Abstract
This article provides a description of the early juvenile stages of Armases rubripes and indicates some differences and additional details found in the megalopa and first juvenile crab stage compared with previous descriptions. The main characters that permit identification of the subsequent juvenile stages were assessed, as well as the moult interval and increment during successive moults and the beginning of development of the secondary sexual features. Megalopae of A. rubripes were collected from the Ubatuba region, São Paulo, Brazil using plankton and neuston nets. Morphological changes in body parts have been recorded for each stage and sex, as well as the duration of the intermoult period was noted. Possible effects of laboratory holding time on growth were checked by means of analysis of covariance. The importance of standardization of the terminology of setae is emphasized.
Introduction
The identification of the species of juvenile brachyurans is often difficult (Martin et al. Citation1984) and has been an impediment to ecological research on their functional importance in estuarine habitats (O’Connor Citation1990). Papers that focus, therefore, on an accurate description of the juveniles of distinct species would permit ecological studies on these often-neglected stages.
According to Hebling et al. (Citation1982), most authors who have raised larvae did not go beyond than the megalopa stage due to rearing difficulties and low survival of juvenile crabs in the laboratory. The available information on post-larval ontogeny of crabs, therefore, is much reduced compared to that on larval development (Guerao and Rotllant Citation2009).
The most detailed literature available on several post-larval stages of crabs are concerned with the acquisition of adult characters, juvenile growth and sexual differentiation (Shen Citation1935; Payen Citation1974; Ingle Citation1977; Hebling et al. Citation1982; Felder et al. Citation1985; Fransozo Citation1987; Fransozo and Negreiros-Fransozo Citation1987; Rieger and Nakagawa Citation1995; Flores et al. Citation1998; Rieger and Beltrão Citation2000; Flores et al. Citation2002; Guimarães and Negreiros-Fransozo Citation2005; Negreiros-Fransozo et al. Citation2007; Bolla Jr et al. Citation2008).
Guerao and Rotllant (Citation2009) emphasized that whilst morphological characteristics of early juveniles have not usually been applied in taxonomic studies of crabs, a description of the early instars is necessary to assist in identification of juvenile stages of crabs collected in field studies (Ingle and Rice Citation1984; Guimarães and Negreiros-Fransozo Citation2005). Felder et al. (Citation1985) have demonstrated the importance of a better knowledge on the first post-larval stages, as it can help in the evaluation of body size, morphology, moulting frequency, growth rate, feeding behaviour and even the elucidation of the phylogeny of some groups of Decapoda, when it is associated with information related to larvae and adults.
Many larval stages of Sesarmidae have been described from American waters over the past 50 years (Costlow Jr and Bookhout Citation1960, Citation1962; Díaz and Ewald Citation1968; Fransozo and Hebling Citation1986; Schubart and Cuesta Citation1998; Cuesta and Anger Citation2001; Cuesta et al. Citation2006), but papers that focus the morphological description of first juvenile stages, as for example Fransozo (1986, 1987), Rieger and Nakagawa (Citation1995), Luppi and Spivak (Citation2007), and Guerao et al. (Citation2007), are still scarce.
Hartnoll (Citation1982) reported the growth of animals that possess a hard exoskeleton, such as crustaceans, as an essentially discontinuous process. There are successive moults separated by intermoult periods and almost all the growth occurs immediately after ecdysis before the hardening of the new tegument. During the intermoult, a little growth may occur due to the flexibility of the membranes that join the sclerites which link the hard exoskeleton.
The moulting frequency and the size increase per moult may be influenced independently by changes in environmental conditions. Thus, to best determine growth rates, both the intermoult duration and the growth over that period need to be measured (Cadman and Weinstein Citation1988).
The present lack of information makes larval studies necessary and important, as they provide a sounder basis for systematic classification and phylogenetics (Guimarães and Negreiros-Fransozo Citation2005), as well as the identification of megalopae and juveniles obtained directly from the field.
The aim of this study is to describe the early juvenile development of Armases rubripes. The main characters that permit the identification of the early juvenile stages are described, as well as the moult interval and the increment during successive moults and the beginning of the external secondary sexual differentiation. This study also provides some additional details on the megalopa and first juvenile description of A. rubripes, as a complement to the original paper by Díaz and Ewald (Citation1968). Using the information obtained about the morphologic characters, it is possible to compare the A. rubripes megalopa and juvenile with other species, from both plankton and benthos.
Materials and methods
Samples were obtained during October, November and December 2005 and February and April 2006. Megalopae were collected using plankton and neuston nets at Itamambuca mangrove (23°24′S; 45°03′W) and at Ubatuba Bay (23°25′S; 45°03′W), both at Ubatuba, São Paulo, Brazil. The collected material was placed in a bucket containing previously aerated seawater. The sampled material was examined and megalopae were removed using a disposable pipette. Subsequently, each megalopa was transferred to a clean acrylic vial containing about 60 mL of aerated seawater, and the megalopae were taken to the laboratory for culture. They were held separately in numbered plastic vials containing seawater at a salinity of 15 at 25°C. Each larva received about 30 nauplii of Artemia sp, daily for feeding. Individuals were daily checked for moult, and the water of the vials was changed every 2 days. The intermoult period was recorded. The exuviae obtained in the process were preserved in correctly identified bottles, containing 80% ethanol and pure glycerin, maintaining 1:1 proportion. Similarly, the dead specimens were identified and preserved in 80% alcohol. For reference, the biological material of A. rubripes is stored in NEBECC larval collection, (numbers: 0141–0170), Zoology Department, Biosciences Institute, Universidade Estadual Paulista, Botucatu, State of São Paulo, Brazil.
After rearing, 130 individuals obtained in the juvenile stages (about 10 stages), only 100 specimens were identified as being A. rubripes according to Melo (Citation1996), and also confirmed as such by G.S. Melo from the Museum of Zoology of São Paulo University (MZUSP). The most important diagnostic features for this species are: carapace narrowed posteriorly; mesogastric and cardiac regions well marked; lateral parts obliquely striated; surface densely punctuated; irregular anteriorly and with short lines of granules; front approximately two-thirds of the carapace width (CW); external angle of the orbit, obtuse and short lacking antero-lateral teeth; chelipeds with the superior face irregular, except the teeth; flat pereopods, being the propodus of the second pereopod wider and more setose than the remainder (Melo Citation1996).
The morphology of megalopa and first juvenile of A. rubripes was examined and changes in anatomy were recorded for each subsequent one by obtained stage and sex. Mortality occurred progressively as the juvenile development proceeded with only 10 crabs reaching the eleventh stage.
About 30 exuviae of megalopae were dissected using sharpened needles under a Zeiss (SV6) stereomicroscope. After this procedure, the appendages were placed on a slide covered with glycerin and the cover was sealed with clear nail varnish. This type of preparation is semi-permanent, and lasts for a number of years.
Using a Zeiss compound microscope (Axioskop 2 plus) equipped with a drawing tube, appendages were analysed and drawn. Later, all the structures and setae were observed under another Zeiss microscope (Axioskop 2) equipped with Normaski differential interference contrast, using 1000X lens under immersion, for observing the details of setae. Illustrations were then transferred to a vegetal paper by means of an ink pen of 0.25 mm.
The terminology adopted in this study uses that suggested by Clark et al. (Citation1996) and Pohle et al. (Citation1999). The setae description agrees with Pohle and Telford (Citation1981). In addition, appendages were described sequentially by proximal article to the distal one, and somites were described from anterior to posterior one. Only the setation pattern (number of setae) was compared with the original paper (Diaz and Ewald 1968).
To check for possible effects of laboratory holding time on growth, the procedure described by Tweedale et al. (Citation1993) was adopted, in which an analyses of covariance (Sokal and Rohlf Citation1995) were used to compare the regression line generated using all moults with the regression line generated using only the first two laboratory moults (the moults presumed to be least affected by laboratory holding time). To define the relationship between moults increment (post-moults CW minus pre-moults CW) and crab size, a regression line was generated, yielding the model Y = a + bX, where Y = moults increment and X = pre-moults CW (Hiatt Citation1948). CW is defined as the distance measured between the antero-lateral spines, under a Zeiss microscope stereoscope equipped with camera and distance measurement system.
Results
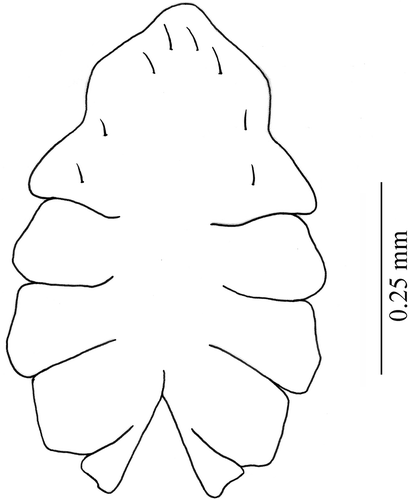
Some setal details and additions to the original description of megalopa (Figures ) and first juvenile ( and ) are summarized in and , respectively. Only the differences concerning setation, level of details or additional structures are described.
Figure 1. Armases rubripes. Pereopods of megalopa stage: (a) cheliped (scale bar 0.2 mm); (b) second pereopod; (c) third pereopod; (d) fourth pereopod and (e) fifth pereopod (scale bar 0.5 mm).
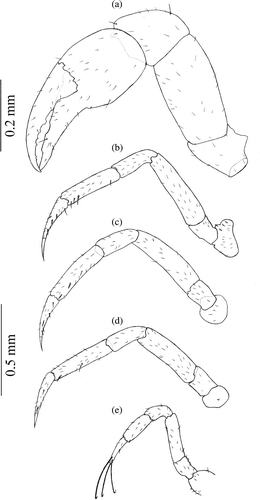
Figure 3. Armases rubripes. Pleopods of megalopa stage: (a) second pleopod; (b) third pleopod; (c) fourth pleopod; (d) fifth pleopod.
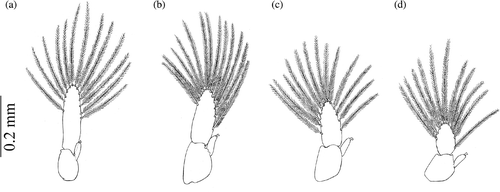
Figure 5. Armases rubripes. Pereopods of the first juvenile stage: (a) cheliped; (b) second pereopod; (c) third pereopod; (d) fourth pereopod; (e) fifth pereopod.
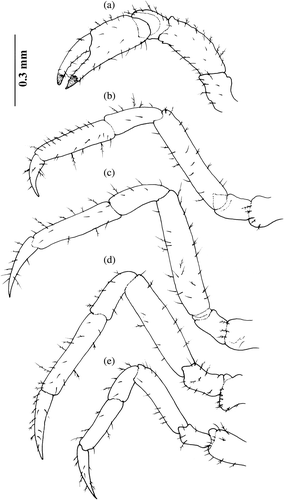
Table 1. Armases rubripes (Rathbun 1897): comparison of the original and the present description of megalopa stage.
Table 2. Armases rubripes (Rathbun 1897): comparison of the original and the present description of the first juvenile stage.
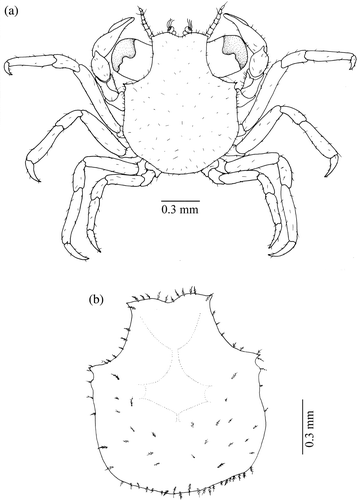
The morphological changes which occurred from second to tenth juvenile stages are shown in Figures . The external morphology of subsequent juvenile stages is slightly modified with the increasing size () and setation of certain structures. The carapace becomes wider than longer throughout the development of stages. The buttons on sternite decrease in size as well as also do ventral depressions on the abdominal sixth somite.
Table 3. Armases rubripes (Rathbun 1897). Measurements of juvenile crabs (mean ± standard deviation; values in millimetres).
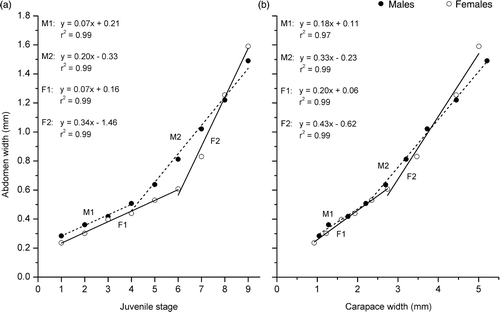
There are no differences in abdominal width between males and females of analysed stages and up to the tenth stage of juvenile development, the abdomen of males and females are very similar and no statistical difference between the growth’ straight line for both sex is observed (p > 0.05) (). Slight differences (although not statistically significant) can be seen in fifth, sixth and seventh juvenile stages can be seen, where abdomen of males are clearly of more width than female ones ().
Figure 6. Armases rubripes. Relationship of the (a) abdomen width vs. the juvenile development stages and the (b) CW for males and females (M1 = male specimens before sexual differentiation; M2 = male specimens after sexual differentiation; F1 = female specimens before sexual differentiation; F2 = female specimens after sexual differentiation).
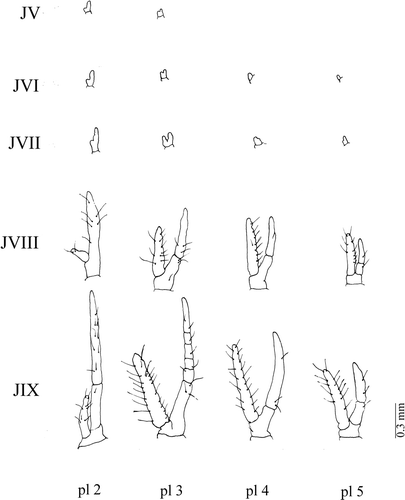
The rudiments of two pairs of pleopods that will become the gonopod appear on the fifth juvenile stage, while male genital openings are visualized only on the eighth stage (). In the ninth juvenile stage, the first pleopod is similar to that of the adult crab in its general shape (). In females, two first pairs of pleopods appear in the fifth stage (). Female genital openings, which are located on thoracic sternites corresponding to the third pair of pereopods, also appear on the fifth juvenile stage. The four pairs of pleopods appear in the sixth stage and are very characteristic in the ninth stage.
Figure 7. Armases rubripes. Ventral view of thoracic sternites of males and females: (a) female fifth juvenile and (b) male eighth juvenile.
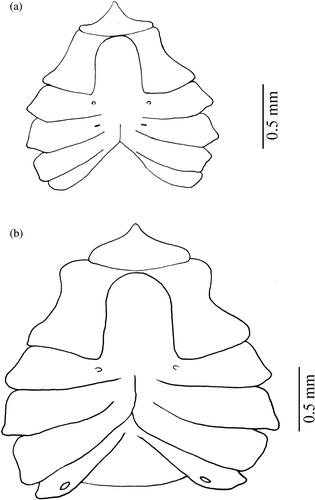
Figure 8. Armases rubripes. Development of male's pleopods. pl 1, first pleopod; pl 2, second pleopod; JV, fifth juvenile; JVI, sixth juvenile; JVII, seventh juvenile; JVIII, eighth juvenile; JIX, ninth juvenile.
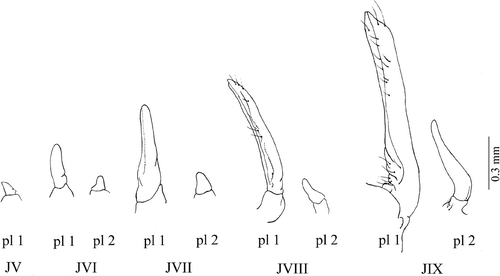
Figure 9. Armases rubripes. Development of female's pleopods. pl 2, second pleopod; pl 3, third pleopod; pl 4, fourth pleopod; pl 5, fifth pleopod; JV, fifth juvenile; JVI, sixth juvenile; JVII, seventh juvenile; JVIII, eighth juvenile; JIX, ninth juvenile.
represents the moult increment (%) versus CW (mm), the variance was high as indicated by the relatively low r 2 value (r 2 = 0.36), and moult increment decreased significantly (p < 0.005) throughout the juvenile development. The time spent between successive moults of A. rubripes increased throughout the juvenile development ( and ), as with other brachyuran species.
Figure 10. Armases rubripes. Relationship of growth parameters to crab size (premoult CW for laboratory-reared juvenile). Dashed lines means 95% confidence intervals.
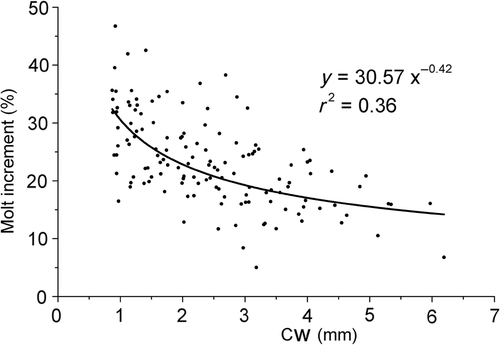
Figure 11. Armases rubripes. Relationship between intermoult period (days) and CW throughout the early juvenile development.
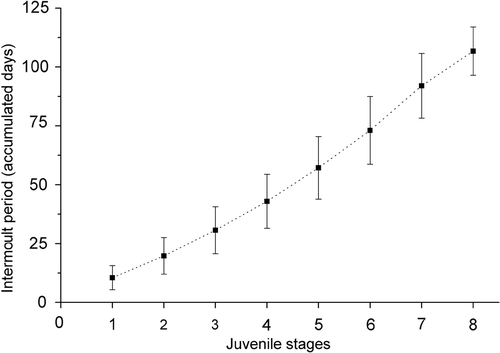
Table 4. Armases angustipes. Intermoult period (days) during the early juvenile stages.
Discussion
The differences observed between the original description of the megalopa and first juvenile (Díaz and Ewald Citation1968) and our observations, as shown in and , consist mainly of the number and level of details in setation. Almost all the setae may have an important function in the megalopa and the setal structure, usually neglected, can be an important diagnostic character and unlike the setal numbers, is not susceptible to loss, neither to significant inter-individual variability (Pohle and Telford Citation1981).
Specimens obtained for the previous description and this study were sampled at different latitudes. The specimens described by Díaz and Ewald (Citation1968) were from Venezuela (lower latitude), whereas the individuals reared here were from the São Paulo state in southern Brazil (higher latitude). It is also clear, however, that there are differences in the method used in order to obtain the megalopae. Díaz and Ewald (Citation1968) obtained the megalopae by rearing the larvae hatched by ovigerous females. In this article, megalopae were sampled in the zooplankton and were later identified.
The phenotypic plasticity of the species was already described by Díaz and Ewald (Citation1968) and Montú et al. (Citation1990) in laboratory reared A. rubripes. These individual variations, as reported by Haynes (Citation1979), Wehrtmann (Citation1989) and Guerao et al. (Citation2006) may also occur in decapod larvae from zooplankton. It is known that the larval rearing, under laboratory conditions, can reveal variations that are also found in their natural environment (Ingle Citation1985; Pohle et al. Citation1999). Montú et al. (Citation1990) suggested that the observed variability, for A. rubripes, can be explained by the different environmental pressure supported by the larvae in their development. They pointed out that the flexibility in larval development can be adaptive once it enables the larvae to find a suitable habitat for the metamorphosis and settling, which could possibly explain some of the observed variability.
The ability in delaying metamorphosis is attributed by Sandifer and Smith (Citation1979) and O′Connor (1990) as an opportunity for the larvae to be transported to other areas with more favourable environmental conditions.
The beginning of pleopod differentiation in Brachyura is not a uniform process, and can start at different stages in juvenile development, independent of the taxonomic group (Hebling and Rieger Citation2003). Early sexual differentiation in Brachyura has been reported in the superfamilies Majoidea (Ingle Citation1977; Flores et al. Citation2002; Guerao and Rotllant Citation2009), Portunoidea (Shen Citation1935; Payen Citation1974; Bolla Jr et al. Citation2008), Xanthoidea (Payen Citation1974; Hebling et al. Citation1982; Fransozo and Negreiros-Fransozo Citation1987; Fransozo et al. Citation1988; Lwin et al. Citation2007) and Calappoidea (Hebling and Rieger Citation2003; Negreiros-Fransozo et al. Citation2007). Among Grapsoidea species, the families Plagusiidae and Grapsidae show the largest megalopae of the superfamily, as well as the fastest juvenile development and the most precocious sexual maturity. On the other hand, Sesarmidae have smaller megalopae and have more stages of juvenile development before reaching the size at which onset of sexual maturity occurs (Flores et al. Citation1998).
Initial male gonopod differentiation at the first stage was recorded by Hartnoll (Citation1992) in Percnon abbreviatum (Dana 1851) and by Muraoka (Citation1965) in Plagusia dentipes (De Haan 1835) and Plagusia depressa (Fabricius 1775), respectively (all representatives of the family Plagusiidae). Flores et al. (Citation1998) verified that pleopod differentiation starts at the second stage of development with Pachygrapsus transversus (Gibbes 1850) (a representative of the family Grapsidae); for Neohelice granulata Dana (1851) (described as Chasmagnathus granulatus) Rieger and Nakagawa (Citation1995) observed that it happens at the third stage, and for Cyrtograpsus angulatus Dana (1851), at the fourth stage (Rieger and Beltrão Citation2000), the last both species belonging to the family Varunidae. In Sesarma rectum Randall (1840), a sesarmid crab, it happens only at the twelfth stage (Fransozo 1986/1987) and for Armases angustipes (Dana 1852), the pleopod differentiation started at sixth stage (Kowalczuk Citation1994). Guerao et al. (Citation2004) have described the complete larval development and the first stage of juvenile development of Perisesarma fasciatum (Lanchester 1900), one of the most conspicuous and species-rich genera of African, Asian and Australian mangroves. The latter authors verified that at the first stage pleopods are reduced and until the fourth stage, juveniles grew in size but no changes related to the appearing of the secondary sexual characters were observed.
This article shows that the beginning of the pleopod differentiation in A. rubripes occurs when individuals reach the fifth stage of juvenile development, which is very similar to that which has been observed for A. angustipes, another species of the same genus, whose sexual differentiation started at the sixth stage of juvenile development (Kowalczuk Citation1994) but different to that observed by Fransozo (1986/1987) with S. rectum. The related species are phylogenetically close (Schubart et al. Citation2002) and show megalopae with sizes that are very similar, so the difference at the beginning of pleopod differentiation among the representatives of the genus Armases and Sesarma may indicate an intrinsic strategy for each species, not a pattern of the family, as suggested by Flores et al. (Citation1998). This is one more character that demonstrates the difficulty that taxonomists must face clarifying, more precisely, the position of each genus and family in the superfamily Grapsoidea that was changed in 2001–2002 (Martin and Davis Citation2001; Schubart et al. Citation2002).
The growth parameters documented in laboratory studies are frequently presumed to be representative of the growth parameters of animals in the wild (Tweedale et al. Citation1993); however, laboratory studies of growth for which there are no comparisons with field observations should be interpreted conservatively (Hartnoll Citation1982). O’Connor (1990) reported that she reared hundreds of field-caught fiddler crab instars up to the seventh juvenile developmental stage and did not observe size differences between juvenile Uca pugnax and Uca pugilator comparable to those seen in crabs reared from eggs in the laboratory. Hartnoll (Citation1982), however, has observed some differences when the growth of crabs reared in laboratory were compared with those caught in the field, namely a decrease in the moult increment and an increase in the intermoult period.
Although no comparative studies on the growth of juveniles reared in the laboratory and those obtained from the nature has been made with A. rubripes, it is possible that what was observed here (decrease in the moult increment and increase in the intermoult period) is present in individuals in the field. Negreiros-Fransozo and Fransozo (Citation1991) have observed the same pattern with other brachyuran species of different families (S. rectum, Eurypanopeus abbreviates, and Eriphia gonagra) and also Hirose and Negreiros-Fransozo (Citation2008) in Uca maracoani.
Acknowledgements
The authors are grateful to the Fundação de Amparo à Pesquisa do Estado de São Paulo – FAPESP – for the financial support (Nos 94/4878-4; 98/3136-4; 04/15194-6), and to FUNDUNESP (No. 00606/05 DFP) to the first author and to FAPESP for a scholarship to the third author. The authors are grateful to anonymous reviewers for their useful suggestions and NEBECC members for their help during field and laboratory activities. Sampling was performed according to Brazilian state and federal laws concerning wild animals.
References
- Bolla , Jr EA , Negreiros-Fransozo , ML and Fransozo , A . 2008 . Juvenile development of Callinectes ornatus Ordway, 1863 (Crustacea: Decapoda: Portunidae), from megalopae obtained in the neuston . Zootaxa , 1788 : 1 – 20 .
- Cadman , LR and Weinstein , MP . 1988 . Effects of temperature and salinity on the growth of laboratory-reared juvenile blue crabs Callinectes sapidus Rathbun . Journal of Experimental Marine Biology and Ecology , 121 : 193 – 207 .
- Clark , PF , Calazans , DK and Pohle , GW . 1996 . Accuracy and standardization of Brachyuran larval descriptions . Invertebrate Reproduction and Development , 33 ( 2–3 ) : 127 – 144 .
- Costlow , Jr JD and Bookhout , CG . 1960 . The complete larval development of Sesarma cinereum (Bosc) reared in the laboratory . Biological Bulletin , 118 ( 2 ) : 203 – 214 .
- Costlow , Jr JD and Bookhout , CG . 1962 . The larval development of Sesarma reticulatum Say reared in the laboratory . Crustaceana , 4 : 281 – 294 .
- Cuesta , JA and Anger , K . 2001 . Larval morphology of the Sesarmid crab Armases angustipes Dana, 1852 (Decapoda, Brachyura, Grapsoidea) . Journal of Crustacean Biology , 21 ( 3 ) : 821 – 838 .
- Cuesta , JA , García-Guerrero , MU , Rodríguez , A and Hendrickx , ME . 2006 . Larval morphology of the sesarmid crab Aratus pisonii (H. Milne Edwards, 1837) (Decapoda, Brachyura, Grapsoidea), from laboratory reared material . Crustaceana , 79 ( 2 ) : 175 – 196 .
- Díaz , H and Ewald , JJ . 1968 . A comparison of the larval development of Metasesarma rubripes (Rathbun) and Sesarma ricordi H. Milne Edwards (Brachyura, Grapsidae) reared under laboratory conditions . Crustaceana , 2 : 225 – 248 .
- Felder , DL , Martin , JW and Goy , JW . 1985 . “ Patterns in early postlarval development of decapods ” . In Crustacean issues II. Larval growth , Edited by: Wenner , AM . 163 – 225 . Boston (MA) : Balkema. .
- Flores , AAV , Marques , FPL and Negreiros-Fransozo , ML . 2002 . Postlarval stages and growth patterns of the spider crab Pyromaia tuberculata (Brachyura, Majidae) from laboratory-reared material . Journal of Crustacean Biology , 22 ( 2 ) : 314 – 327 .
- Flores , AAV , Negreiros-Fransozo , ML and Fransozo , A . 1998 . The megalopa and juvenile development of Pachygrapsus transversus (Gibbes 1850) (Decapoda, Brachyura) compared with other grapsid crabs . Crustaceana , 71 ( 2 ) : 197 – 222 .
- Fransozo , A . 1986/1987 . Desenvolvimento dos estágios juvenis de Sesarma (Holometopus) rectum Randall, 1840 (Decapoda, Grapsidae) obtidos em laboratório . Naturalia , 11/12 : 77 – 87 .
- Fransozo , A and Hebling , NJ . 1986 . Desenvolvimento larval de Sesarma (Holometopus) rectum, Randall 1840 (Decapoda, Grapsidae) em laboratório . Revista Brasileira de Biologia , 46 ( 2 ) : 353 – 364 .
- Fransozo , A and Negreiros-Fransozo , ML . 1987 . Morfologia dos primeros estágios juvenis de Eriphia gonagra (Fabricius 1781) e Eurypanopeus abbreviatus (Stimpson 1860) (Crustacea, Decapoda, Xanthidae), obtidos em laboratório . Papéis Avulsos de Zoologia , 36 ( 22 ) : 257 – 277 .
- Fransozo , A , Negreiros-Fransozo , ML and Hiyodo , CM . 1988 . Développement juvénile de Menippe nodifrons Stimpson, 1859 (Crustacea, Decapoda, Xanthidae) au laboratoire . Revue de Hydrobiologie Tropical , 21 ( 4 ) : 297 – 308 .
- Guerao , G , Abelló , P and dos Santos , A . 2006 . Morphological variability of the megalopa of Liocarcinus depurator (Brachyura: Portunidae) in Mediterranean and Atlantic populations . Journal of Natural History , 40 ( 32–34 ) : 1851 – 1866 .
- Guerao , G , Anger , K , Nettelmann , UWE and Schubart , CD . 2004 . Complete larval and early juvenile development of the mangrove crab Perisesarma fasciatum (Crustacea: Brachyura: Sesarmidae) from Singapore, with a larval comparison of Parasesarma and Perisesarma . Journal of Plankton Research , 26 ( 12 ) : 1389 – 1408 .
- Guerao , G , Anger , K and Schubart , CD . 2007 . Larvae and first-stage juveniles of the American genus Armases Abele 1992 (Brachyura: Sesarmidae): a morphological description of two complete developments and one first zoeal stage . Journal of Natural History , 41 ( 29–32 ) : 1811 – 1839 .
- Guerao , G and Rotllant , G . 2009 . Post-larval development and sexual dimorphism of the spider crab Maja brachydactyla (Brachyura: Majidae) . Scientia Marina , 73 ( 4 ) : 797 – 808 .
- Guimarães , FJ and Negreiros-Fransozo , ML . 2005 . Juvenile development and growth patterns in the mud crab Eurytium limosum (Say 1818) (Decapoda, Brachyura, Xanthidae) under laboratory conditions . Journal of Natural History , 39 ( 23 ) : 2145 – 2161 .
- Hartnoll , RG . 1982 . “ Growth ” . In The biology of crustacea , Edited by: Abele , LG . Vol. 2 , 111 – 196 . New York : Academic Press .
- Hartnoll , RG . 1992 . Megalopae and early post-larval stages of East African Percnon abbreviatum (Decapoda: Brachyura: Grapsidae) . Journal of Zoology , 228 : 51 – 67 .
- Haynes , E . 1979 . Description of larvae of the northern shrimp, Pandalus borealis, reared in situ in Kachemak Bay, Alaska . Fishery Bulletin , 77 : 157 – 173 .
- Hebling , NJ , Fransozo , A and Negreiros-Fransozo , ML . 1982 . Desenvolvimento dos primeiros estágios juvenis de Panopeus herbstii H. Milne Edwards, 1834 (Crustacea, Decapoda, Xanthidae), criados em laboratório . Naturalia , 7 : 177 – 188 .
- Hebling , NJ and Rieger , PJ . 2003 . Desenvolvimento juvenil de Hepatus pudibundus (Herbst) (Crustacea, Decapoda, Calappidae), em laboratório . Revista Brasileira de Zoologia , 20 ( 3 ) : 531 – 539 .
- Hiatt , RW . 1948 . The biology of the lined shore crab Pachygrapsus crassipes Randall . Pacific Science , 11 : 135 – 212 .
- Hirose , GL and Negreiros-Fransozo , ML . 2008 . Growth and juvenile development of Uca maracoani Latreille, 1802-1803 in laboratory conditions (Crustacea, Decapoda, Brachyura, Ocypodidae) . Senckenbergiana biologica , 88 ( 2 ) : 161 – 168 .
- Ingle , RW . 1977 . The larval and post-larval development of the scorpion spider crab, Inachus dorsettensis (Pennant) (family: Majidae), reared in the laboratory . Bulletin of the British Museum of Natural History (Zoology) , 30 : 331 – 348 .
- Ingle , RW and Rice , AL . 1984 . The juvenile stages of eight swimming crab species (Crustacea: Brachyura: Portunidae): a comparative study . Bulletin of the British Museum of Natural History (Zoology) , 46 ( 4 ) : 345 – 354 .
- Ingle , RW . 1985 . Larval development of the mud crab Panopeus occidentalis de Saussure, from Bermuda (Crustacea: Xanthoidea: Panopeidae) . Bulletin of the British Museum of Natural History (Zoology) , 48 ( 4 ) : 233 – 248 .
- Kowalczuk VGL. 1994. Estrutura populacional de Armases angustipes (Dana 1852) (Decapoda: Brachyura: Grapsidae) da Ilha do Farol, Caiobá, PR e seu desenvolvimento pós-embrionário sob condições de laboratório [unpublished MSc dissertation]. [Curitiba, Brazil]: Universidade Federal do Paraná, p. 100.
- Luppi , IA and Spivak , ED . 2007 . Morphology of megalopa and first crab of Cyrtograpsus angulatus, with comments on the presence of an anomalous first crab stage on Brachyuran crabs . Journal of Crustacean Biology , 27 ( 1 ) : 80 – 89 .
- Lwin , TT , Doi , W , Yokota , M , Strussmann , CA and Watanabe , S . 2007 . Juvenile morphology of the xanthid crab Leptodius exaratus (H. Milne-Edwards 1834) (Decapoda: Brachyura), with notes on the appearance of sexual dimorphism . Invertebrate Reproduction and Development , 50 ( 4 ) : 191 – 201 .
- Martin , JW and Davis , GE . 2001 . An updated classification of the recent Crustacea . Natural Museum of Los Angeles County, Science Series , 39 : 1 – 124 .
- Martin , JW , Felder , DL and Truesdale , FM . 1984 . A comparative study of morphology and ontogeny in juvenile stages of four Western Atlantic xanthoid crabs (Crustacea: Decapoda: Brachyura) . Philosophical Transactions of the Royal Society of London , 303 : 537 – 604 .
- Melo , GAS . 1996 . Manual de identificação dos Brachyura (Caranguejos e Siris) do litoral brasileiro , 603 São Paulo : Plêiade/FAPESP .
- Montú , M , Anger , K and Bakker , C . 1990 . Variability in the larval development of Metasesarma rubripes (Decapoda, Grapsidae) reared in the laboratory . Nerítica , 5 ( 1 ) : 113 – 128 .
- Muraoka , K . 1965 . On the post-larval stage of Plagusia depressa . Research on Crustaceans , 2 : 83 – 90 .
- Negreiros-Fransozo , ML and Fransozo , A . 1991 . Growth and age determination of three juvenile crab species (Crustacea, Decapoda, Brachyura) . Papéis Avulsos de Zoologia , 37 ( 18 ) : 277 – 283 .
- Negreiros-Fransozo , ML , Wenner , EL , Knott , DM and Fransozo , A . 2007 . The megalopa and early juvenile stages of Calappa tortugae Rathbun, 1933 (Crustacea, Brachyura) reared in the laboratory from South Carolina neuston samples . Proceedings of the Biological Society of Washington , 120 ( 4 ) : 469 – 485 .
- O’Connor , NJ . 1990 . Morphological differentiation and moulting of juvenile fiddler crabs (Uca pugilator and U. pugnax) . Journal of Crustacean Biology , 10 ( 4 ) : 608 – 612 .
- Payen , G . 1974 . Morphogenèse sexuelle de quelques Brachyoures (Cyclométopes) au cours du développement embryonnaire, larvaire et postlarvaire . Bulletin du Museum Nationale d’Histoire Naturelle, (3n série), 209, Zoology. , 139 : 202 – 261 .
- Pohle , G , Mantelatto , FLM , Negreiros-Fransozo , ML and fransozo , A . 1999 . “ Larval Decapoda (Brachyura) ” . In South Atlantic Zooplankton , Edited by: Boltovskoy , D . Vol. 2 , 1280 – 1351 . Rotterdam, , The Netherlands : Backhuys .
- Pohle , G and Telford , M . 1981 . Morphology and classification of decapod crustacean larval setae: a scanning electron microscope study of Dissodactylus crinitichelis Moreira, 1901 (Brachyura, Pinnotheridae) . Bulletin of Marine Science , 31 ( 3 ) : 736 – 752 .
- Rieger , PJ and Beltrão , R . 2000 . Desenvolvimento juvenil de Cyrtograpsus angulatus Dana (Crustacea, Decapoda, Grapsidae), em laboratório . Revista Brasileira de Zoologia , 17 ( 2 ) : 405 – 420 .
- Rieger , PJ and Nakagawa , C . 1995 . Desenvolvimento juvenil de Chasmagnathus granulata Dana, 1851 (Crustacea, Decapoda, Grapsidae), em laboratório . Nauplius , 3 : 59 – 74 .
- Sandifer , PA and Smith , IJ . 1979 . Possible significance of variation in the larval development of Palaemonid shrimp . Journal of Experimental Marine Biology and Ecology , 39 : 55 – 64 .
- Schubart , CD and Cuesta , JA . 1998 . First zoeal stages of four species of Sesarma from Panama (Crustacea, Brachyura, Grapsidae) described from the laboratory hatched material with a identification key and phylogenetic remarks . Journal of Plankton Research , 20 ( 1 ) : 61 – 84 .
- Schubart , CD , Cuesta , JA and Diesel , R . 2002 . Glyptograpsidae, a new brachyuran family from Central America: larval and adult morphology, and a molecular phylogeny of the Grapsoidea . Journal of Crustacean Biology , 22 : 28 – 44 .
- Shen , CJ . 1935 . An investigation of the post-larval development of the shore-crab Carcinus maenas, with the special reference to the external secondary sexual characters . Proceedings of the Zoological Society of London. , 1 : 1 – 33 .
- Sokal , RR and Rohlf , FJ . 1995 . Biometry: the principles and practice of statistic in biological research , 887 New York : W. H. Freeman .
- Tweedale , WA , Bert , TM and Brown , SD . 1993 . Growth of postsettlement juveniles of the Florida stone crab, Menippe mercenaria (Say) (Decapoda: Xanthidae) in the laboratory . Bulletin of Marine Science , 52 ( 3 ) : 873 – 885 .
- Wehrtmann , I . 1989 . Seasonal occurrence and abundance of caridean larvae of Helgoland. German Bight . Helgoländer Meeresunters , 43 : 87 – 112 .