Abstract
The decline of tissue regenerative potential with the loss of stem cell function is a hallmark of mammalian aging. We study Botryllus schlosseri, a colonial chordate which exhibits robust stem cell-mediated regeneration capacities throughout life. Larvae, derived by sexual reproduction and chordate development, metamorphose to clonal founders that undergo weekly formation of new individuals by budding from stem cells. Individuals are transient structures which die through massive apoptosis, and successive buds mature to replicate an entire new body. As a result, their stem cells, which are the only self-renewing cells in a tissue, are the only cells which remain through the entire life of the genotype and retain the effects of time. During aging, a significant decrease in the colonies’ regenerative potential is observed and both sexual and asexual reproductions will eventually halt. When a parent colony is experimentally separated into a number of clonal replicates, they frequently undergo senescence simultaneously, suggesting a heritable factor that determines lifespan in these colonies. The availability of the recently published B. schlosseri genome coupled with its unique life cycle features promotes the use of this model organism for the study of the evolution of aging, stem cells, and mechanisms of regeneration.
Introduction
The aging process can be defined as a general loss in biological competence for both the individual cell and the organism as a whole. At the cellular level, it is expressed as decreasing replicative ability in proliferating cells and decreasing functional activity in post-mitotic cells (Geiger & Van Zant Citation2002; Van Zant & Liang Citation2003). Adult stem cells divide to produce new progeny that undergo programs of differentiation and maturation required to replace old or damaged tissues (Weissman Citation2000). The decline in tissue regeneration capacity has been attributed to a diminished responsiveness of tissue-specific stem and progenitor cells (Rossi et al. Citation2008; Conboy & Rando Citation2012; Signer & Morrison Citation2013). Adult stem cells are subjected to the effects of aging, which limit their ability to self-renew, differentiate, and maintain tissue homeostasis leading to loss of function (Conboy & Rando Citation2012; Signer & Morrison Citation2013). Short telomeres, oxidative stress, DNA damage, impaired metabolism, and deregulation of gene expression are all ‘hallmarks’ of aging that account for the impaired stem/progenitor cell function (Rossi et al. Citation2008; Beerman et al. Citation2014; Sousounis et al. Citation2014). Studying invertebrate models which are closely related to vertebrates support robust regeneration activities, and exhibit extensive stem cell-mediated regeneration capacities throughout adult life can provide fundamental insights into the process of aging and regeneration (Austad Citation2009). The potential of studying colonial organisms for aging studies was further emphasized by Skold and Obst (Citation2011). The colonial ascidian Botryllus schlosseri belongs to the chordate subphylum Tunicata or Urochordata, a taxonomic group considered to be the closest living invertebrate relative of vertebrates (Figure (e), Delsuc et al. Citation2006; Voskoboynik, Neff, et al. Citation2013). It is an organism with several characteristics that render it a valuable model for the study of aging. This includes: (i) homeostasis, involving weekly, de novo robust tissue regeneration with adult stem cells mediating formation of all body organs including the heart, nervous system, respiration system, digestive system, endostyle, ovary and testis, throughout their life (ii) identified adult stem cells (Laird et al. Citation2005) and their niches (Voskoboynik et al. Citation2008; Rinkevich et al. Citation2013), (iii) colony life cycle including both sexual and asexual reproductions (Review in Manni & Burighel Citation2006; Manni et al. Citation2014), (iv) clonal replicates which demonstrate simultaneous senescence (Rinkevich et al. Citation1992; Lauzon et al. Citation2000), and can be altered under various conditions and treatments (Boyd et al. Citation1986; Voskoboynik et al. Citation2002, Citation2004), (v) a colonial life history which allows separation of one genotype (colony) into several clonal replicates and a serial collection of whole body samples along their entire lifespan, offering a unique approach to characterize genetic changes during aging. Here, we review studies conducted over six decades that examined the B. schlosseri life cycle, with special emphasis on research that led to the discovery of the nature of the cells in colonial ascidians that mediate budding and highlight its potential for stem cell and aging research. The life histories of B. schlosseri and the availability of its genome (Voskoboynik, Neff, et al. Citation2013) further promote B. schlosseri as a model organism for the study of aging and the evolution of stem cell-mediated regeneration.
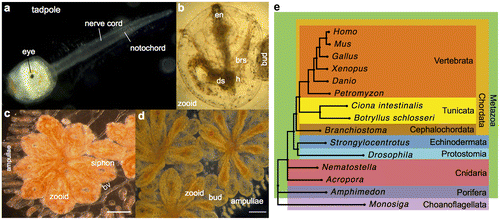
Figure 1. B. schlosseri anatomy, life cycle, and phylogeny. B. schlosseri reproduces both through sexual and asexual (budding) pathways, giving rise to virtually identical adult body plans. Upon settlement, the tadpole phase of the B. schlosseri lifecycle (a) will metamorphose into a founder individual (oozooid) (b), which through asexual budding, generates a colony. The colony includes three overlapping generations: an adult zooid, a primary bud, and a secondary bud, all of which are connected via a vascular network (bv) embedded within a gelatinous matrix (termed tunic). The common vasculature terminates in finger-like protrusions (ampullae; b–d). (c). Through budding, B. schlosseri generates its entire body, including digestive (ds) and respiratory (brs) systems, a simple tube-like heart (h), an endostyle (en) that harbors a stem cell niche, a primitive neural complex, and siphons used for feeding, waste, and releasing larvae (b–d). Each week, successive buds grow (d) and complete replication of all zooids in the colony, replacing the previous generation’s zooids, which die through a massive apoptosis. (e) A phylogenomic tree produced from the analysis of 521 nuclear genes (40,798 aligned amino acids) from 15 species, including B. schlosseri. Scale bar-1 mm.
Source: DOI: 10.7554/eLife.00569.003.
B. schlosseri alternative reproduction pathways
The life cycle of B. schlosseri includes both sexual and asexual reproduction pathways (Review in Manni & Burighel Citation2006; Manni et al. Citation2014). Despite differences in initiation, these two reproduction modes give rise to the same adult body plan (Figure ). Sexual reproduction starts with fertilization and progresses through classic embryonic stages into a tadpole larva featuring chordate characteristics such as tail, notochord, neural tube, and striated musculature (Figure (a)). Upon hatching, the motile tadpole settles on a substrate and metamorphoses into an oozooid, with a sessile body plan, losing most of its morphological chordate characteristics (Figure (b)). The oozooid begins a cyclical budding process of asexual reproduction, forming a colony of genetically identical zooids and buds. The colony grows when more than one bud replaces the parent zooid. All zooids and buds are connected through a vasculature network and are embedded within a gelatinous tunic (Figure ). A mature colony includes three overlapping generations: an adult zooid, a primary bud, and a secondary bud. Each zooid is an autonomous, filter-feeding individual comprising a heart, digestive tract, endostyle, branchial sac, neural complex, oral, and atrial siphons. Sexual reproduction commences upon the formation of ovaries and testis (Burighel & Cloney Citation1997; Figure ). Asexual (palleal) budding begins with the formation of a small vesicle that breaks off from the parent epidermis and peribranchial epithelium and segregates into a blastula-like structure. As the cell proliferates, the vesicle undergoes a series of invaginations, differentiates, and is developed into an adult zooid (Manni et al. Citation2014).
The replacement of the zooids’ generation occurs through a synchronized wave of massive apoptosis, in which the adult zooids are destroyed and taken over by their primary buds (Lauzon et al. Citation1993; Cima et al. Citation2010; Figure (d)). Palleal budding is divided into several major developmental stages and is synchronized in continuous cycles throughout the colony (Berrill Citation1951; Sabbadin Citation1955), a cycle that continues throughout the entire life of the colony. Unlike most species, where the body is long lived and maintained by cellular replacements, B. schlosseri regenerates new colonial units on a weekly basis ─ its stem cells remain for life.
Under certain conditions, B. schlosseri can regenerate its entire body from the vasculature alone (vascular budding; Sabbadin et al. Citation1975; Voskoboynik et al. Citation2007). When all the developing buds and zooids are removed from a colony, regions of the vasculature become niches for budding. The entire body can be regenerated even if no pre-existing zooids are present. This suggests that pluripotent and/or multipotent stem and progenitor cells that initiate budding can migrate and that regions within the colony vasculature are suitable niches (Voskoboynik et al. Citation2007).
Stem cell-mediated chimerism and budding
In addition to their high regeneration activity, B. schlosseri stem cells can be mobilized and transplanted between genetically compatible individuals, inducing natural chimerism. Once transplanted, donor pluripotent stem cells proliferate, differentiate, home, and replace germline and or somatic host tissues (termed cell parasitism; Sabbadin & Zaniolo Citation1979; Pancer et al. Citation1995; Stoner & Weissman Citation1996; Stoner et al. Citation1999; Laird et al. Citation2005; Voskoboynik et al. Citation2008; Rinkevich et al. Citation2013). The ability of colonies to undergo a natural transplantation reaction and exchange stem cells is determined by a single polymorphic histocompatibility locus (Oka & Watanabe Citation1957; Sabbadin Citation1962; Scofield et al. Citation1982; Voskoboynik, Newman, et al. Citation2013). Colonies that share at least one allele will anastomose vessels upon contact (fusion), creating a chimera or natural parabiont. Pairs that do not share alleles develop an inflammatory immune response (rejection) between the colonies (Oka & Watanabe Citation1957; Sabbadin Citation1962; Scofield et al. Citation1982; Voskoboynik, Newman, et al. Citation2013).
The Botryllus histocompatibility gene (BHF) was isolated, encoding a 252-amino acid protein that is highly charged, partially unstructured, and has no recognizable domains or motifs (Voskoboynik, Newman, et al. Citation2013). Allelic variation of this polymorphic gene predicts fusion/rejection outcomes, where one amino acid difference can lead to rejection. Thus, molecular discrimination of sequence variation within this gene provides a barrier to chimerism and transfer of stem cells between rejecting colonies (Voskoboynik, Newman, et al. Citation2013). BHF recognition represents an ancestral recognition system regulating blood-based chimerism; future investigation of this gene will likely reveal new mechanisms of recognition.
The long-term ability of cells from one genotype to replace the germline or soma of the host led to the hypothesis that chimerism, cell parasitism, and budding are mediated by stem cells (Stoner & Weissman Citation1996; Stoner et al. Citation1999). When somatic cell parasitism in B. schlosseri induces the development of a foreign entity bud within the host colony, chimerism serves as a platform to test whether asexual budding is mediated by stem cells. In a set of serial engraftment assays, Laird et al. (Citation2005) demonstrated that adult stem cells are responsible for a stable long-term chimerism and budding in B. schlosseri, by transplanting a single cell which expressed high enzymatic activity of aldehyde dehydrogenase. Using in vivo cell labeling, cell engraftment, and time lapse imaging, we (Voskoboynik et al. Citation2008) further demonstrated that the anterior ventral region of the sub-endostyle sinus (termed endostyle niche) harbors and exports somatic stem cells in B. schlosseri colonies. As few as 5–20 engrafted cells transplanted from the donor endostyle niche sufficed to generate somatic chimerism in compatible hosts. However, no germline chimerism was observed in this transplantation assay (Voskoboynik et al. Citation2008). Germline chimerism was detected following transplantation of cells from a cluster of cells flanking the endostyle, termed ventral cell islands (Rinkevich et al. Citation2013).
Induction of chimerism demonstrates the remarkable stemness capacity of the cells in these tissues (Voskoboynik et al. Citation2008; Rinkevich et al. Citation2013). In adult mammalian tissues, stem cells are stably maintained in their niche throughout most, if not all, of an organism’s life. (Zhou et al. Citation2013). The niche achieves long-term maintenance of stem cells and controls their self-renewal by orchestrating multiple signaling inputs that prevent differentiation (Morrison & Spradling Citation2008). Although somatic tissues undergo weekly waves of apoptosis and phagocytosis, B. schlosseri colonies undergo regeneration and live for years. Repeated trafficking of stem cells into new niches prevents their destruction by degenerating tissues and enables self-preservation throughout life.
Programmed life span in B. schlosseri colonies
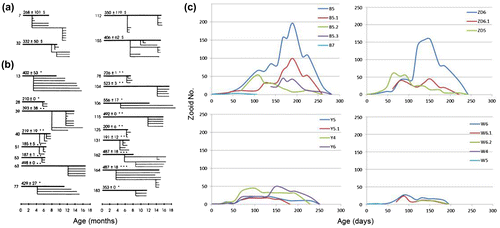
The rate of aging in B. schlosseri is plastic and amenable to change. Colonies grown in the field are characterized by short, sub-annual life spans (Grosberg Citation1988; Chadwick-Furman & Weissman Citation1995a, Citation1995b). For instance, life span in colonies raised in Monterey Bay varied significantly in response to seasonal variation (temperature, light, and nutrients), from about 3 months for spring-born colonies to 8 months for fall-born colonies (Chadwick-Furman & Weissman Citation1995a, Citation1995b). Laboratory-maintained colonies exhibit three broad groups of lifespan, either short (<0.5 year), medium (0.5–2 years), or long (2–8+ years; Sabbadin Citation1969; Boyd et al. Citation1986; Rinkevich et al. Citation1992; Lauzon et al. Citation2000). However, when a specific parent colony (genet) is experimentally separated into a number of clonal replicates (subclones), subclones frequently undergo senescence simultaneously (Figure ; Rinkevich et al. Citation1992; Lauzon et al. Citation2000). We have found that in independent genotypes, senescence could manifest itself in either random or synchronized fashion (Figure ; Rinkevich et al. Citation1992; Lauzon et al. Citation2000). Simultaneous senescence suggests that a heritable factor determines lifespan in B. schlosseri colonies.
Figure 2. Longevity of ramets subcloned from four representative colonies of B. schlosseri undergoing random mortality (a) and the 17 genets expressing synchronized mortality (b). The colony numbers are marked on the left margins of the lines. The original part of each colony is marked by a thick horizontal line; a subclone subcloned from the original part, by a thin line. When more than one subclone was subcloned on a given time, their longevities are marked by parallel horizontal lines. The right-hand end of the horizontal line represents the age at death of each subclone derived from a specific genet. Numbers above each colony represent the average +−SD of all subclones belonging to a specific genet. Levels of significance between the absolute deviation in mortalities of subclones from each genet, compared with the sample mean of the 41 parents genets, are *p < 0.05; **p < 0.01; ***p < 0.001; $, not significant (taken from Rinkevich et al. Citation1992). (c) Growth chart, as measured by zooid number of subclones from four different genotypes studied.
Lifespan and regeneration capacities in B. schlosseri
B. schlosseri colonies grow exponentially as juveniles, reaching a maximum size towards the last third period of its life. Toward the end of its life span, asexual reproduction begins to slow down and the number of individuals in the colony is reduced (Figure (c)). This suggests that an individual dies when it is no longer able to replace tissues. Bancroft (Citation1903) reported that field colonies of B. schlosseri exhibited a series of regressive changes prior to death including decreased budding potential and shrinkage of individual zooids. Our studies on wild and laboratory-raised colonies confirmed his observations and further described the unique morphological changes that occur systemically during colonial senescence (Rinkevich et al. Citation1992; Chadwick-Furman & Weissman Citation1995a, Citation1995b; Lauzon et al. Citation2000). While nonrandom senescence occurs quickly, completed in 7 days from the onset of morphological changes, random senescence exhibits a gradual deterioration over a prolonged period of weeks and months (Lauzon et al. Citation2000).
Long-lived B. schlosseri colonies exhibit significantly higher regeneration capacities compared with short-lived colonies (Voskoboynik and Ishizuka unpublished data). Exposure to physiological stimuli such as the antioxidant Butylated Hydroxytoluene (BHT) affects both regeneration capacities and longevity (Voskoboynik et al. Citation2002, Citation2004). We demonstrated enhanced growth rates and dramatically increased the life span in B. schlosseri colonies in response to a single, short-term treatment with the antioxidant BHT (Voskoboynik et al. Citation2002). The BHT treatment also affected the structure of the endostyle and the composition of cells in its sinuses (Voskoboynik et al. Citation2004). These studies suggest that animals able to sustain regeneration activities throughout adult life, and maintain the ability to support robust regeneration activities, experience increased longevity.
As an organism ages, its phenotype undergoes modification in its functionality and morphology. Due to the complexity of each organism and the vast amount of aging-related phenomena that occur, it is very difficult to distinguish between phenotypes that cause aging and those which are the effect of it. The decline of tissue regenerative potential is a hallmark of mammalian aging. In colonial organisms, like B. schlosseri, individuals are transient structures; every week, some individuals die through massive apoptosis, while others are ‘born’ as buds of stem cells that replicate an entire new body. As a result, their stem cells, the only self-renewing cells in a tissue, remain throughout the entire life of the genotype and are the only cells that preserve the effects of time. Therefore, aging of the colony is, by definition, aging of the stem cells; most other cells are regenerated from these on a weekly basis. In this model organism, we can clearly define that stem cell aging is the root cause of senescence of the entire colony. Utilizing B. schlosseri as an invertebrate model organism, which is closely related to vertebrates and exhibits robust stem cell-mediated regeneration capacities throughout life, will provide an opportunity to study the correlation between robust regeneration and aging and to discover additional genes and key pathways that might be relevant to human aging.
Acknowledgments
We thank K.J. Palmeri and K.J. Ishizuka for thoughtful edits.The Botryllus schlosseri genome is publicly available at NCBI BioProject http://www.ncbi. nlm.nih.gov/bioproject/. A Botryllus genome browser is maintained and updated by our lab at http://botryllus.stanford.edu/botryllusgenome/browse/.
Additional information
Funding
References
- Austad SN. 2009. Is there a role for new invertebrate models for aging research? The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 64A:192–194.10.1093/gerona/gln059
- Bancroft FW. 1903. Variation and fusion in compound variation and fusion in compound ascidians. Proceedings of the California Academy of Sciences. 3:137–186.
- Beerman I, Seita J, Inlay MA, Weissman IL, Rossi DJ. 2014. Quiescent hematopoietic stem cells accumulate DNA damage during aging that is repaired upon entry into cell cycle. Cell Stem Cell. 15:37–50.
- Berrill NJ. 1951. Regeneration and budding in tunicates. Biological Reviews. 26:456–475.10.1111/brv.1951.26.issue-4
- Boyd HC, Brown SK, Harp JA, Weissman IL. 1986. Growth and sexual maturation of laboratory-cultured Monterey Botryllus schlosseri. Biological Bulletin. 170:91–109.10.2307/1541383
- Burighel P, Cloney RA. 1997. Urochordata: Ascidiacea. In: Harrison FW, Ruppert EE, editors. Microscopic anatomy of invertebrates. New York (NY): Wiley-Liss; p. 221–347.
- Chadwick-Furman NE, Weissman IL. 1995a. Life histories and senescence of Botryllus schlosseri (Chordata, Ascidiacea) in Monterey Bay. Biological Bulletin. 189:36–41.10.2307/1542199
- Chadwick-Furman NE, Weissman IL. 1995b. Life history plasticity in chimaeras of the colonial ascidian Botryllus schlosseri. Proceedings of the Royal Society B: Biological Sciences. 262:157–162.10.1098/rspb.1995.0190
- Cima F, Manni L, Basso G, Fortunato E, Accordi B, Schiavon F, Ballarin L. 2010. Hovering between death and life: natural apoptosis and phagocytes in the blastogenetic cycle of the colonial ascidian Botryllus schlosseri. Developmental and Comparative Immunology. 34:272–285.10.1016/j.dci.2009.10.005
- Conboy IM, Rando TA. 2012. Heterochronic parabiosis for the study of the effects of aging on stem cells and their niches. Cell Cycle. 11:2260–2267.10.4161/cc
- Delsuc F, Brinkmann H, Chourrout D, Philippe H. 2006. Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature. 439:965–968.10.1038/nature04336
- Geiger H, Van Zant G. 2002. The aging of lympho-hematopoietic stem cells. Nature Immunology. 3:329–333.10.1038/ni0402-329
- Grosberg RK. 1988. Life-history variation within a population of the colonial ascidian botryllus-schlosseri I. The genetic and environmental control of seasonal variation. Evolution. 42:900–920.10.2307/2408907
- Laird DJ, De Tomaso AW, Weissman IL. 2005. Stem cells are units of natural selection in a colonial ascidian. Cell. 123:1351–1360.10.1016/j.cell.2005.10.026
- Lauzon RJ, Patton CW, Weissman IL. 1993. A morphological and immunohistochemical study of programmed cell death in Botryllus schlosseri (Tunicata, Ascidiacea). Cell and Tissue Research. 272:115–127.10.1007/BF00323577
- Lauzon RJ, Rinkevich B, Patton CW, Weissman IL. 2000. A morphological study of nonrandom senescence in a colonial urochordate. Biological Bulletin. 198:367–378.10.2307/1542692
- Manni L, Burighel P. 2006. Common and divergent pathways in alternative developmental processes of ascidians. BioEssays. 28:902–912.10.1002/(ISSN)1521-1878
- Manni L, Gasparini F, Hotta K, Ishizuka KJ, Ricci L, Tiozzo S, Voskoboynik A, Dauga D. 2014. Ontology for the asexual development and anatomy of the colonial chordate Botryllus schlosseri. PLoS ONE. 9:e96434.10.1371/journal.pone.0096434
- Morrison SJ, Spradling AC. 2008. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 132:598–611.10.1016/j.cell.2008.01.038
- Oka H, Watanabe H. 1957. Colony specificity in compound ascidians as tested by fusion experiments (a preliminary report). Proceedings Japan Academy. 33:657–659.
- Pancer Z, Gershon H, Rinkevich B. 1995. Coexistence and possible parasitism of somatic and germ cell lines in chimeras of the colonial urochordate Botryllus schlosseri. Biological Bulletin. 189:106–112.10.2307/1542460
- Rinkevich B, Lauzon RJ, Brown BW, Weissman IL. 1992. Evidence for a programmed life span in a colonial protochordate. Proceedings of the National Academy of Sciences of the United States of America. 89:3546–3550.10.1073/pnas.89.8.3546
- Rinkevich Y, Voskoboynik A, Rosner A, Rabinowitz C, Paz G, Oren M, Douek J, Alfassi G, Moiseeva E, Ishizuka KJ, et al. 2013. Repeated, long-term cycling of putative stem cells between niches in a basal chordate. Developmental Cell. 24:76–88.10.1016/j.devcel.2012.11.010
- Rossi DJ, Jamieson CH, Weissman IL. 2008. Stems cells and the pathways to aging and cancer. Cell. 132:681–696.10.1016/j.cell.2008.01.036
- Sabbadin A. 1955. Osservazioni sullo svi- luppo, l’accrescimento e la riproduzione di Botryllus schlosseri (Pallas), in condi- zioni di laboratorio. Bolletino di zoologia. 243–263.10.1080/11250005509439204
- Sabbadin A. 1962. Le basi geneticha della capacita di fusion fra colonies in Botryllus schlosseri (Ascidiacea). Rend Accad Naz Lincei Ser. 32:1031–1035.
- Sabbadin A. 1969. The compound ascidian Botryllus-schlosseri in the field and in the laboratory. Pubblicazioni della Stazione Zoologica di Napoli. 37:62–72.
- Sabbadin A, Zaniolo G. 1979. Sexual differentiation and germ cell transfer in the colonial ascidian Botryllus schlosseri. Journal of Experimental Zoology. 207:289–304.10.1002/(ISSN)1097-010X
- Sabbadin A, Zaniolo G, Majone F. 1975. Determination of polarity and bilateral asymmetry in palleal and vascular buds of the ascidian Botryllus schlosseri. Developmental Biology. 46:79–87.10.1016/0012-1606(75)90088-3
- Scofield VL, Schlumpberger JM, West LA, Weissman IL. 1982. Protochordate allorecognition is controlled by a MHC-like gene system. Nature. 295:499–502.10.1038/295499a0
- Signer RA, Morrison SJ. 2013. Mechanisms that regulate stem cell aging and life span. Cell Stem Cell. 12:152–165.10.1016/j.stem.2013.01.001
- Skold HN, Obst M. 2011. Potential for clonal animals in longevity and ageing studies. Biogerontology. 12:387–396.10.1007/s10522-011-9333-8
- Sousounis K, Baddour JA, Tsonis PA. 2014. Chapter eight – aging and regeneration in vertebrates. Current Topics in Developmental Biology. 108:217–246.10.1016/B978-0-12-391498-9.00008-5
- Stoner DS, Rinkevich B, Weissman IL. 1999. Heritable germ and somatic cell lineage competitions in chimeric colonial protochordates. Proceedings of the National Academy of Sciences of the United States of America. 96:9148–9153.10.1073/pnas.96.16.9148
- Stoner DS, Weissman IL. 1996. Somatic and germ cell parasitism in a colonial ascidian: possible role for a highly polymorphic allorecognition system. Proceedings of the National Academy of Sciences of the United States of America. 93:15254–15259.10.1073/pnas.93.26.15254
- Van Zant G, Liang Y. 2003. The role of stem cells in aging. Experimental Hematology. 31:659–672.10.1016/S0301-472X(03)00088-2
- Voskoboynik A, Neff NF, Sahoo D, Newman AM, Pushkarev D, Koh W, Passarelli B, Fan HC, Mantalas GL, Palmeri KJ, et al. 2013. The genome sequence of the colonial chordate, Botryllus schlosseri. Elife. 2:e00569.10.7554/eLife.00569
- Voskoboynik A, Newman AM, Corey DM, Sahoo D, Pushkarev D, Neff NF, Passarelli B, Koh W, Ishizuka KJ, Palmeri KJ, et al. 2013. Identification of a colonial chordate histocompatibility gene. Science. 341:384–387.10.1126/science.1238036
- Voskoboynik A, Reznick AZ, Rinkevich B. 2002. Rejuvenescence and extension of an urochordate life span following a single acute administration of an anti-oxidant Butylated hydroxytoluene. Mechanisms of Ageing and Development. 123:1203–1210.10.1016/S0047-6374(02)00002-7
- Voskoboynik A, Rinkevich B, Weiss A, Moiseeva E, Reznick AZ. 2004. Macrophage involvement for successful degeneration of apoptotic organs in the colonial urochordate Botryllus schlosseri. Journal of Experimental Biology. 207:2409–2416.10.1242/jeb.01045
- Voskoboynik A, Simon-Blecher N, Soen Y, Rinkevich B, De Tomaso AW, Ishizuka KJ, Weissman IL. 2007. Striving for normality: whole body regeneration through a series of abnormal generations. The FASEB Journal. 21:1335–1344.10.1096/fj.06-7337com
- Voskoboynik A, Soen Y, Rinkevich Y, Rosner A, Ueno H, Reshef R, Ishizuka KJ, Palmeri KJ, Moiseeva E, Rinkevich B. 2008. Identification of the endostyle as a stem cell niche in a colonial chordate. Cell Stem Cell. 3:456–464.10.1016/j.stem.2008.07.023
- Weissman IL. 2000. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 100:157–168.10.1016/S0092-8674(00)81692-X
- Zhou Y, Lewallen M, Xie T. 2013. Stem cells’ exodus: a journey to immortality. Developmental Cell. 24:113–114.10.1016/j.devcel.2013.01.001
