in species of the genus Nematocarcinus A. Milne Edwards, 1881 could be explained by the greater longevity of the females.
The reproductive period is regarded as the interval of time in which the females of a given population become ovigerous. Peaks in the frequency of OFs occur, associated with variations in factors such as temperature, light, and food availability (Sastry Citation1983). The highest percentages of OFs, mainly in the spring, were probably associated with larger amounts of available food, since in this period the South Atlantic Central Water (SACW) intrudes along the northern São Paulo coast. This water mass is the main source of nutrient transport into the study region, and has an nitrogen and phosphorus (N : P) ratio of approximately 16 : 1, which favors primary productivity (Odebrecht and Castello Citation2001). According to Aidar et al. (Citation1993), during the spring and summer, the ocean chlorophyll-a content (phytoplankton production) is generally higher because of the penetration of the SACW over the inner continental shelf. The penetration of this water mass into the lower layer of the coastal zone enriches the water of the euphotic zone with nutrients, leading to an increase of photoplankton primary productivity. In turn, this primary production supports a larger biomass of herbivorous zooplankton and consequently creates better conditions for the survival of larvae of benthic animals (Pires-Vanin and Matsuura Citation1993).
The SACW is characterized by temperatures lower than 18°C and salinities less than 36 (Castro-Filho et al. Citation1987), and it can penetrate coastal regions to depths of 10–15 m (Pires-Vanin and Matsuura Citation1993). The transect sampled for this study was located at the limit of extension of this water mass, and because the area is exposed, the surface and bottom waters mix more readily. As a result, the mean bottom temperatures during summer were 27.8 ± 0.3°C in 1998 and 28.4 ± 0.4°C in 1999. Although the hydrodynamic conditions on the sampling transect did not show the influence of the SACW, it is notable that Bertini et al. (Citation2001) and Costa et al. (Citation2005) observed the intrusion of this water mass over other transects in the same region and during the same period as this study.
In spite of the lack of correlation between the water temperature and the percentage of OFs, this environmental factor is presumably a considerable stimulus for the ovarian development of the females of N. schmitti. According to Bauer (Citation1992), seasonal variations in water temperature and/or other environmental variables have been considered important proximate factors or environmental stimuli acting on the physiological system of marine invertebrates, for example on gametogenesis, in this way defining the reproductive period of these invertebrates.
The higher percentages of juveniles recorded in autumn and winter are responses to the higher proportions of OFs observed in the spring. Moreover, the decrease observed in abundance of N. schmitti in spring (October–December) and the larger decrease in summer (January–March; ) may indicate that these shrimps perform difficult seasonal migration and capture of juvenile and adult specimens. Seasonal migrations of caridean shrimps have been mentioned by some authors (Spaargaren Citation2000; Temming and Damm Citation2002; Siegel et al. Citation2005; Henderson et al. Citation2006; Bilgin et al. Citation2008; Janas and Spicer Citation2008), who point to environmental variables (temperature and salinity) as factors in habitat selection by juveniles and adults. Additionally, the absence of individuals smaller than 6.8 mm CL may be related to the size of the net mesh utilized during the samples. The nets used were probably the most appropriate tools to capture adults but not necessarily for the sampling of juveniles. Thus, the design of fishing net would need to be considered to gain better understanding of recruitment patterns of N. schmitti.
The presence of juveniles and OFs during the entire sampling period suggests that N. schmitti has a pattern of continuous reproduction in the study region. According to Bauer (Citation1992), the relatively constant temperature conditions in tropical regions are the cause of continuous reproduction in a variety of marine invertebrates. This same pattern was recorded by Bauer (Citation1989) for nine species of caridean shrimps in the Northern Hemisphere, by Silva and Silva (Citation1989) for Caridina fernandoi Arud. and Costa, 1962; by Mossolin and Bueno (Citation2002) for M. Olfersi; by Galvão and Bueno (Citation2000) for A. scabra; and by Braga (Citation2006) for E. oplophoroides. For penaeid shrimps, continuous reproduction was also observed by Bauer and Rivera Vega (Citation1992) for Sicyonia parri (Burkenroad 1934) and Sicyonia laevigata Stimpson, 1871; by Costa and Fransozo (Citation2004) for R. Constrictus; by Castilho et al. (2007a) for A. longinaris; and by Castilho et al. (Citation2008a, 2008b) for P. muelleri and S. dorsalis, respectively.
In general, the results of this study demonstrate that the aspects of population biology observed for N. schmitti on the northern coast of the state of São Paulo can be characterized by the migration of the population with regard to the use of different regions during its life cycle. Nevertheless, future investigations focusing on characteristics such as natality, growth, mortality, ecology, and larval development, macro- and microscopic analysis of the gonad development in order to determine sexual maturity, fecundity, and better fishing gear for sampling young individuals will provide essential information for understanding the population dynamics of N. schmitti, providing useful information the development of strategies for the maintenance and preservation of this species in the study region.
Acknowledgements
The authors are grateful to the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for providing financial support (Grant nos. 94/4878–8, 97/12108–6, 97/12106–3, 97/12107–0, and 98/3134–6). The authors thank the NEBECC co-workers for their help during the fieldwork and Dr Janet W. Reid (Virginia Museum of Natural History, Virginia) for her great help with English language. All sampling in this study have been conducted in compliance with current applicable state and federal laws.
References
- Aidar , E , Gaeta , SA , Gianesenella-Galvão , SMF , Kutner , MBB and Teixeira , C . 1993 . Ecossistema costeiro subtropical: nutrientes dissolvidos, fitoplâncton e clorofila-a e suas relações com as condições oceanográficas na região de Ubatuba, SP . Publicação Especial do Instituto Oceanográfico , 10 : 9 – 43 .
- Almeida AC. 2008. Biologia e ecologia do camarão barriga branca Nematopalaemon schmitti (Holthuis 1950) (Crustacea, Caridea, Palaemonidae) na região de Ubatuba, litoral norte do Estado de São Paulo [Dissertação de Mestrado]. [Botucatu, São Paulo, (Brasil)]: Universidade Estadual Paulista.
- Bauer , RT . 1989 . Continuous reproduction and episodic recruitment in nine shrimp species inhabiting a tropical seagrass meadow . Journal of Experimental Marine Biology and Ecology , 127 : 177 – 187 .
- Bauer , RT . 1991 . “ Sperm transfer and storage structures in penaeoid shrimps ” . In Crustacean sexual biology , Edited by: Bauer , RT and Martin , JW . 183 – 207 . New York : Columbia University Press. .
- Bauer , RT . 1992 . Testing generalizations about latitudinal variation in reproduction end recruitment patterns with sicyoniid and caridean shrimp species . Invertebrate Reproduction and Development , 22 ( 1–3 ) : 193 – 202 .
- Bauer , RT . 2002 . Reproductive ecology of a protandric simultaneous hermaphrodite, the shrimp Lysmata wurdemanni (Decapoda: Caridea: Hippolytidae) . Journal of Crustacean Biology , 22 ( 4 ) : 742 – 749 .
- Bauer , RT . 2004 . Remarkable shrimps: adaptations and natural history of the Carideans , Norman : University of Oklahoma Press .
- Bauer , RT and Abdalla , JH . 2001 . Male mating tactics in the shrimp Palaemonetes pugio (Decapoda, Caridea): precopulatory mate guarding vs. pure searching . Ethology , 107 : 185 – 199 .
- Bauer , RT and Rivera Vega , LW . 1992 . Pattern of reproduction and recruitment in two sicyoniid shrimp species (Deapoda: Penaeoidea) from a tropical seagrass habitat . Journal of Experimental Marine Biology and Ecology , 161 : 223 – 240 .
- Benetti , AS , Negreiros-Fransozo , ML and Costa , TM . 2007 . Population and reproductive biology of the crab Uca burgersi (Crustcaea: Ocypodidae) in three subtropical mangrove forest . Revista de Biología Tropical , 55 ( 1 ) : 55 – 70 .
- Bertini , G , Fransozo , A and Costa , RC . 2001 . Ecological distribution of three species of Persephona (Brachyura: Leucosiidae) in the Ubatuba region, São Paulo, Brazil . Nauplius , 9 ( 1 ) : 31 – 42 .
- Bhattacharya , CG . 1967 . A simple method of resolution of a distribution into Gaussian components . Biometrics , 23 : 115 – 135 .
- Bilgin , S , Ozen , O and Ates , AS . 2008 . Spatial and temporal variation of Palaemon adspersus, Palaemon elegans, and Crangon crangon (Decapoda: Caridea) in the southern Black Sea . Estuarine, Coastal and Shelf Science , 79 : 671 – 678 .
- Bilgin , S , Ozen , O and Samsun , O . 2009 . Sexual seasonal growth variation and reproduction of the rock pool prawn, Palaemon elegans (Decapoda: Palaemonidae) in the southern Black Sea . Scientia Marina , 73 ( 2 ) : 239 – 247 .
- Braga , ACA . 2006 . Biologia e Ecologia do camarão-espinho Exhippolysmata oplophoroides (Holthuis, 1948) (Caridea, Alpheoidea, Hippolytidae) na Região de Ubatuba, Litoral Norte Paulista , Tese de Doutorado. Botucatu, São Paulo, Brasil : Universidade Estadual Paulista .
- Braga , AA , López-Greco , LS , Santos , DC and Fransozo , A . 2009 . Morphological evidence for protandric simultaneous hermaphroditism in the caridean Exhippolysmata oplophoroides . Journal of Crustacean Biology , 29 ( 1 ) : 34 – 41 .
- Castilho , AL , Costa , RC , Fransozo , A and Boschi , EE . 2007a . Reproductive pattern of the South American endemic shrimp Artemesia longinaris (Decapoda: Penaeoidea) off São Paulo State, Brazil . Revista de Biología Tropical , 55 ( 1 ) : 39 – 48 .
- Castilho , AL , Fransozo , A and Costa , RC . 2008a . Pattern of reproduction and recruitment of the South American red shrimp (Pleoticus muelleri), off the subtropical coast of Brazil . Marine Biology Research , 4 : 361 – 368 .
- Castilho , AL , Furlan , M , Costa , RC and Fransozo , V . 2008b . Reproductive biology of the rock shrimp Sicyonia dorsalis (Decapoda: Penaeoidea) from the southeastern coast of Brazil . Invertebrate, Reproduction and Development , 52 ( 1–2 ) : 59 – 68 .
- Castilho , AL , Gavio , MA , Costa , RC , Boschi , EE , Bauer , RT and Fransozo , A . 2007b . Latitudinal variation in population structure and reproductive pattern of the endemic South America shrimp Artemesia longinaris (Decapoda: Penaeoidea) . Journal of Crustacean Biology , 27 ( 4 ) : 548 – 552 .
- Castro , RH , Costa , RC , Fransozo , A and Mantelatto , FLM . 2005 . Population structure of the seabob shrimp Xiphopenaeus kroyeri (Heller 1862) (Crustacea: Penaeoidea) in the littoral of São Paulo, Brazil . Scientia Marina , 69 ( 1 ) : 105 – 112 .
- Castro-Filho , BM , Miranda , LB and Myao , SY . 1987 . Condições hidrográficas na plataforma continental ao largo de Ubatuba: variações sazonais e em média escala . Boletim do Instituto Oceanográfico , 35 ( 2 ) : 135 – 151 .
- Chacur , MM and Negreiros-Fransozo , ML . 1998 . Aspectos biológicos do camarão-espinho Exhippolysmata oplophoroides (Holthuis 1948) (Crustacea, Caridea, Hippolytidae) . Revista Brasileira de Biologia , 59 ( 1 ) : 173 – 181 .
- Chilari , A , Thessalou-Legaki , M and Petrakis , G . 2005 . Population structure and reproduction of the deep-water shrimp Plesionika martia (Decapoda: Pandalidae) from de eastern Ionian Sea . Journal of Crustacean Biology , 25 ( 2 ) : 233 – 241 .
- Correa , C , Baeza , JA , Dupré , E , Hinojosa , IA and Thiel , M . 2000 . Mating behavior and fertilization success of three ontogenetic stages of male rock shrimp Rhynchocinetes typus (Decapoda: Caridea) . Journal of Crustacean Biology , 20 ( 4 ) : 628 – 640 .
- Correa , C and Thiel , M . 2003 . Mating systems in caridean shrimp (Decapoda: Caridea) and their evolutionary consequences for sexual dimorphism and reproductive biology . Revista Chilena de Historia Natural , 76 : 187 – 203 .
- Costa , RC and Fransozo , A . 2004 . Reproductive biology of the shrimp Rimapenaeus constrictus (Decapoda, Penaeidae) in the Ubatuba region of Brazil . Journal of Crustacean Biology , 24 ( 2 ) : 274 – 281 .
- Costa , RC , Fransozo , A , Castilho , AL and Freire , FAM . 2005 . Annual, seasonal and spatial variation of abundance of the shrimp Artemesia longinaris (Decapoda, Penaeoidea) in south-eastern Brazil . Journal of the Marine Biological Association of the United Kingdom , 85 : 107 – 112 .
- Costa , TM and Negreiros-Fransozo , ML . 2003 . Population biology of Uca thayeri Rathibun, 1900 (Brachyura, Ocypodidae) in a subtropical South American mangrove area: results from transect and catch-per-unit-effort techniques . Crustaceana , 75 ( 10 ) : 1201 – 1218 .
- Crocos , PJ and van der Velde , TD . 1995 . Seasonal, spatial and interannual variability in the reproduction dynamics of the grooved tiger prawn Penaeus semisulcatus in Albatross Bay, Gulf of Carpentaria, Australia: the concept of effective spawning . Marine Biology , 122 : 557 – 570 .
- Díaz , H and Conde , JE . 1989 . Population dynamics and life history of the mangrove crab Aratus pisonii (Brachyura, Grapsidae) in a marine environment . Bulletin of Marine Science , 45 ( 1 ) : 148 – 163 .
- Doi , W , Yokota , M , Strüssmann , CA and Watanabe , S . 2008 . Growth and reproduction of the portunid crab Charybdis bimaculata (Decapoda: Brachyura) in Tokyo Bay . Journal of Crustacean Biology , 28 ( 4 ) : 641 – 651 .
- Enin , UI , Lowenberg , U and Kunzel , T . 1996 . Population dynamics of the estuarine prawn (Nematopaleomon hastatus Aurivillius 1898) off the southeast coast of Nigeria . Fisheries Research , 26 : 17 – 35 .
- Fernandes-Goés , LC , Fransozo , A and Góes , JM . 2005 . Population dynamics of Dardanus insignis (Saussure, 1858) (Crustacea, Anomura, Diogenidae) in the Ubatuba region, São Paulo, Brazil . Nauplius , 13 ( 2 ) : 191 – 196 .
- Fisher , RA . 1958 . The genetical theory of natural selection, , 2nd , New York : Dover .
- Fransozo , A and Bertini , G . 2001 . Population structure and breeding period of Pachycheles monilifer (Dana) (Anomura, Porcellanidae) inhabiting sabellariid sand reefs from the littoral coast of São Paulo, State, Brazil . Revista Brasileira de Zoologia , 18 ( 1 ) : 197 – 203 .
- Fransozo , V , Castilho , AL , Freire , FAM , Furlan , M , Almeida , AC , Teixeira , GM and Baeza , JA . 2009 . Spatial and temporal distribution of the shrimp Nematopalaemon schmitti (Decapoda, Caridea, Palaemonidae) at a subtropical enclosed bay in South America . Journal of the Marine Biological Association of the United Kingdom , 89 ( 8 ) : 1581 – 1587 .
- Fransozo , V , Costa , RC , Bertini , G and Cobo , VJ . 2005 . Population biology of spine shrimp Exhippolysmata oplophoroides (Holthuis) (Caridea, Hippolytidae) in a subtropical region, São Paulo, Brazil . Revista Brasileira de Zoologia , 22 ( 4 ) : 1078 – 1084 .
- Fransozo , A and Mantelatto , FLM . 1998 . Population structure and reproductive period of the tropical hermit crab Calcinus tibicen (Decapoda: Diogenidae) in the region of Ubatuba, São Paulo, Brazil . Journal of Crustacean Biology , 18 ( 4 ) : 738 – 745 .
- Fransozo , A , Rodrigues , FD , Freire , FAM and Costa , RC . 2004 . Reproductive biology of the freshwater prawn Macrobrachium iheringi (Ortmann 1897) (Decapoda: Caridea: Palaemonidae) in the Botucatu region, São Paulo, Brazil . Nauplius , 12 ( 2 ) : 119 – 126 .
- Galvão , R and Bueno , SLS . 2000 . Population structure and reproductive biology of the Camacuto shrimp, Atya scabra (Decapoda, Caridea, Atyidae), from São Sebastião, Brazil . Crustacea Issues , 12 : 291 – 299 .
- Henderson , PA , Seaby , RM and Somes , JR . 2006 . A 25-year study of climatic and density-dependent population regulation of common shrimp Crangon crangon (Crustacea: Caridea) in the Bristol Channel . Journal of the Marine Biological Association of the United Kingdom , 86 : 287 – 298 .
- Janas , U and Spicer , JI . 2008 . Does the effect of low temperature on osmoregulation by the prawn Palaemon elegans Rathke, 1837 explain winter migration offshore? . Marine Biology , 153 : 937 – 943 .
- Kim , S . 2005 . Population structure, growth, mortality, and size at sexual maturity of Palaemon gravieri (Decapoda: Caridea: Palaemonidae) . Journal of Crustacean Biology , 25 ( 2 ) : 226 – 232 .
- Lima , GV , Silveira , CM and Oshiro , LMY . 2006 . Estrutura populacional dos camarões simpátricos Potimirim glabra e Potimirim potimirim (Crustacea, Decapoda, Atyidae) no rio Sahy, Rio de Janeiro, Brasil . Iheringia , 96 ( 1 ) : 81 – 87 .
- Litulo , C . 2006 . Population and reproductive biology of the fiddler crab Uca chlorophthalmus (Brachyura: Ocypodidae) from Inhaca Island, southern Mozambique . Journal of the Marine Biological Association of the United Kingdom , 86 : 737 – 742 .
- Mantelatto , FLM , Fransozo , A and Negreiros-Fransozo , ML . 1995 . Population structure of Hepatus pudibundus (Decapoda: Calappidae) in Fortaleza Bay, Brazil . Revista de Biología Tropical , 43 ( 1–3 ) : 265 – 270 .
- Martinelli , JM , Mantelatto , FLM and Fransozo , A . 2002 . Population structure and breeding season of the South Atlantic hermit crab, Loxopagurus loxochelis (Anomura, Diogenidae) from the Ubatuba Region, Brazil . Crustaceana , 75 ( 6 ) : 791 – 802 .
- Mossolin , EC and Bueno , SLS . 2002 . Reproductive biology of Macrobrachium olfersi (Decapoda, Palaemonidae) in São Sebastião, Brazil . Journal of Crustacean Biology , 22 ( 2 ) : 367 – 376 .
- Mossolin , EC , Shimizu , RM and Bueno , SLS . 2006 . Population structure of Alpheus armillatus (Decapoda, Alpheidae) in São Sebastião and Ilha Bela, southeastern Brazil . Journal of Crustacean Biology , 26 ( 1 ) : 48 – 54 .
- Nakagaki , JM and Negreiros-Fransozo , ML . 1998 . Population biology of Xiphopenaeus kroyeri (Heller 1862) (Decapoda: Penaeidae) from Ubatuba Bay, São Paulo Brazil . Journal of Shellfish Research , 17 ( 4 ) : 931 – 935 .
- Negreiros-Fransozo , ML , Gonzáles-Gordillo , JI and Fransozo , A . 2002 . First larval stage of Exhippolysmata oplophoroides (Holthuis 1948) (Decapoda, Caridea, Hippolytidae) obtained in laboratory . Nauplius , 10 ( 1 ) : 67 – 71 .
- Negreiros-Fransozo , ML , Mantelatto , FLM and Fransozo , A . 1999 . Population biology of Callinectes ornatus Ordway, 1863 (Decapoda, Portunidae) from Ubatuba (SP), Brazil . Scientia Marina , 63 ( 2 ) : 157 – 163 .
- Odebrecht , C and Castello , JP . 2001 . The convergence ecosystem in the Southwest Atlantic . Ecological Studies , 144 : 147 – 165 .
- Oh , CW , Hartnoll , RG and Nash , RDM . 1999 . Population dynamics of the common shrimp, Crangon crangon (L.), in Port Erin Bay, Isle of Man, Irish Sea . Journal of Marine Science , 56 : 718 – 733 .
- Pires-Vanin , AMS and Matsuura , Y . 1993 . Estrutura e função do ecossistema de plataforma continental da região de Ubatuba, Estado de São Paulo: uma introdução . Boletim do Instituto Oceanográfico , 10 : 1 – 8 .
- Pralon , BGN and Negreiros-Fransozo , ML . 2006 . Population biology of Palaemon (Palaeander) northropi Rankin, 1898 (Crustacea, Decapoda, Palaemonidae) in a tropical South American estuary . Acta Limnologica Brasiliensia , 18 ( 1 ) : 77 – 87 .
- Ra’anan , Z and Sagi , A . 1985 . Alternative mating strategies in male morphotypes of the freshwater prawn Macrobrachium rosenbergii (De Man) . Biological Bulletin , 169 : 592 – 601 .
- Ramirez-Llodra , E , Company , JB , Camps , M and Rotllant , G . 2007 . Spatio-temporal variations in reproductive patterns and population structure of Pasiphaea multidentata (Decapoda: Caridea) in the Blanes canyon and adjacent margin, North-western Mediterranean Sea . Marine Ecology , 28 : 470 – 479 .
- Ramos-Porto , M and Coelho , PA . 1998 . “ Malacostraca. Eucarida. Caridea (Alpheoidea excluded) ” . In Catalogue of crustacea of Brazil , Edited by: Young , PS . 325 – 350 . Rio de Janeiro : Museu Nacional. .
- Ricklefs , RE and Miller , GL . 1999 . Ecology, , 4th , New York : WH. Freeman .
- Sastry , AN . 1983 . “ Ecological aspects of reproduction ” . In The biology of the Crustacea environmental adaptations , Edited by: Vernberg , FJ and Vernberg , WB . Vol. 8 , 179 – 270 . New York : Academic Press .
- Siegel , V , Gröger , J , Neudecker , T , Damm , U and Jansen , S . 2005 . Long-term variation in the abundance of the brown shrimp Crangon crangon (L.) population of the German Bight and possible causes for its interannual variability . Fisheries Oceanography , 14 ( 1 ) : 1 – 16 .
- Silva , PK and Silva , KHGM . 1989 . Aspects of the population ecology of a tropical freshwater atyid shrimp Caridina fernandoi Arud. and Costa, 1962 (Crustacea: Decapoda: Caridea) . Archiv für Hydrobiologie , 117 ( 2 ) : 237 – 253 .
- Sokal , RR and Rohlf , FJ . 1995 . Biometry, , 3rd , New York : WH. Freeman .
- Spaargaren , DH . 2000 . Seasonal and annual variations in the catches of Crangon crangon (L., 1758) (Decapoda, Natantia) near the coast of Texel, the Netherlands . Crustaceana , 73 ( 5 ) : 547 – 563 .
- Sparre , P and Venema , SC . 1998 . Introduction to tropical fish stock assessment. Part 1. Manual. , Rome : FAO . FAO Fisheries Technical Paper No. 306/1, Rev. 2
- Teixeira , GM , Fransozo , V , Cobo , VJ and Hiyodo , CM . 2009 . Population features of the spider crab Acanthonyx scutiformis (Dana 1851) (Crustacea, Majoidea, Epialtidae) associated with rocky-shore algae from southeastern Brazil . Pan-American Journal of Aquatic Sciences , 4 ( 1 ) : 87 – 95 .
- Temming , A and Damm , U . 2002 . Life cycle of Crangon crangon in the North Sea: a simulation of timing of recruitment as a function of the seasonal temperature signal . Fisheries Oceanography , 11 ( 1 ) : 45 – 58 .
- Viegas , I , Martinho , F , Neto , J and Pardal , N . 2007 . Population dynamics, distribution and secondary production of the brown shrimp Crangon crangon (L.) in a southern European estuary. Latitudinal variations . Scientia Marina , 71 ( 3 ) : 451 – 460 .
- Wenner , AM . 1972 . Sex ratio as a function of size in marine Crustacea . The American Naturalist , 106 ( 949 ) : 321 – 350 .
- Wenner , EL . 1979 . Distribution and reproduction of nematocarcinid shrimp (Decapoda: Caridea) from the northwestern North Atlantic . Bulletin of Marine Science , 29 ( 3 ) : 380 – 393 .
- Willson , MF and Pianka , ER . 1963 . Sexual selection, sex ratio and mating system . The American Naturalist , 97 : 405 – 407 .
- Yamada , R , Kodama , K , Yamakawa , T , Horiguchi , T and Auki , I . 2007 . Growth and reproductive biology of the small penaeid shrimp Trachysalambria curvirostris in Tokyo Bay . Marine Biology , 151 : 961 – 971 .