Abstract
Curcumin feeding of Drosophila larvae or young adults inhibits TOR and other known longevity genes and induces an extended health span in a normal-lived Ra strain adult. Combining larval curcumin feeding with an adult dietary restriction (DR) diet does not yield an additive effect. The age-specific mortality rate is decreased and is comparable with that of genetically selected long-lived La animals. Feeding Ra adults with the drug their whole life, or only during the senescent span, results in a weak negative effect on median longevity with no increase in maximum lifespan. The La strain shows no response to this DR mimetic. Thus, curcumin acts in a life stage-specific manner to extend the health span. Histone deacetylase inhibitors decrease the longevity of Ra animals if administered over the health span only or over the entire adult lifespan, but these inhibitors increase longevity when administered in the transition or senescent spans. Their major effect is a reduction in the mortality rate of older flies, raising the possibility of reducing frailty in older organisms. Their life stage-specific effects are complementary to that of curcumin. Use of stage-specific drugs may enable targeted increases in health or senescent spans, and thus selectively increase the quality of life.
Introduction
The adult lifespan of Drosophila consists of health, transition, and senescent spans (Arking et al. Citation2002). Each is characterized by different gene expression patterns, such that the health span is defined by tightly regulated gene expression patterns which maximize tissue function; the transition phase is characterized by a qualitative decrease in the regulatory ability of cells, and the senescent span is characterized by a stochastic pattern of degradation of the gene expression network (Pletcher et al. Citation2002; Arking Citation2009). Certain small molecules exist which, when given orally to an organism, affect the known genes regulating longevity. Examples of such effects are the longevity-enhancing effects of 4-phenyl butyrate (4PB) on flies (Kang et al. Citation2002), resveratrol on flies (Antosh et al. Citation2011) or humans (Timmers et al. Citation2011), rapamycin on flies (Harrison et al. Citation2009; Bjedov et al. Citation2010) and mice (Harrison et al. Citation2009; Miller et al. Citation2010), tetrahydrocurcumin on mice (Kitani et al. Citation2007), and/or curcumin on flies (Suckow and Suckow Citation2006; Lee et al. Citation2010; Soh et al. Citation2013).
We tested the effects of curcumin on the longevity of our normal-lived Ra and long-lived La strains (Soh et al. Citation2013). Curcumin is known to inhibit the TOR complex of flies in vivo (Bjedov et al. Citation2010) although it also downregulates other targets (Das et al. Citation2010; Sun et al. Citation2011). Longevity alterations in our fly strains have been described (Arking et al. Citation2004). In summary, the La strains were derived by selection from the Ra strains over a period of 21 generations (Luckinbill et al. Citation1984; Arking Citation1987). The longevity extension in this strain is due almost entirely to the extension of the health span only, and it is brought about by multiple physiological changes (Arking et al. Citation2002). We now report that curcumin has beneficial effects in the normal-lived Ra strain such that it increases longevity when administered in the developmental or health span stages, but has a negative effect when administered over the entire adult lifespan or over the senescent stage only.
Both male and female Ra control adults, not treated with curcumin and raised on AL food, had a normal-lived AL-type phenotype, while those raised on DR food had a long-lived DR-type phenotype (Soh et al. Citation2013). These results are consistent with our previous studies of dietary restriction in these animals (Soh et al. Citation2007). Importantly, Ra females or males treated with 100-mM curcumin as larvae and raised on either AL or DR food have a long-lived phenotype which is diet insensitive since the two survival curves are statistically indistinguishable (Soh et al. Citation2013). This suggests that the curcumin effect is epistatic to the DR effect.
The pro-longevity effects of phenylbutyrate (Kang et al. Citation2002), a histone deacetylase (HDAc) inhibitor, led us to test sodium butyrate (NaBu). We showed elsewhere that NaBu and related compounds have significant negative effects if administered during the health span but has beneficial effects if administered only during the transition and senescent stages (McDonald et al. Citation2013). This HDAc inhibitor is a mid- to late-life acting pro-longevity drug, and so it complements the early acting pro-longevity effects of curcumin described above.
Materials and methods
The various reagents and techniques involved in these experiments were discussed in Soh et al. (Citation2013) and McDonald et al. (Citation2013), and should be consulted for those details as well as for the data mentioned but not shown here. The survival statistical data cited herein are based on the log-rank test as specified in the GraphPad Prism 5.04 software; the relevant parameters are in the figure legends or text. Mortality kinetics were calculated using multiple likelihood analysis; the calculations are available in the supplementary data of the original publications.
Results
Dose response
A dose-response test was done on the Ra and La strains by testing the effect of 0, 10, 100, and 200-mM curcumin food fed to larvae only, and assaying the subsequent longevity of the adult flies raised on the AL standard food. The optimal extended longevity response was expressed by the Ra adults fed with 100-mM curcumin as larvae (days −9 to −5; Soh et al. Citation2013; data not shown). Other labs used lower doses and obtained significantly smaller effects on the longevity. The La animals showed no significant response to curcumin. Curcumin is genome specific in that it extends the longevity of normal-lived but not long-lived strains. We thus concentrated our investigations on the Ra strain.
Curcumin effect is stage specific: whole adult life feeding
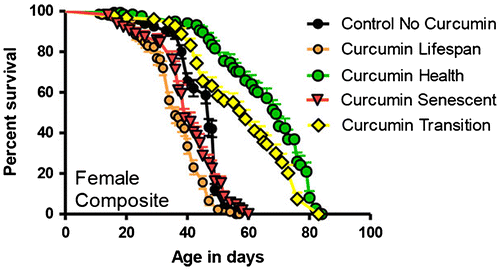
When treated with 100-mM curcumin during their entire adult life span (e.g. from days 5 to 65), both males and females responded with an obvious decrease in the median longevity. This decrease is borderline significant in the Ra males but is highly significant in the females (Figure ).
Figure 1. Survival curves of females fed with curcumin at the indicated phases of the lifespan. This is a composite graph of female longevities following curcumin feeding during the entire lifespan (N = 147, median lifespan = 36 days) or the untreated control (N = 230, median = 47) or in just one of the three different adult life stages. Comparing control to health (N = 126, median = 69, X2 = 127.7, and p < 0.0001); to transition (N = 126, median = 59, X2 = 59.4, and p < 0.0001); to senescent (N = 125, median = 39, X2 = 7.567, and p = 0.0059). Both sexes show the same stage-specific response in that only pre-midlife feedings (<42 days) increased lifespan relative to controls (from Soh et al. Citation2013).
Curcumin effect is stage specific: health span feeding
When females were fed an AL diet with curcumin only during their health span (days 5–27; defined as the time in which the survival is >90%), and then transferred to an AL diet with no curcumin for the rest of their lives; they displayed a delayed onset of senescence, which resulted in a significant increase in their median (30%) and maximum longevities (38%) (Figure ). Males showed comparable effects (Supplementary Figure 1).
Curcumin effect is stage specific: transitional span feeding
When Ra females were fed an AL diet with curcumin during their transition span (days 27–40; defined as the time from 90% survival to 80% survival (e.g. the onset of senescence)); and fed with an AL diet with no curcumin before and after that period, they displayed a 20% decrease in their median longevity and no change in their maximum longevity, relative to controls (Figure ). Males showed comparable effects (Supplementary Figure 1).
Curcumin effect is stage specific: senescent span feeding
When Ra females were fed an AL diet with curcumin only during their senescent span (days 38–89; defined as the period from 80% survivorship until death), and fed an AL diet with no curcumin prior to day 38, then they displayed a 20% decrease in median longevity and ~3% decrease in maximum longevity (Figure ). Males showed comparable effects (Supplementary Figure 1).
These data make clear that whole life feeding of curcumin to either sex is ineffective at best. But feeding curcumin only during the health span yields a robust delayed onset of senescence in both sexes which yields an adult longevity comparable to that induced by DR treatments. The effective periods of curcumin treatment are the larval stage and the health span portion of the adult stage. Changes in longevity flow from changes in mortality. Curcumin-fed Ra larvae yield adults of both sexes with significantly altered mortality kinetics such that the Ra adults have a Gompertz slope statistically equivalent to that of the long-lived La adults (Supplementary Figure 2).
Effect of continuous exposure to NaBu: Ra strain adults
Continuous high-dose NaBu treatment of Ra adults significantly decreases their maximum longevity compared to the control (p < 0.0001), apparently via a decreased length of late life (i.e. post-45-day longevity). At the low dose, an obvious but borderline significant (p = 0.0589) shortening of the late life span is also noticed. We conclude that the Ra animals are negatively affected by this treatment.
Effect of continuous exposure to NaBu: La strain adults
Continuous high-dose NaBu treatment of the long-lived La animals results in a significant decrease in their longevity relative to the control at the high dose (p = 0.0244), but not at the low doses (p = 0.2869) (McDonald et al. Citation2013; data not shown). We conclude that the La animals are significantly and negatively affected by this treatment, without any indication of a positive effect on their mortality or longevity.
Continuous larval + adult exposure to NaBu: Ra strain
Continuous high-dose NaBu treatment of the Ra animals induced a significantly decreased longevity which involved a 10-day decrease in the median lifespan. However, in the low-dose group, the treatment induced a significantly longer lifespan (p > 0.0001) which involves an alteration of the survival pattern such that there is a nine-day increase in the median lifespan. These data suggest that the low dose slowed the mortality rate during the transition and senescent periods, while the high dose accelerated it in these same life stages.
Continuous larval + adult exposure to NaBu: La strain
Continuous high-dose NaBu exposure of the La strain induces a significant decrease in longevity relative to the controls, and this decrease also seems to begin at the approximate end of the health span. However, the low-dose group is not statistically different from the control (McDonald et al. Citation2013; data not shown). The La animals are apparently not as severely affected by NaBu as are the Ra animals.
Effect of stage-specific exposure to NaBu: Ra strain
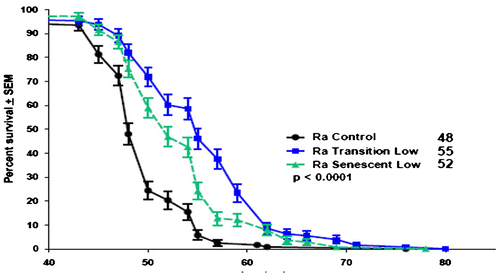
Both doses of the drug decreased longevity when applied during the health span only (1–21 days) (data not shown). However, treatment with NaBu during the transition span (21–42 days; Figure ) yielded a highly significant increase in longevity (p < 0.0001) at either dose. Treatment during the senescent span (43–64 days) also yielded a highly significant increase in longevity (p < 0.0001) at either dose (Figure ). Overall, the largest increase in median lifespan (+12.5% to 54 days) was induced by NaBu feeding at low dose during the transition phase. Figure shows the effects of low dose, mid- or late-life intervention on survival during the senescent span (days 43 ff) only. It is clear that intervention during mid-life (21–42 days) is significantly more effective than that during late life (days 43 ff) (X2 = 10.31, 1 df, p = 0.0013). Both the median and maximum lifespans increased (LT10 estimated at ~55 days for the control, ~60 days for the senescent low dose, and ~61.6 days for the transition low dose (Figure ). Analysis of the Gompertz curves (Supplementary Figure 3) showed that both the low- and high-dose mid-life interventions significantly reduced the age-specific mortality rate, but the low-dose intervention yielded the greatest increase in median longevity since it results in a reduction in both the slopes and the intercepts. The NaBu effect does not appear in flies pretreated with larval curcumin, suggesting that the response of the senescent span is dependent on the prior state of the health span. It is not clear if this represents an intrinsic limit to longevity or simply the failure to induce appropriate combinations of pathways.
Figure 2. Survival curves of females fed 10 mM NaBu at 21 days (start of transition span (<90 days)) or 42 days (start of senescent span (<80% survival)). The graph is rescaled so as to better show the late-life effects of NaBu. Both control vs. transition (log rank test, X2 = 52.98, 1 df, p < 0.0001) or control vs. senescent (X2 = 38.52, 1 df, p < 0.0001) are highly significant, but treatment starting with the transition span has a greater positive effect on late-life longevity than if given in later life (from McDonald et al. Citation2013).
Effect of stage-specific exposure to NaBu: La strain
NaBu had a deleterious effect on the lifespan of the La strain when administered at a low dose during each life stage. The high-dose NaBu treatment was significantly deleterious only when administered during the health or senescent spans (McDonald et al. Citation2013; data not shown). The immediate deleterious effect of NaBu on the long-lived adults during their health span suggests that this drug is inhibiting some essential processes in this strain at this stage. SAHA has effects similar to those of NaBu, but at a significantly lower dosage (McDonald et al. Citation2013).
Discussion
Curcumin
Taken together, these data suggest that (1) larval or adult feeding of curcumin to normal-lived animals acts to significantly extend their health span and delay the onset of senescence and (2) there exists a curcumin-sensitive component of development that has long-term delayed effects on the adult animal. This confirms our earlier report that larval crowding induced extended longevity in the adults (Buck et al. Citation1993). Understanding the mechanistic nature of that process would likely provide new insights into the nature of longevity extension mechanisms. Curcumin treatment results in the inhibition of at least nine genes known to be variously involved in the longevity treatment; we tentatively attributed that to the inhibition of mTOR (Soh et al. Citation2013). Chandrashekara and Sakarad (personal communication, 2014) reported that low-dose curcumin increased the level of AKT1-phosphorylation, and presumably its activity. However, we have obtained data suggesting that curcumin-induced longevity might actually stem from the inhibition of AKT, a finding consistent with the literature (Mercken et al. Citation2013; Nojima et al. Citation2013) and so this issue is still unsettled.
The fact that both the drugs have complementary stage-specific sensitive periods suggests that such pharmacological traits may not be rare, at least in epigenetically active drugs. Our data support a paradigm whereby genotropic drugs would be effective only during those life cycle stages during which their target molecules are actually available. A result of ineffectiveness derived from a whole life feeding regime may be a false negative result (Strong et al. Citation2013). Our data also support the addition of curcumin to the roster of those interventions which are known to robustly delay the onset of senescence, and thereby significantly extend longevity. Characterizing the mechanisms involved in stage-specific longevity extension should be of great value in aiding our deeper understanding of inducible longevity pathways.
NaBu
NaBu and SAHA, both of which are HDAc inhibitors with a similar spectrum of effects (Zhou et al. Citation2011) are mid- or late-life drugs which bring about healthy senescence in normal-lived animals as indicated by lower levels of age-specific mortality and a significant extension of the senescent span. We conclude that these data, taken together, present a proof of principle for the hypothesis that mid-life treatment with these HDAc inhibitors can significantly reduce late-life mortality and lead to healthier senescence.
Supplemental data
Supplemental data for this article can be accessed here. [http://dx.doi.org/10.1080/10.1080/07924259.2014.978028]
TINV_A_978028_SUPPL.docx
Download MS Word (309.4 KB)Acknowledgments
This work was made possible by the efforts of the colleagues responsible for the original papers on which this review was based: Jung-Won Soh, Nicholas Marowsky, Thomas J. Nichols, Abid M. Rahman, Tayaba Miah, Paraminder Sarao, Phillip McDonald, Brian M. Maizi Rawia Khasawneh PhD, Archana Unnikrishnan, PhD, Ahmad R. Heydari PhD, and Robert B. Silver PhD. I am indebted to Scott Pletcher PhD and the Drosophila Aging Core of the Nathan Shock Center of Excellence in Biology of Aging at the University of Michigan (funded by NIA P30-AG-013283) for assistance with the calculation of the mortality statistics.
References
- Antosh M, Whitaker R, Kroll A, Hosier S, Chang C, Bauer J, Cooper L, Neretti N, Helfand SL. 2011. Comparative transcriptional pathway bioinformatic analysis of dietary restriction, Sir2, p53 and resveratrol life span extension in Drosophila. Cell Cycle. 10:904–911.
- Arking R. 1987. Successful selection for increased longevity in Drosophila: analysis of the survival data and presentation of a hypothesis on the genetic regulation of longevity. Experimental Gerontology. 22:199–220.10.1016/0531-5565(87)90040-4
- Arking R. 2009. Overview of the genomic architecture of longevity. In: Sell C, Lorenzini A, Brown-Borg HM, editors. Life span extension: single cell organisms to man. Dordrecht: Humana Press, Springer; p. 59–73.10.1007/978-1-60327-507-1
- Arking R, Buck S, Novoseltsev VN, Hwangbo D-S, Lane M. 2002. Genomic plasticity, energy allocations, and the extended longevity phenotypes of Drosophila. Ageing Research Reviews. 1:209–228.10.1016/S1568-1637(01)00010-1
- Arking R, Novoseltsev V, Novoseltseva J. 2004. The human life span is not that limited: effects of multiple longevity phenotypes. Journals of Gerontology Series A-Biological Sciences and Medical Sciences. 59A:697–704.
- Bjedov I, Toivonen JM, Kerr F, Slack C, Jacobson J, Foley A, Partridge L. 2010. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metabolism. 11:35–46.10.1016/j.cmet.2009.11.010
- Buck S, Nicholson M, Dudas S, Wells R, Force A, Baker GT III, Arking R. 1993. Larval regulation of adult longevity in a genetically-selected long-lived strain of Drosophila. Heredity. 71:23–32.10.1038/hdy.1993.103
- Das T, Sa G, Saha B, Das K. 2010. Multifocal signal modulation therapy of cancer: ancient weapon, modern targets. Molecular and Cellular Biochemistry. 336:85–95.
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, et al. 2009. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 460:392–395.
- Kang HL, Benzer S, Min KT. 2002. Life extension in Drosophila by feeding a drug. Proceedings of the National Academy of Sciences of the United States of America. 99:838–843.10.1073/pnas.022631999
- Kitani K, Osawa T, Yokozawa T. 2007. The effects of tetrahydrocurcumin and green tea polyphenol on the survival of male C57BL/6 mice. Biogerontology. 8:567–573.
- Lee K-S, Lee B-S, Semnani S, Avanesian A, Um C-Y, Jeon H-J, Seong K-M, Yu K, Min K-J, Jafari M. 2010. Curcumin extends life span, improves health span, and modulates the expression of age-associated aging genes in Drosophila melanogaster. Rejuvenation Research. 13:561–570.10.1089/rej.2010.1031
- Luckinbill LS, Arking R, Clare M, Cirocco W, Buck S. 1984. Selection for delayed senescence in Drosophila melanogaster. Evolution. 38:996–1003.10.2307/2408433
- McDonald P, Maizi BM, Arking R. 2013. Chemical regulation of mid- and late-life longevities in Drosophila. Experimental Gerontology. 48:240–249.10.1016/j.exger.2012.09.006
- Mercken EM, Crosby SD, Lamming DW, JeBailey L, Krzysik-Walker S, Villareal DT, Capri M, Franceschi C, Zhang Y, Becker Kevin, et al. 2013. Calorie restriction in humans inhibits the PI3K/AKT pathway and induces a younger transcription profile. Aging Cell. 12:645–651.10.1111/acel.2013.12.issue-4
- Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, et al. 2010. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 66A:191–201.10.1093/gerona/glq178
- Nojima A, Yamashita M, Yoshida Y, Shimizu I, Ichimiya H, Kamimura N, Kobayashi Y, Ohta S, Ishii N, Minamino T. 2013. Haploinsufficiency of Akt1 prolongs the lifespan of mice. PLoS ONE. 8:e69178.10.1371/journal.pone.0069178
- Pletcher SD, Macdonald SJ, Marguerie R, Certa U, Stearns SC, Goldstein DB, Partridge L. 2002. Genome-wide transcript profiles in aging and calorically restricted Drosophila melanogaster. Current Biology. 12:712–723.10.1016/S0960-9822(02)00808-4
- Soh J-W, Hotic S, Arking R. 2007. Dietary restriction in Drosophila is dependent on mitochondrial efficiency and constrained by pre-existing extended longevity. Mechanisms of Ageing and Development. 128:581–593.10.1016/j.mad.2007.08.004
- Soh J-W, Marowsky N, Nichols TJ, Rahman AB, Miah T, Sarao P, Khasawneh R, Unnikrishnan A, Heydari AR, Silver RB, Arking R. 2013. Curcumin is an early-acting stage-specific inducer of extended functional longevity in Drosophila. Experimental Gerontology. 48:229–239.10.1016/j.exger.2012.09.007
- Strong R, Miller RA, Astle CM, Baur JA, de Cabo R, Fernandez E, Guo W, Javors M, Kirkland JL, Nelson JF, et al. 2013. Evaluation of resveratrol, green tea extract, curcumin, oxaloacetic acid, and medium-chain triglyceride oil on life span of genetically heterogeneous mice. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 68:6–16.
- Suckow BK, Suckow MA. 2006. Lifespan extension by the antioxidant curcumin in Drosophila melanogaster. International Journal of Biomedical Science. 2:401–404.
- Sun ZJ, Chen G, Zhang W, Hu X, Liu Y, Zhou Q, Zhu LX, Zhao YF. 2011. Curcumin dually inhibits both mammalian target of rapamycin and nuclear factor-κB pathways through a crossed phosphatidylinositol 3-kinase/Akt/IκB kinase complex signaling axis in adenoid cystic carcinoma. Molecular Pharmacology. 79:106–118.
- Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D, Kersten S, et al. 2011. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metabolism. 14:612–622.10.1016/j.cmet.2011.10.002
- Zhou Q, Dalgard CL, Wynder C, Doughty ML. 2011. Histone deacetylase inhibitors SAHA and sodium butyrate block G1-to-S cell cycle progression in neurosphere formation by adult subventricular cells. BMC Neuroscience. 12:50.
