Abstract
The morphological development and the sequence of organogenesis from glochidium to the early juvenile stage of the freshwater pearl mussel, Hyriopsis bialatus, were observed. Larvae of H. bialatus were cultured in standard tissue culture medium (M199) supplemented with common carp (Cyprinus carpio) plasma and they showed transformation within 10 days. Larval samples were collected every 2 days during glochidia development and subjected to histological processing. Three types of cell masses were developed during this period: the ventral plate (the foot rudiment), lateral pits (the gill rudiment), and the oral plate or endodermic sac (the origin of the digestive tract). The ventral plate gave rise to two foot lobes which fused into a single lobe. The gills were developed from the lateral pits next to the ventral plate, forming a pair of gill buds that became elongated and turned into gill bars. The digestive tract began with the formation of mouth by invagination of the oral plate (or endodermic sac) and formation of a tube underneath the growing foot. Several controversial aspects of organogenesis have been inferred, e.g., de novo formation of the anterior and posterior juvenile adductors, the fate of the mushroom body structure, and foot lobe formation from two separate precursor lobes. A mushroom body protruded into the mantle cavity and remained there throughout the transformation period. Moreover, the evidence of a supporting band (mucoid structure) in the mature glochidium of H. bialatus has never been reported in other freshwater mussel species, and its function and composition need to be further investigated.
Introduction
Hyriopsis bialatus Simpson, 1900 (order Unionoida, family Unionidae) is a freshwater pearl mussel, widely distributed in the bottom of the reservoirs and rivers in Thailand, Laos, and Cambodia (Brandt Citation1974; Kovitvadhi and Kovitvadhi Citation2002). The nacreous mussel shell can be used for making pearl-inlaid furniture, as well as for making ornaments, kitchen utensils, and souvenirs. The shell can also be used for human bone implantation (nacreous implantation; Lopez et al. Citation1994; Liao et al. Citation2002), and the meat is a source of protein for humans and animals. Mussels are filter feeders, siphoning nutrients, and suspended particles from the water column while antioxidant (or biotransformation) enzymes in their digestive gland detoxify substances in water. These activities contribute to maintaining a clean ecosystem (Birmelin et al. Citation1999) and make mussels useful bioindicators for toxicity testing, especially during their juvenile stages (Keller and Zam Citation1990; Keller et al. Citation1998). The life cycle of freshwater pearl mussels is quite complex. Fertilized eggs develop into larvae (or glochidia) within marsupia. There are major morphological changes of the demibranch of the mature female to accommodate the large number of these larvae. When glochidia are released from the female gills, they come into contact with a passing fish and attach themselves to the gills, fins, or body of the fish. After a few days to several weeks, glochidia leave the host, drift to the bottom substratum, and begin their lives as juvenile mussels. It may take several years before juveniles become reproductively mature (Pennak Citation1989).
Juvenile freshwater mussels have been successfully cultured in the laboratory by attaching glochidia to fish until they reach the juvenile stage (Fukuhara et al. Citation1990; Panha Citation1992; Buddensiek Citation1995; Kovitvadhi et al. Citation2003; Hanlon and Neves Citation2006). It is also possible to use sterilized artificial media for culturing glochidia (to bypass the parasitic stage; Isom and Hudson Citation1984; Keller and Zam Citation1990; Kovitvadhi et al. Citation2001b, Citation2002; Fisher and Dimock Citation2002a, Citation2002b). According to Kovitvadhi et al. (Citation2002, Citation2006), Areekijseree et al. (Citation2006) and Supannapong et al. (Citation2008), the survival and transformation percentages of glochidia Hyriopsis myersiana and H. bialatus were increased when the artificial medium contained a mixture of M199, fish plasma (common carp, Cyprinus carpio), and antibiotics/antimycotic. The percentage of survival of glochidia was >93% (97% of surviving glochidia transformed to juveniles). The duration of transformation was approximately 10 days. Furthermore, H. bialatus possesses the advantageous property of rapid reproduction and can spawn year-round (Jindamongkol et al. Citation2003). For these reasons, the species H. bialatus and culturing glochidia in artificial media for mass production were used for this histological study.
Although several studies have been carried out on the morphology of glochidia, juvenile, and adult freshwater mussels, as well as the morphological development of juveniles through adults (Kovitvadhi et al. Citation2001a, Citation2007; Araujo et al. Citation2002; Fisher and Dimock Citation2002a, Citation2002b), only a few studies have focused on the sequences of organogenesis from glochidium to the early juvenile stage. This is the most crucial and vulnerable stage of their lives. Blystad (Citation1923) and Arey (Citation1932a, Citation1932b) proposed three consecutive stages of metamorphosis of hooked Pyganodon corpulenta (=grandis) (Say) and hookless Lampsilis luteola (=siliquoidea) (Lamarck) glochidia reared on a fish host: the encystment stage (when the larva is attached to the fish); the mushroom body stage (during which the larva receives nourishment from its host through absorption); and the final stage, which includes the organogenesis of juveniles. Araujo et al. (Citation2002) studied the metamorphosis of Margaritifera auricularia glochidia in the gills of a fish host (Acipenser baeri), and reported that organogenesis of the glochidia including foot, rudiments of the digestive glands, gills, and the two adductor muscles was observed. Fisher and Dimock (Citation2002a, Citation2002b) suggested that metamorphosis of the hooked glochidium Utterbackia imbecillis cultured in artificial medium is composed of two stages. The first stage occurs within the first 3–4 days; this consists of larval adductor muscle degeneration and formation of the mushroom body by the larval mantle cells. The second stage occurs during the 4 days before transformation into juveniles. During this stage the stomach, intestine, digestive gland, foot, gill bars, nerve cords, and other juvenile structures are formed. However, some aspects of metamorphosis have received less attention, namely the gill and foot formation.
The aim of this study was to investigate the histological structure of organogenesis in glochidia to the juvenile stage of the freshwater pearl mussel H. bialatus. This knowledge will contribute significantly to a better understanding of larval development and lead to the further potential application of enhancing the transformation of glochidia into juveniles while simultaneously increasing their survival rate during settlement.
Materials and methods
Glochidia culture in artificial media
Preparation of glochidia
Adult H. bialatus were collected from the Mun River basin in Si Sa Ket province, Thailand. These individuals had an average weight of 37.51 ± 6.49 g, width of 1.88 ± 0.20 m, length of 8.28 ± 2.80 cm, and height of 6.28 ± 0.30 cm. The mussels were sexed by microscopic observation of sperm and ova in fluid removed by suction from gonads. They were cultured in a circular net (45 cm in diameter and 30 cm in height) in an earthen pond at the Department of Aquaculture, Faculty of Fisheries, Kasetsart University, Bangkok, Thailand. The mussels were fed with natural food in the pond. To prepare the mussels for collection of glochidia, soil and algae were thoroughly removed from the outside shells of gravid mussels with dechlorinated tap water. Only gravid mussels with completely brown marsupia were selected for glochidia culture (Kovitvadhi et al. Citation2002).
Glochidia culture
The culture method for H. bialatus larvae was modified from that of Kovitvadhi et al. (Citation2002). About 50–100 mature glochidia were cultured in tissue culture dishes (60 × 15 mm) containing 3.5 mL of an artificial medium composed of Medium 199 (Gibco, No. 6231100-035), fish plasma (common carp, C. carpio), and antibiotics/antimycotic (100 µg/mL carbenicillin, 100 µg/mL gentamycin sulfate, 100 µg/mL rifampin, and 5 µg/mL amphotericin B) in a ratio of 2:1:0.5, respectively.
All culture dishes were placed in plastic boxes inside an incubator with a constant supply of 5% CO2 and ambient humidity. The internal temperature in the box was kept at 25°C. The culture medium was removed and replaced with fresh medium on day 5. Finally, 1 mL of sterilized distilled water was added to the culture dish on day 9 to stimulate the transformation of glochidia into juveniles.
Preparation of glochidia samples for light microscopic observations
Samples were collected on days 0, 2, 4, 6, 8, and 10 of glochidia development. They were fixed in 10% neutral buffered formalin for 2 h, and stored in 5% neutral buffered formalin for further processing. Fixed samples were placed in a decalcifying solution (5% EDTA) for 3 h to soften the tissues. The specimens were thoroughly washed under running water for 30 min, and placed in 10% ethanol. Paraffin embedding was performed by dehydrating the specimen using a series of ethanol at 10%, 30%, 50%, and 70% for 10 min each, twice in 95% ethanol for 10 min, and twice in absolute ethanol for 15 min. Clearing was done twice with xylene for 5 min, while infiltration was done twice in molten paraffin (Paraplast) for 15 min at 60°C in a hot air oven. Finally, the samples were embedded in molten Paraplast. Tissue blocks were cut at 5–6 µm thickness using a rotary microtome. Sections were attached onto glass slides and stained with Harris hematoxylin and eosin. The specimens were examined under a light microscope. The subsequent serial sections were stained with Masson's trichrome for collagen fiber and muscular fiber, and periodic acid Schiff-hematoxylin (PAS-H) for neutral mucin and chitin.
Results
Morphological development of glochidia to early juvenile stage
The glochidia of H. bialatus were completely transformed within 10 days, with a survival rate of 97.5 ± 2.1%. All surviving larvae transformed into the juvenile stage. During the transformation process, the larvae retained their semi-oval shape and did not significantly change in size until the last day of transformation. There were, however, several distinctive changes in the principal developing structures leading to foot formation, gill bars, digestive tract, anterior and posterior juvenile adductors, and mantle. Rudimentary organs such as the pericardium, kidney, heart, and nervous ganglia were not observed immediately following transformation.
Mature glochidia (day 0 glochidia)
Mature glochidia were collected from the outer demibranch of gravid mussels. The glochidia were semi-ovoid in shape and contained an equivalved shell that completely enclosed the body. These two valves were equilateral and hookless type. The rim of glochidia valves, particularly in the ventral region, showed different arrangements of numerous spines or microscopic teeth which were eosinophilic stained. The average size of these valves (n = 50) was 190 ± 0.02 µm in length, 230 ± 0.02 µm in height, and 61 ± 0.86 µm in width (). The valves were joined by a straight hinge and had a muscle bundle called a “larval adductor” extending transversely between the valves (). The larval adductor consisted of several smooth muscle cells; each cell was spindle-shaped with an elongated concentric nucleus. All the soft parts of the glochidium were enclosed within the larval shell. There were two layers of larval mantle cells lining the internal glochidial shell surface (). The outer layer was very thin and inconspicuous. It adhered directly to the shell by connective tissue. The inner layer, on the other hand, was composed of low columnar cells containing concentric nuclei and numerous eosinophilic granules in the cytoplasm. Between the inner shell surface and the outer layer of the larval mantle, a thick conspicuous band of mucopolysaccharide was found as a supporting band which was positively stained with PAS and Masson's trichrome (). The mantle cavity was found between the inner mantle cells of each valve (). The mantle contained three types of cell masses: the ventral plate, the lateral pits, and the oral plate (endodermic sac). These cell masses were further developed into foot rudiment, gill buds, and digestive tract rudiment, respectively.
Figure 1. Mature glochidia of H. bialatus at day 0 showing living specimen (a), cross-sections (b, d, f, g, and h), and sagittal sections (c, e, and i). Ci, cilia; ELT, external larval thread; H, hinge; ILT, internal larval thread; IMC, inner mantle cells; LAM, larval adductor muscle; LP, lateral pits; LS, larval shell; MC, mantle cavity; N, nucleus; OMC, outer mantle cells; OP, oral plate; SB, supporting band; SHC, sensory hair cell; SP, spines; TG, thread gland; and VP, ventral plate. Bars = 25 µm.
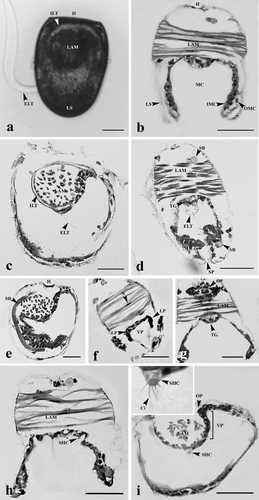
Table 1. Comparison of glochidial dimensions of the hookless Unionids.
The ventral plate was found at the posterior end, adjacent to the adductor muscle (). It was characterized by 1–2 layers of cuboidal cells that were darkly basophilic stained. The plate was invaginated to become a pair of grooves known as lateral pits (). The oral plate was near the shell hinge (). These irregular-shaped cell masses were formed by a loose aggregation of cells.
A few sensory hair cells containing several cilia were found on the larval mantle (). They were somewhat rounded cells containing concentric nuclei, and also had several cilia on their free ends.
An external thread and a thread gland were found in the central region of the ventral plate (). In addition, an internal thread was found located around the larval adductor muscle (). Both external and internal threads were non-cellular structures. They were stained magenta with PAS and stained blue with Masson's trichrome. The thread gland, on the other hand, consisted of a few polyhedral-shaped cells ().
Development of digestive tract
The proximal digestive tract (mouth) was formed at day 2 by invagination of the oral plate (). At day 4, the digestive tract was extended and the layers of the wall of the tract became thicker along the hinge line (). At day 6, the epithelium of the digestive tract contained numerous cilia stained pink with eosin (). At day 8, the stomach, digestive gland, style sac, and intestine became apparent (). At day 10 (early juvenile stage), the digestive system was completely developed showing the mouth opening into a ciliated esophagus, leading to the stomach, style sac, intestine, and terminating at the rectum (). However, a crystalline style was not observed at this stage ().
Figure 2. Glochidia of H. bialatus at day 2 showing cross-sections (a, b, d, and e), and sagittal section (c). Ci, cilia; DMC, degenerating muscle cells; DT, digestive tract; FL, foot lobe; IMC, inner mantle cells; LAM, larval adductor muscle; LP, lateral pits; OP, oral plate; SB, supporting band; Sp, spines; and VP, ventral plate. Bars = 25 µm.
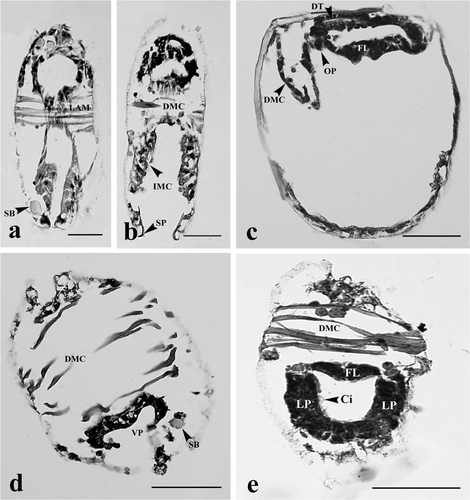
Figure 3. Glochidia of H. bialatus at day 4 showing sagittal section (a), and cross-sections (b, c, and d). DMC, degenerating muscle cells; DT, digestive tract; FL, foot lobe; GB, gill bud; H, hinge; IMC, inner mantle cells; M, mouth; OMC, outer mantle cells; R, rectum; and Sp, spines. Bars = 25 µm.
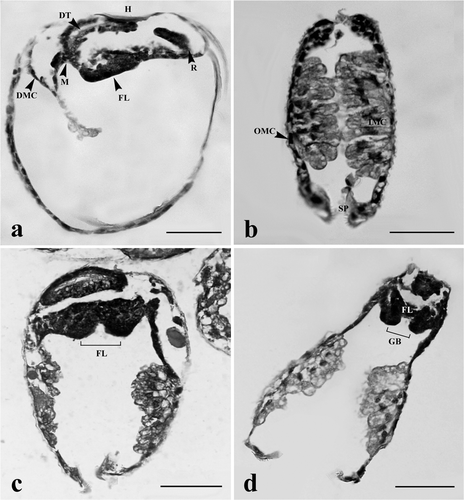
Figure 4. Glochidia of H. bialatus at day 6 showing sagittal sections (a and b), and cross-section (c and d). AJA, anterior juvenile adductor; DT, digestive tract; F, foot; GBa, gill bar; IMC, inner mantle cells; MB, mushroom body; PJA, posterior juvenile adductor; and Sp, spines. Bars = 25 µm.
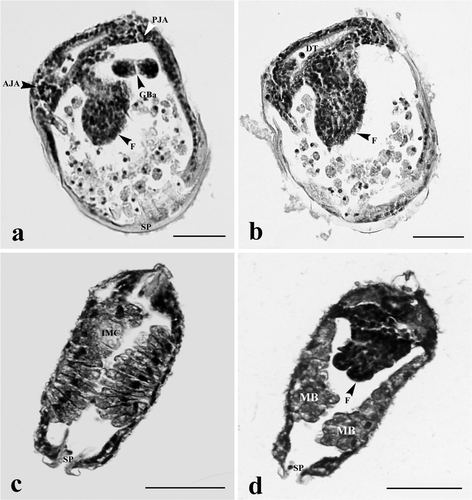
Figure 5. Glochidia of H. bialatus at day 8 showing sagittal sections (a and b) and cross-sections (c–h). AJA, anterior juvenile adductor; DG, digestive gland; F, foot; GBa, gill bar; I, intestine; MB, mushroom body; PJA, posterior juvenile adductor; S, stomach; Sp, spines; and SS, style sac. Bars = 25 µm.
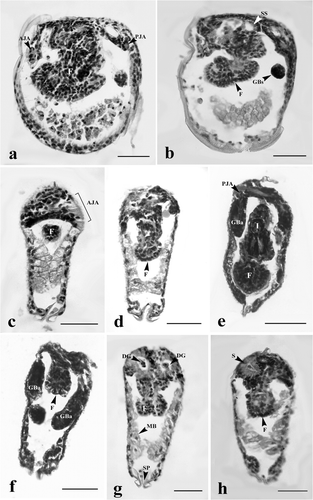
Figure 6. Early juvenile stages of H. bialatus showing sagittal section (a), and cross-sections (b–e). AJA, anterior juvenile adductor; Ci, cilia; DM, definitive mantle; F, foot; GBa, gill bar; I, intestine; MCD, mushroom body cells debris; PJA, posterior juvenile adductor; Sp, spines; and SS, style sac. Bars = 25 µm.
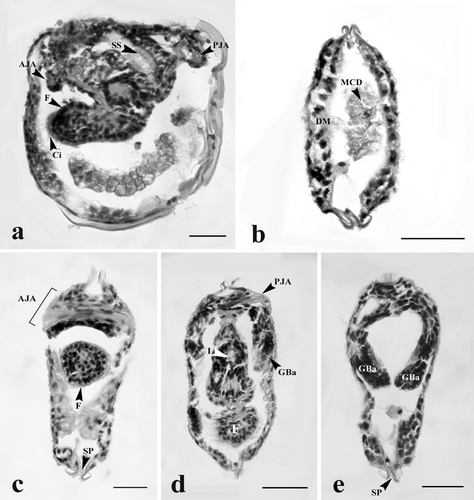
Table 2. Morphological development of H. bialatus glochidia cultured in artificial media.
Development of adductor muscle
The larval adductor muscle began to degenerate during the first 2 days of glochidium development, as seen from the deterioration of muscle cells and the remnants which still remained attached to the larval mantle layer (). The larval adductor muscle completely disintegrated within 4 days, causing the mantle cavity to become larger in size (). The muscle bundle at this point migrated to the anterior end and degenerated ().
The anterior and posterior adductor muscles began to develop within 6 days. They were comprised of densely aggregated smooth muscle cells, and were located at the corners of the hinge line. The anterior adductor muscle was near the foot, whereas the posterior adductor muscle was close to the gill bud or gill bar (). Between 8 and 10 days after development, the anterior adductor muscle was larger than the posterior one ( and ). The muscle cells were spindle-shaped and had concentric nuclei. These muscle cells stained red with Masson's trichrome, and they were surrounded by groups of cells which were darkly stained with hematoxylin.
Development of gills
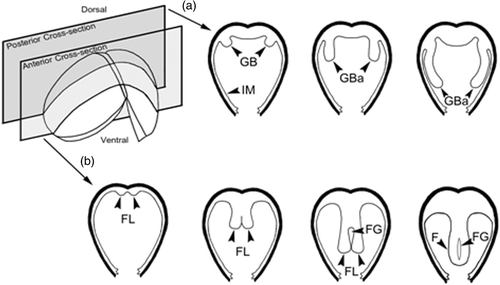
The lateral pits were composed of several cell layers. The cells that lined the mantle cavity had numerous cilia (). Gill buds began to form, consisting of darkly hematoxylin-stained cells (). The boundary of these cells was not clearly identified. They protruded into the mantle cavity on the dorso-posterior shell within 2 days of glochidial development, and became distinctive gill buds at day 4 (). At day 6, gill buds were larger in size and continued to elongate to form gill bars. The cell boundaries were distinct. These cells were cuboidal in shape and lined parallel to both sides of the foot (). At days 8–10, gill bars were extended toward the mid-body and located next to the mantle ( and ), close to the posterior adductor muscle ( and ). From histological observations, this study has produced a general outline of gill development from glochidium through the early juvenile stage ().
Development of foot
At day 2, the cells near the ventral plate region were highly proliferated and became elongated. These cells were darkly basophilic stained (). At day 4, the mid-region of the ventral plate was invaginated to form two lobes known as “foot lobes” (). The foot lobes continued to develop and extended into the mantle cavity, located between a pair of gill bars. At day 6, the foot lobes became larger and fused to form a single foot (). Meanwhile, the mushroom body flattened out near the shell (, , and ). From day 8 onward, the foot became distinct, and was located in the middle of two gill bars (, , and ). The distal foot was covered with stratified ciliated cuboidal epithelium ( and ). Inside the foot region, some part of the intestine was observed (). Histological observations in this study have produced a general outline of foot development from glochidium through the early juvenile stage ().
Development of mantle
At days 2–4, the inner larval mantle cells gradually hypertrophied and protruded into the mantle cavity ( and ). These cells were grouped together and appeared as a mushroom body when viewed from the frontal section at the end of day 4 (). The larval mantle cells were clearly seen at day 6. These cells were club-shaped, and extended into the mantle cavity. Their nuclei were found near the base of the cell (). At days 8–10, the inner larval mantle cells started to change their shape from club-shaped to low columnar with condensed nuclei ( and ). Some mushroom body cell debris was also found in the mantle cavity ().
Discussion
Reports on glochidial development, especially histological observations from glochidia to the juvenile stage, are rarely found (Araujo et al. Citation2002; Fisher and Dimock Citation2002a, 2002b). Our use of H. bialatus cultured in vitro in artificial medium, therefore, provides sufficient samples to follow with ease the transformation of glochidia into juveniles, in the same manner as Fisher and Dimock (Citation2002a) has done on the metamorphosis of U. imbecillis glochidia in a modified cell culture medium.
Shape and size of mature glochidia
Two types of unionoidean glochidia, one hookless and another with hooked shells, have been distinguished (Jeong et al. Citation1992; Panha and Eongprakornkeaw Citation1995; Hoggarth Citation1999). The general appearance of the glochidium of H. bialatus resembles other unionid larvae, but is most similar to H. myersiana (Kovitvadhi et al. Citation2001a). Both species have semi-oval and equilateral-valved shells. During transformation, these two species remain in a semi-oval shape until they are completely transformed. The size of H. bialatus is somewhat smaller than that of H. myersiana, but quite different compared to other unionids ().
Attachment organs
Two types of attachment organ have been found in H. bialatus glochidia: numerous spines (or microscopic teeth) and two larval threads (internal and external threads). These attachment organs play an important role in host attachment (Wood Citation1974). However, the precise function of the internal thread is unknown; it may support and protect the internal organs of glochidia. The microscopic teeth along the shell margin play a significant role in the interpretation of relationships among unionid species (Panha and Eongprakornkeaw Citation1995; Jupiter and Byrne Citation1997; Hoggarth Citation1999). As seen in H. myersiana, the rim of the glochidial valves of H. bialatus is lined with numerous spines (Kovitvadhi et al. Citation2001a). During transformation, these small spines cover the ventral larval shell margin until the early juvenile stage. The external larval thread of the mature glochidia of H. bialatus rising from the thread gland is up to 2 mm long, sticky, and pliable, and can attach itself to rough surface organs such as the fin or gill of a host fish (Wood Citation1974). The internal larval thread is located around the larval adductor muscle. These threads consist of collagen fibers, neutral mucopolysaccharide, or mucin, as seen from the positive staining with Masson's trichrome and PAS. This supports the observations of Wood (Citation1974) on the glochidia of Anodonta cygnea stained only with PAS. Transmission electron microscopy observation of Anodonta arcaeformis, as reported by Jeong et al. (Citation1992), confirmed that the larval thread is non-cellular and not bounded by a membrane, while the matrix of the larval thread does not contain any cellular organelles other than numerous microtubule-like structures. It was speculated that these structures were related to the movement of the larval thread (Lodish et al. Citation1995). Although there was no larval thread observed inside the glochidia of M. auricularia, fine thread-like structures or “hair” were occasionally found between the eggs in the conglutinate (Araujo and Ramos Citation1998).
Organogenesis
To prepare for the free-living conditions at the juvenile stage, glochidia begin to form essential structures such as a mantle, foot, gill bar, and digestive tract. In the glochidia of H. bialatus, the rudiments of all organs arise as temporary aggregations of cells. The adductor muscle and the ciliated organs (ventral plate and lateral pits) are the most conspicuous cell masses (Araujo and Ramos Citation1998). Other rudimentary organs such as the pericardium, kidney, heart, or gonad have not been observed in the glochidia or at the early juvenile stages of H. bialatus, H. myersiana, U. imbecillis, Margaritifera margaritifera, and M. auricularia (Araujo and Ramos Citation1998; Kovitvadhi et al. Citation2001a; Fisher and Dimock Citation2002a).
Similar to the glochidia of M. auricularia, no respiratory organs have developed in H. bialatus immediately following transformation, so the cilia of the lateral pits and ventral plate are probably used to aerate the larva (Araujo and Ramos Citation1998). The ventral plate and lateral pits are the origins of the foot and gills, as also described by Lillie (Citation1895). Autoradiographic data on U. imbecillis has indicated that these structures are the areas of high-rate RNA, DNA, and protein synthesis during transformation (Fisher and Dimock Citation2002a). These active cell masses of H. bialatus were darkly basophilic stained because they contained numerous basophilic molecules such as DNA, RNA, and proteins.
Gill formation
There are two gill buds (left and right) in the developing glochidia of H. bialatus, similar to those of H. myersiana as reported by Kovitvadhi et al. (Citation2001a, Citation2007). These two gill buds could not fully function for filter feeding at this stage. As pedal-mantle feeders, these glochidia have numerous cilia covering the juvenile foot and gill bars, as well as the mantle, and can move them very quickly and vigorously to transfer food into the mantle cavity (Kovitvadhi et al. Citation2001a, 2007; Fisher and Dimock Citation2002a).
Foot formation
Foot formation requires different periods of time, depending on the freshwater mussel species. Both H. bialatus and H. myersiana take 8–10 days (Kovitvadhi et al. Citation2001a), while U. imbecillis takes only 6–7 days to form the juvenile foot (Fisher and Dimock Citation2002a). Scanning electron microscopy observation of A. cygnea, as reported by Lima et al. (Citation2006), confirmed that the foot lobes fuse to form a bifurcated structure until completely joined as a definitive foot.
Digestive tract formation
As the larval adductor muscle slides into the anterior part, the continuous tube of the digestive tract is formed underneath the growing foot, where numerous eosinophilic granules are found. These yolk granules might be the source of nutrition for glochidial development, especially of the foot and digestive tract. The complete digestive system of H. bialatus is formed within 10 days, while the glochidial digestive tract of U. imbecillis is completely formed within only 6–7 days in a culture medium (Fisher and Dimock Citation2002a).
Mantle development
The presence of two layers of mantle cells has been reported in several species including P. corpulenta (=grandis), L. luteola (=siliquoidea; Blystad Citation1923; Arey Citation1932b), M. auricularia (Araujo and Ramos Citation1998; Araujo et al. Citation2002), U. imbecillis (Fisher and Dimock Citation2002a), and M. margaritifera (Linnaeus) (Karna and Millemann Citation1978).
It is interesting to note the occurrence of a supporting band located between the outer mantle epithelium and the larval shell. This structure is a neutral and structural mucopolysaccharide resembling the chitin for constructing the new young shell of juvenile as a fundamental framework which will support other further organic and mineral structures to be deposited (Machado et al. Citation1988a, Citation1988b, Citation1990). The growth of new juvenile shell probably begins from inside between the outer mantle epithelium and the original larval shell.
In H. bialatus, the larval mantle cells were found to form the mushroom body, as also seen in the glochidia of U. imbecillis, and persist until the end of metamorphosis (Fisher and Dimock Citation2002a, Citation2002b). Arey (Citation1932b) also described this structure in both L. luteola (=siliquoidea) and P. corpulenta (=grandis), suggesting that the mushroom body persists after the end of parasitism in the hookless glochidia, whereas it disappears toward the end of parasitism in the hooked glochidia.
The mushroom body is not necessary for free-living juveniles because it only facilitates the glochidial acquisition of nutrients from the mussels’ host (Blystad Citation1923; Arey Citation1932b). However, the fate of larval mantle cells in freshwater mussels during metamorphosis is still inconclusive. It is not clear whether the cells are digested by the animal itself or they are sloughed off during the final transition to juvenile. However, this study found that some parts on the free end of the mushroom body of H. bialatus were seen as debris sloughing off in the mantle cavity. Arey (Citation1932b) suggested that the decrease in the height of the larval mantles of L. luteola (=siliquoidea) and P. corpulenta (=grandis) during metamorphosis was possibly caused by the pressure from the growing foot and independent shortening of the mantle itself. It seems that the processes which decrease the larval mantle in H. bialatus are similar to those described by Arey (Citation1932b), in addition to pressure from the gill bars exerted onto the mantle.
Adductor muscle development
The adductor of H. bialatus glochidia in artificial culture medium and in the host fish was found to contract consistently while the valves remained closed until the juvenile was completely formed. This was also seen in U. imbecillis (Fisher and Dimock Citation2002a). Histochemical and actin-specific staining of U. imbecillis showed the complete degradation of the larval adductor muscle during the first few days of metamorphosis. This phenomenon was also observed by Zsolnai-Nagy and Lábos (Citation1969), indicating that the larval and adult adductor muscles of A. cygnea were two distinctly different structures. The adult adductor muscles were initially very small, and presumably enlarged as development continued. In contrast, Fukuhara et al. (Citation1990) suggested that the adult anterior adductor muscle in Anodonta woodiana was derived in part from the larval muscle, which was quite different from the reports of other research groups.
Nervous ganglia and sensory hair cells
Like the glochidia of M. auricularia and M. margaritifera, nervous ganglia in the glochidia of H. bialatus are not present. The contractile response is, therefore, transmitted by the sensory hair tufts or hair cells (Pekkarinen and Valovirta Citation1996). The ultrastructures of A. arcaeformis show two types of sensory hair cells in the larval mantle (Jeong et al. Citation1992), with one type consisting of three solitarily positioned and highly specialized cells in the mantle. Each cell possesses a bunch of protruding hairs. This type presumably perceives chemical stimuli (Wood Citation1974). The function of the other type of hair cells located in the posterior margin near the lateral pits is still unknown. This second type of hair cell was found to be variable in number and has been used for taxonomic classification in some species. For example, there are two pairs in both M. margaritifera and M. auricularia instead of the four pairs found in most other Unionoid glochidia (Lefevre and Curtis Citation1912; Pekkarinen and Englund Citation1995; Pekkarinen and Valovirta Citation1996). Unfortunately, in the genus Hyriopsis, the number and distribution of this type of hair cell in individual specimens of H. bialatus and H. myersiana are quite varied (Kovitvadhi et al. Citation2002); it is not, therefore, a good indicator for taxonomic classification of this genus.
The present observation found that during the transformation from glochidia to early juvenile stage the sensory hair cells, larval thread, thread gland, and larval adductor degenerated, whereas the shell and numerous spines persisted. Simultaneously, the juvenile adductor, foot, gill, and digestive tract developed.
Acknowledgements
We would like to thank the Department of Aquaculture, Faculty of Fisheries, Kasetsart University, for providing a pond for culturing mussels. We are also grateful to Mr Adrian Robert Hillman from the Graduate School, Kasetsart University, for his revision of our manuscript.
References
- Araujo , R , Cámara , N and Ramos , MA . 2002 . Glochidium metamorphosis in the endangered freshwater mussel Margaritifera auricularia (Spengler 1793): a histological and scanning electron microscopy study . Journal of Morphology , 254 : 259 – 265 .
- Araujo , R and Ramos , MA . 1998 . Description of the glochidium of Margaritifera auricularia (Spengler 1793) (Bivalvia, Unionoidea) . Philosophical Transactions of the Royal Society of London B , 353 : 1553 – 1559 .
- Areekijseree , M , Engkagul , A , Kovitvadhi , S , Kovitvadhi , U , Thongpan , A and Rungruangsak-Torrissen , K . 2006 . Development of digestive enzymes and in vitro digestibility of different species of phytoplankton for culture of early juveniles of the freshwater pearl mussel, Hyriopsis bialatus Simpson, 1900 . Invertebrate Reproduction and Development , 49 : 255 – 262 .
- Arey , LB . 1932a . The formation and structure of the glochidial cyst . Biological Bulletin , 62 : 212 – 221 .
- Arey , LB . 1932b . The nutrition of glochidia during metamorphosis. A microscopical study of the sources and manner of utilization of nutritive substances . Journal of Morphology , 53 : 201 – 221 .
- Birmelin , C , Pipe , RK , Goldfarb , PS and Livingstone , DR . 1999 . Primary cell-culture of the digestive gland of the marine mussel Mytilus edulis: a time-course study of antioxidant- and biotransformation enzyme activity and ultrastructure changes . Marine Biology , 135 : 65 – 75 .
- Blystad , CN . 1923 . Significance of larval mantle of fresh-water mussels during parasitism, with notes on a new mantle condition exhibited by Lampsilis luteola . Bulletin of the United States Bureau of Fisheries , 39 : 203 – 219 .
- Brandt , RAM . 1974 . The non-marine aquatic mollusca of Thailand . Archiv für Molluskenkunde , 105 : 1 – 423 .
- Buddensiek , V . 1995 . The culture of juvenile freshwater pearl mussels Margaritifera margaritifera L. in cages: a contribution to conservation programmes and the knowledge of habitat requirements . Biology Conservation , 74 : 33 – 40 .
- Fisher , GR and Dimock , RV . 2002a . Morphological and molecular changes during metamorphosis of Utterbackia imbecillis (Bivalvia: Unionidae) . Journal of Molluscan Studies , 68 : 159 – 164 .
- Fisher , GR and Dimock , RV . 2002b . Ultrastructure of the mushroom body: digestion during metamorphosis of Utterbackia imbecillis (Bivalvia: Unionidae) . Invertebrate Biology , 121 : 126 – 135 .
- Fukuhara , S , Nakai , I and Nagata , Y . 1990 . Development of larvae of Anodonta woodiana (Bivalvia) parasitized on host fish . Venus , 49 : 54 – 61 .
- Hanlon , SD and Neves , RJ . 2006 . Seasonal growth and mortality of juveniles of Lampsilis fasciola (Bivalvia: Unionidae) released to a fish hatchery raceway . American Malacological Bulletin , 21 : 45 – 49 .
- Hoggarth , MA . 1999 . Description of some of the glochidia of the Unionidae (Mollusca: Bivalvia) . Malacologia , 41 : 180 – 298 .
- Isom BG, Hudson RG. 1984. Culture of freshwater mussel glochidia in an artificial habitat utilizing complex liquid growth media. US Patent 4,449,480; p. 18.
- Jeong , KH , Min , BJ and Chung , PR . 1992 . An anatomical and ultrastructural study of the glochidium of Anodonta arcaeformis . Malacological Review , 25 : 71 – 79 .
- Jindamongkol , T , Kovitvadhi , U , Kovitvadhi , S , Thongpan , A and Chatchavalvanich , K . 2003 . Duration and frequency of glochidia development of freshwater pearl mussel, Hyriopsis bialatus Simpson, 1900 . Proceedings of the Annual Conference . 2003 , Bangkok, Thailand. pp. 171 – 178 [in Thai] . Kasetsart University, Fisheries Section .
- Jupiter , SD and Byrne , M . 1997 . Light and scanning electron microscopy of the embryos and glochidia larvae of the Australian freshwater bivalve Hyridella depressa (Hyriidae) . Invertebrate Reproduction and Development , 32 : 177 – 186 .
- Karna , DW and Millemann , RE . 1978 . Glochidiosis of salmonid fishes. III. Comparative susceptibility to natural infection with Margaritifera margaritifera (L.) (Pelecypoda: Margaritanidae) and associated histopathology . Journal of Parasitology , 64 : 528 – 537 .
- Keller , AE , Ruesslerand , DS and Chaffee , CM . 1998 . Testing the toxicity of sediments contaminated with diesel fuel using glochidia and juvenile mussels (Bivalvia, Unionidae) . Aquatic Ecosystem Health and Management , 1 : 37 – 47 .
- Keller , AE and Zam , SG . 1990 . Simplification of in vitro culture techniques for freshwater mussels . Environmental Toxicology and Chemistry , 9 : 1291 – 1296 .
- Kovitvadhi , U , Chatchavalvanich , K , Noparatnaraporn , N and Machado , J . 2001a . Scanning electron microscopy of glochidia and juveniles of the freshwater mussel, Hyriopsis myersiana . Invertebrate Reproduction and Development , 40 : 143 – 151 .
- Kovitvadhi U, Kovitvadhi S. 2002. Collection and identification of freshwater amblemid mussels in the Mun River Basin for culture of glochidia in artificial media. Report for National Research Council of Thailand; p. 155 [in Thai].
- Kovitvadhi , S , Kovitvadhi , U , Sawangwong , P and Machaado , J . 2007 . Morphological development of the juvenile through to the adult in the freshwater pearl mussel, Hyriopsis (Limnoscapha) myersiana, under artificial culture . Invertebrate Reproduction and Development , 50 : 207 – 218 .
- Kovitvadhi , S , Kovitvadhi , U , Sawangwong , P , Thongpan , A and Machado , J . 2006 . Optimization of diet and culture environment for larvae and juvenile freshwater pearl mussels, Hyriopsis (Limnoscapha) myersiana Lea, 1856 . Invertebrate Reproduction and Development , 49 : 61 – 70 .
- Kovitvadhi , U , Noparatnaraporn , N and Machado , J . 2001b . Culture of glochidia of the freshwater pearl mussel Hyriopsis myersiana (Lea 1856) in artificial media . Aquaculture , 195 : 61 – 69 .
- Kovitvadhi , U , Pakkong , P , Noparatnaraporn , N , Vilarinho , L and Machado , J . 2002 . Studies of a suitable fish plasma for in vitro culture of glochidia Hyriopsis myersiana (Lea 1856) . Aquaculture , 209 : 197 – 208 .
- Kovitvadhi , U , Pakkong , P , Noparatnaraporn , N , Vilarinho , L and Machado , J . 2003 . Studies on the plasma composition of fish hosts of the freshwater mussel, Hyriopsis myersiana, with implications for improvement of the medium for culture of glochidia . Invertebrate Reproduction and Development , 44 : 53 – 61 .
- Lefevre , G and Curtis , WC . 1912 . Studies on the reproduction and artificial propagation of fresh-water mussels . Bulletin of the United States Bureau of Fisheries , 30 : 105 – 201 .
- Liao , H , Mutvei , H , Hammarstrom , L , Wurtz , T and Li , J . 2002 . Tissue responses to nacreous implants in rat femur: an in situ hybridization and histochemical study . Biomaterials , 23 : 2693 – 2701 .
- Lillie , FR . 1895 . The embryology of the Unionidae: a study in cell-lineage . Journal of Morphology , 10 : 1 – 100 .
- Lima , P , Kovitvadhi , U , Kovitvadhi , S and Machado , J . 2006 . In vitro culture of glochidia from the freshwater mussel Anodonta cygnea . Invertebrate Biology , 125 : 34 – 44 .
- Lodish , H , Baltimore , D , Berk , A , Zipursky , SL , Matsudaira , P and Darnell , J . 1995 . Molecular cell biology, , 3rd , 1344 New York : Scientific American Press .
- Lopez , E , Berland , S and Faou , AL . 1994 . Mother of pearl can repair human skeletons . La Recherché , 25 : 208 – 210 .
- Machado , J , Castilho , F , Coimbra , J , Monteiro , E , Sá , C and Reis , M . 1988a . Ultrastructural and cytochemical studies in the mantle of Anodonta cygnea . Tissue and Cell , 20 : 797 – 807 .
- Machado , J , Coimbra , J , Castilho , F and Sá , C . 1990 . Effects of diflubenzuron on shell formation of the freshwater clam, Anodonta cygnea . Archives of Environmental Contamination and Toxicology , 19 : 35 – 39 .
- Machado , J , Coimbra , J , Sá , C and Cardoso , I . 1988b . Shell thickening in Anodonta cygnea by induced acidosis . Comparative Biochemistry Physiolology , 91 A : 645 – 651 .
- Panha , S . 1992 . Infection experiment of glochidium of a freshwater pearl mussel Hyriopsis (Limnoscapha) myersiana (Lea, 1856) . Venus , 51 : 303 – 314 .
- Panha , S and Eongprakornkeaw , A . 1995 . Glochidium shell morphology of Thai amblemid mussels . Venus , 54 : 225 – 236 .
- Pekkarinen , M and Englund , VPM . 1995 . Description of unionacean glochidia in Finland, with a table aiding in their identification . Archiv für Hydrobiologie , 134 : 515 – 531 .
- Pekkarinen , M and Valovirta , I . 1996 . Anatomy of the glochidia of the freshwater pearl mussel, Margaritifera margaritifera (L.) . Archiv für Hydrobiologie , 137 : 411 – 423 .
- Pennak , RW . 1989 . Fresh-water invertebrate of the United States: protozoa to Mollusca, , 3rd , 628 New York (NY) : John Wiley & Sons Inc. .
- Supannapong , P , Pimsalee , T , A-komol , T , Engkagul , A , Kovitvadhi , U , Kovitvadhi , S and Rungruangsak-Torrissen , K . 2008 . Digestive enzymes and in vitro digestibility of different species of phytoplankton for culture of the freshwater pearl mussel, Hyriopsis bialatus . Aquaculture International , 16 : 437 – 453 .
- Wood , EM . 1974 . Development and morphology of the glochidium larva of Anodonta cygnea (Mollusca: Bivalvia) . Journal of Zoology (London) , 173 : 1 – 13 .
- Zsolnai-Nagy , I and Lábos , E . 1969 . Light and electron microscopical investigations on the adductor muscle and nervous elements in the larva of Anodonta cygnea L . Annales Biologie Tihany , 36 : 123 – 133 .