ABSTRACT
In the present study, videos were recorded to observe oviposition in living, mature worms of Tubifex tubifex. After the formation of a lemon shaped cocoon around the clitellum at segments 11 and 12, mature eggs that have been stored in the ovisac at segments 12–14 begin to move into segments 11–12 through the constricted posterior end of the cocoon. Within a second or so, ovisac eggs emerge out of the posterior margin and return to the posterior segments. Such movements are repeated several times before the ovisac eggs settle in the ovarian coelom of segment 11. Within a few seconds of the eggs moving into the ovarian coelom, the clitellar region begins to change its contour slightly; it looks as if something round is tapping on the coelomic wall of this region. As this tapping ceases, the coelomic eggs begin to move to the space between the cocoon and the clitellum. Coelomic eggs are individually squeezed out of the female gonopores located near the midpoint of the cocoon. This process, referred to as egg deposition, is accomplished within 30 s. The egg deposition is followed by the backward withdrawal of the anterior segments from the cocoon.
Introduction
Oviposition in clitellate annelids (i.e., oligochaetes and leeches) is the process during which eggs stored in the ovisac or those fertilized internally are deposited outside the body. One of the characteristics of clitellate oviposition is that the deposited eggs rest in a capsule or cocoon that is formed by the secretion of the clitellum (Foot Citation1898; Grove and Cowley Citation1926; Penners Citation1933; Hirao Citation1965; Suzutani Citation1977; Suzutani-Shiota Citation1980; Romdhane et al. Citation2017; Saidel et al. Citation2017). It has also been demonstrated that at the final phase of clitellate oviposition, the body anterior to and including the clitellum is withdrawn backwards from the posterior margin of the cocoon (); Foot and Strobell Citation1902; Grove and Cowley Citation1926; Hirao Citation1965). Thus, it has generally been accepted that oviposition in clitellates begins with cocoon formation and terminates with the withdrawal of the anterior segments, including the head, through the cocoon (Barnes Citation1968; Brusca and Brusca Citation2003).
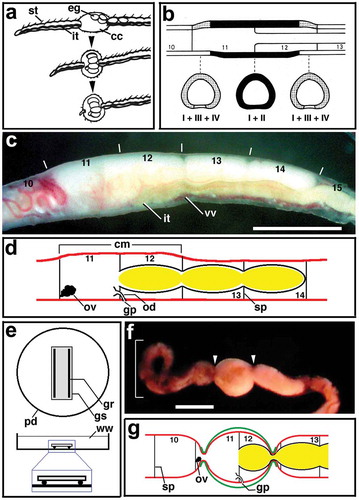
Figure 1. (a) Semi-diagrammatic illustration showing the successive steps of cocoon deposition in Tubifex. Anterior is to the left. cc, cocoon; eg, egg; it, intestine; st, seta. Adapted from Hirao (Citation1965). (b) Diagram showing the distribution of gland cells of Types I–IV in the clitellum in Tubifex. Dark area, Types I and II; dotted area, Types I, III and IV. Upper, sagittal section of segments 10–13; lower, transverse sections at three levels indicated by broken line. Adapted from Suzutani (Citation1977). (c) Lateral view of a living worm of T. tubifex with mature eggs in the ovisac at segments 12–14. Anterior is to the left; dorsal is to the top. Numerals indicate the segment number. Vertical lines indicate the segment boundary. Ovisac eggs are seen on the dorsal side of each segment. Note that the clitellar region (segments 11 and 12) is slightly thicker than other regions. it, intestine; vv, ventral vessel. Scale bar = 1 mm. (d) Diagram showing the arrangement of the female reproductive system in Tubifex. Numerals indicate the segment number. cm, clitellum; gp, female gonopore; ov, ovary; od, oviduct; sp, septum. (e) Diagram showing the apparatus designed for the observations of oviposition behaviour. Upper, overhead view; lower, side view. gr, glass rod; gs, glass slide; pd, petri dish; ww, well water. (f) A representative living worm undergoing oviposition in the oviposition apparatus shown in (e). Note the constricted margins (arrowhead) of the clitellar region and the formation of a loop in the anterior portion (square bracket). Scale bar = 1 mm. (g) Diagram showing the relative position of the constricted ends of the cocoon (green line) and the female reproductive system. Numerals indicate the segment number. gp, female gonopore; ov, ovary; sp, septum.
As has been well known in oligochaetes, upon cocoon deposition, both ends of the cocoon are sealed with the ‘cocoon plug’ and the lumen is filled with ‘cocoon fluid’ and deposited eggs. Based on morphological examinations of the clitellar epithelium and cocoon, it has been suggested that both the cocoon plugs and the cocoon fluid are secreted from specific types of gland cells in the clitellum, at the time of cocoon formation (Suzutani-Shiota Citation1980). In contrast, there is very limited information on the behaviour of eggs during oviposition. To date, ‘direct’ observations on the oviposition behaviour in living oligochaetes have been performed only in a few species (Foot and Strobell Citation1902; Grove and Cowley Citation1926; Hirao Citation1965). In their articles, however, these authors did not touch upon the behaviour of individual eggs while describing the worms’ movements accompanying cocoon deposition (e.g. backward withdrawal of the body). Thus, details of the behaviour of individual eggs during oviposition remain to be elucidated.
The present study was undertaken to gain an insight into the behaviour of eggs during oviposition in oligochaete clitellates. Hence, I made video recordings of oviposition behaviours in the freshwater oligochaete Tubifex tubifex. The results show that oviposition behaviour in Tubifex is divided into four successive phases, i.e. cocoon formation around the clitellum, movement of ovisac eggs to the ovarian coelom of segment 11, deposition of coelomic eggs to the outside within the cocoon, and release of the formed cocoon from the worm. In this article, I describe the behaviour of eggs during these events, by presenting supplemental movies.
Materials and methods
Animals
In this study I used the freshwater oligochaete, Tubifex tubifex. Adult worms were obtained from a laboratory breeding colony, following the instructions that have been described previously (Shimizu Citation1982). Mature worms for the experiments were collected through a two-step selection process. First, worms with large oocytes (eggs) in the ovisac of segments 12–14 were selected with the naked eye. Next, these worms were examined under a stereomicroscope, to find ovisac eggs with germinal vesicles. The selected worms did not exhibit any trace of germinal vesicles in the ovisac ()). Each worm was placed in a separate plastic petri dish with well water, into the darkness overnight (at least for 10 h before the experiments were to begin). Unless otherwise stated, all the experiments were carried out at room temperature (21–23°C).
Preparation of oviposition apparatus (OA)
To provide the animals mentioned above with an appropriate space for reproduction, I prepared an oviposition apparatus (OA) that consisted of a glass slide fragment (25 mm x 10 mm) and a pair of glass rods (approx. 0.7 mm in diameter); the latter was glued to the former with epoxy resin. When this apparatus is placed in a plastic petri dish with the glass rods downward, a space about 0.7 mm thick is made between the glass fragment and the bottom of the dish ()).
Observation of oviposition behaviour
To observe the oviposition behaviour, animals that had been in isolation overnight were transferred individually to the petri dish with the OA and well water ()). Then, they were placed under a red light and checked at 15 min intervals, for reproductive activity. When worms undergoing oviposition were found, they were recorded on a VHS video tape. Since the worms are very sensitive to slight vibrations of the water, great care was taken to avoid any vibration of the petri dishes that contained the experimental worms. The desk lamp (for red light) and the stereomicroscope equipped with a video camera were both placed on a different table than that on which the petri dishes were arranged.
A brief note on the cellular basis of cocoon formation in Tubifex
The clitellum in Tubifex is located at segments 11 and 12 ()). The clitellar epithelium contains four types (Types I–IV) of granular cells, in addition to the supporting cells and mucous cells generally found in the epidermis (Suzutani Citation1977; Suzutani-Shiota Citation1980). Among these types, only Type I cells are distributed throughout the clitellum ()). In contrast, Type II cells are found only in the intermediate part of the clitellum, and cells of Types III and IV are limited to the anterior and posterior terminal parts of the clitellum ()). Based on electron microscopic observations of granular cells undergoing discharge of secretory granules, Suzutani-Shiota (Citation1980) has specified the functions of cells of Types I–III as follows: The secretions of Type I cells provide materials for the future cocoon membrane; the secretions of Type II cells constitute a colloid in the cocoon lumen; the secretory products of Type III cells form the cocoon plug at both ends of the cocoon. Based on the fact that the secretions from Type IV cells precede those from Type I, it has been suggested that the secretions from Type IV cells provide accumulations of tubular components that are observed on the outer surface of the cocoon membrane at both ends of the cocoon (Suzutani-Shiota Citation1980).
Results and discussion
Summary of the previous observations on the female reproductive system
A brief review of the anatomy of the female reproductive system and the clitellum in Tubifex is presented here as a background for the observations described below. The female reproductive system of Tubifex consists of a pair of ovaries, a single ovisac and a pair of oviducts (Dixon Citation1915; )). The ovaries are situated in segment 11 on the lower part of the anterior septum and project into the coelom. The ovisac is a coelomic pouch that projects backward from the posterior septum of the ovarian segment through three segments (i.e. segments 12–14). Young oocytes that are released from the ovary move into the ovisac and undergo growth and maturation (Hirao Citation1964). The internal opening of the oviduct is through a ciliated funnel located on the posterior septum of the ovarian segment. From the funnel a tubule extends backward penetrating the septum and opens on the ventral surface of the body near the anterior septum of segment 12 (Jamieson Citation2006; )).
General features of mature worms under laboratory conditions
When mature worms that had been in isolation overnight were transferred to a petri dish with an oviposition apparatus (OA) and well water, they moved around for a while. When they came across the space made between the OA and the bottom of the dish, they proceeded into it until the anterior half of their body settled into the space.
In a preliminary experiment, mature worms (50 in total) that were cultured in the OA were examined with the naked eye to check for the newly deposited cocoons, at one-hour intervals. Seventy percent of the worms (35 out of 50) accomplished cocoon deposition during the first 6 h, and nine did so during the next 6 h. If the mature worms were continuously cultured in isolation without the OA, it was found that there were no worms that had deposited cocoons during the 24 h of culture. This indicates that the OA introduced in the present study plays a role in triggering the process of oviposition in mature worms. However, it seems unlikely that the presence of the OA specifies even the timing of oviposition, because mature worms that had begun to ‘use’ the OA at a similar timing underwent cocoon deposition at different times. In such a system, effectively recording videos of the process requires identifying worms beginning or undergoing oviposition using the naked eye, beforehand. During the preliminary observation, 5–9 min before the completion of cocoon deposition, the body portion posterior to the clitellar region exhibited a wave-like motion, which was recognizable with the naked eye ()).
To record videos of the oviposition behaviour in Tubifex, 10 petri dishes (per day), each containing a mature worm and the OA were arranged on the table under a red light and examined for worms exhibiting the wave-like movement in the region posterior to the clitellum, at 15 min intervals. When such worms were found, the stereomicroscope equipped with a video camera was set over the worms and video recordings began immediately. One hundred worms were set in the petri dishes with the OA and the oviposition behaviour was recorded from 25 worms.
Oviposition in Tubifex begins with cocoon formation
When the worms exhibiting the wave-like movement were viewed at higher magnifications, it was found that the clitellar region of the worm assumed a lemon shape with constricted anterior and posterior ends ()). This shape is very similar to that of the deposited cocoon. It is assumed that this lemon shape of the clitellar region is brought about by the nascent cocoon that is closely associated with the worm’s body surface and is constricted at its ends ()). In such worms, the formation of the cocoon membrane and the constriction of the cocoon ends have already been completed. It seems likely that the secretory products from Type IV gland cells that accumulate on the outer surface of the cocoon membrane at both ends of the cocoon play a role in constricting them (see Suzutani-Shiota Citation1980).
Cocoon formation is soon followed by the movement of ovisac eggs to the ovarian coelom
In the worms that exhibited the wave-like movement in the region posterior to the clitellum, eggs were still seen in the ovisac, though they moved between segments 12–14 through the constricted posterior end of the cocoon (; Movie S1). Typically, ovisac eggs present in segments 13 and 14 are moved as a mass into the preceding segment 12 through the constricted posterior end of the cocoon ()). As a result of this ‘forward’ movement, ovisac eggs disappeared from segments 13 and 14. However, within a second or so, ovisac eggs emerged out of the posterior end of the cocoon and returned to segments 13 and 14 by ‘backward’ movement ()).
Figure 2. Repeated back and forth movements of ovisac eggs in a living worm undergoing oviposition. Anterior is to the left. (a-f) segments 10–14; (g-z) segments 12–14. Numerals shown in the upper right of each panel indicate the time (sec) after the onset of the ‘first’ forward movement of the ovisac eggs (square bracket) seen in (a). Arrowheads in (a), (g) and (q) indicate the posterior margin of the clitellar region (cr). The position of the posterior margin of the clitellar region is adjusted to the same level in each column of panels. Open circles indicate the posterior margin of the ovisac egg mass at the time it has just extended posteriorly. Asterisks indicate the posterior end of the ovisac egg mass at the time it has just shrunken anteriorly. it, intestine. Scale bar = 1 mm.
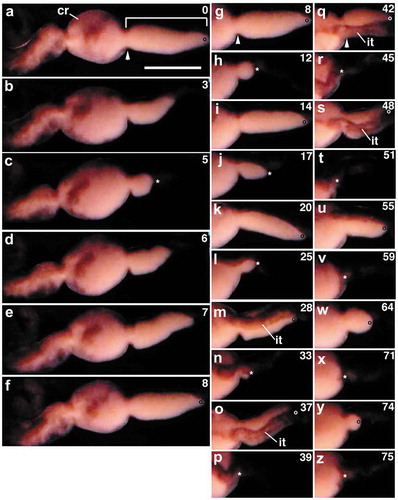
The returned eggs appeared to be packed closely; it is possible that they were still wrapped within the ovisac membrane. Subsequently, such ovisac eggs repeated the forward and backward movements several times () before they underwent a ‘final’ forward movement that was not be followed by a backward movement (). During this process, three things were noticed. First, the forward movement gave rise to the bulk displacement of ovisac eggs to segment 12. Second, when ovisac eggs moved forward, the intestine and the blood vessels underlying the ovisac moved into segment 12, together with the ovisac egg mass. Third, the ovisac egg mass that returned to the posterior segments became smaller in size as the eggs repeated the movements. This suggests that the ovisac eggs are settled, one by one, in the ovarian coelom of segment 11. Hereafter, eggs that had settled in the ovarian coelom are referred to as ‘coelomic eggs’.
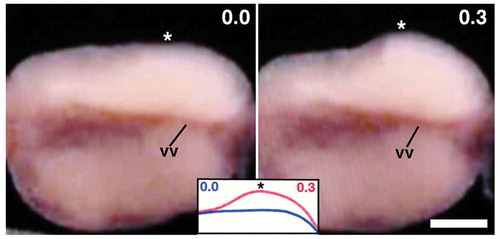
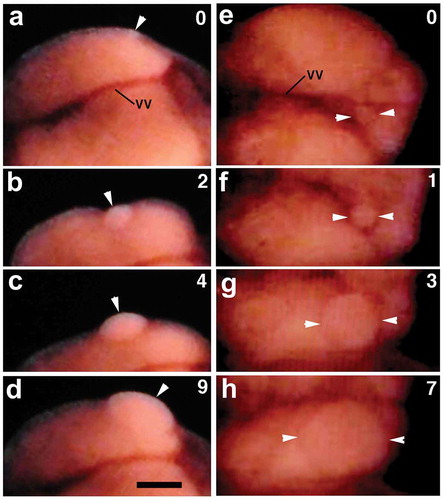
When the aforementioned movements of ovisac eggs ceased, the clitellar surface (including cocoon membrane) began to extrude slightly and quickly at various spots (; Movie S2). Such surface distortions were not easy to record photographically. It looks as if something round tapped on the coelomic wall. There is also a possibility that such surface distortions were generated via the contraction of the body muscles.
Figure 3. Ventral view of the clitellar region of a worm shortly after the completion of ovisac eggs’ movement to the ovarian coelom. Anterior is to the left. Numerals shown in the upper right of each panel indicate the time (sec) after the onset of the surface distortion. Note the quick shape change in the portion indicated by asterisks. vv, ventral vessel. Scale bar = 0.2 mm.
Coelomic eggs are squeezed out of the female gonopores (‘egg deposition’)
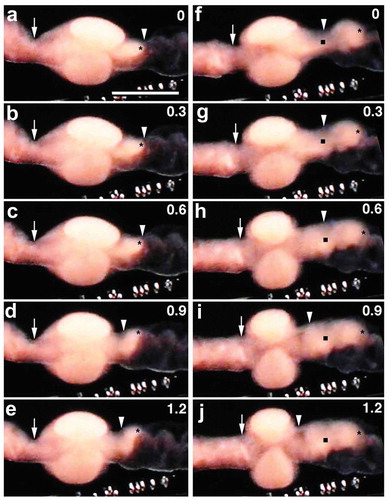
Soon after the surface events mentioned above ceased, coelomic eggs began to move outside the body. The first sign of movement was the appearance of a small, white spot on the ventral surface near the midpoint of the clitellar region ()). This spot corresponded to the discharged (or ‘outer’) portion (OP) of the coelomic egg. The remaining or ‘inner’ portion (IP) of the egg, which was still located within the coelom, assumed a slightly oblong shape, running along the ventral vessel ()). It looks as if a small sphere (i.e. the OP of the egg) was attached to the posterior margin of the IP ()). Thereafter, the OP of the egg grew in size over time (); Movie S3). In contrast, the IP of the egg became smaller over time. It should be mentioned that during the first 2 s, the anterior margin of the IP of the egg appeared not to change its position relative to the ventral vessel ()), while it was displaced by approximately 200 µm posteriorly during the next 2 s ()). When the OP of the egg was viewed from the side, it looked like a growing dome (); Movie S4). When viewed end on, the egressing egg (i.e. the OP of the egg) looked like a growing disc (); Movie S5). This was a rapid process, accomplished within a few seconds. During the next second, the dome shape of the OP of the egg disappeared, and its apical surface flattened as much as the clitellar surface. This movement of the coelomic eggs’ to the outside of the body (i.e. the space between the cocoon membrane and the clitellar epithelium) is, hereafter, referred to as ‘egg deposition’.
Figure 4. Ventral views of the clitellar region of a worm undergoing egg deposition. Anterior is to the left. (b-j) show the boxed region in (a) at higher magnification. Numerals shown in the upper right of each panel indicate the time (sec) after the onset of discharge of the egg. Arrowheads indicate the apical surface of the egg portion located outside the body. Asterisks indicate the anterior margin of the egg portion still located within the coelom. Arrows in (a) indicate the constricted margins of the clitellar region. Crosses indicate the small sphere of unidentified tissue. Scale bar in (a) = 1 mm (for a); 0.4 mm (for b-j).
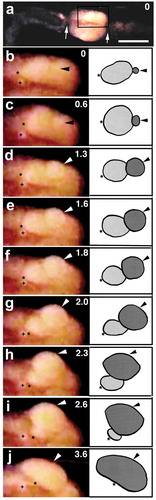
Figure 5. Ventral views of the clitellar region of worms undergoing egg deposition. Anterior is to the left. Numerals shown in the upper right of each panel indicate the time (sec) after the onset of discharge of the egg. (a-d) Lateral views of an emerging egg. Arrowheads indicate the apical surface of the egg. (e-h) End on views of another emerging egg. Paired arrowheads indicate the egg. vv, ventral vessel. Scale bar = 0.2 mm.
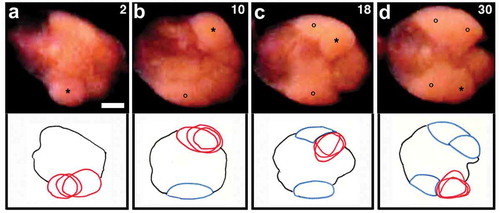
Once the egg deposition commenced, coelomic eggs in a given worm came out of the gonopore one after another (). The typical mature worms, which possessed 4–6 ovisac eggs each, accomplished egg deposition within a period of 30 s. Upon completion, the clitellar portion and adjacent regions became nearly motionless except for occasional and very slow wave-like movements. This calm state lasted for about 30 s before the worms began stretching movements at the regions near both ends of the cocoon.
Figure 6. Ventral views of the clitellar region of a worm undergoing deposition of four eggs. Anterior is to the left. (a) first egg; (b) second egg; (c) third egg; (d) fourth egg. Numerals shown in the upper right of each panel indicate the time (sec) after the onset of discharge of the first egg. Asterisks indicate the egg that is emerging. Open circles indicate the egg that has already been deposited. Line drawings shown in the lower part of each panel are tracings of the videos, highlighting the emerging egg (red lines) at the three time points. Eggs outlined in blue are those which have already been deposited. Scale bar = 0.2 mm.
The worms withdraw backwards out of the cocoon (‘cocoon deposition’)
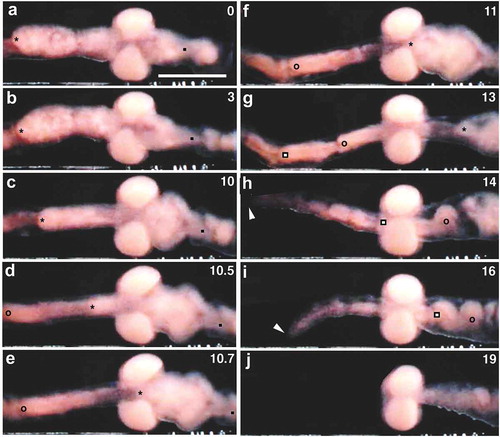
Twenty to 30 seconds after egg deposition completion, the region adjacent to the posterior end of the cocoon began to pull the posterior half of the cocoon rather vigorously. As these jerk-like movements kept repeating, coelomic cellular contents such as young oocytes that were seen in the posterior region of the cocoon gradually got closer to the posterior margin of the cocoon. Ultimately, a portion of segment 12 containing such cellular structures popped out of the constricted posterior end of the cocoon (); Movie S6). Soon after, segment 11 that remained in the cocoon began to be pulled and eventually popped out (); Movie S6).
Figure 7. Clitellar region of a worm undergoing cocoon deposition. Anterior is to the left. Numerals shown in the upper right of each panel indicate the time (sec) after the onset of the movement of the segments. (a-e) and (f-j) show the backward withdrawal of segments 12 and 11, respectively, through the posterior margin (arrowhead) of the cocoon. Asterisks indicate the posterior margin of segment 12. Solid squares indicate the posterior margin of segment 11. vv, ventral vessel. Scale bar = 1 mm.
As a result of the ‘massive’ movements of segments 11 and 12, the posterior end of the cocoon came near segment 10; the cocoon, which had assumed a lemon shape, now had a barrel shape ()). This change in the clitellar region was soon followed by the third ‘massive’ backward movement of the portion corresponding to segments 9 and 10 (); Movie S6). Since segment 10 was located adjacent to the anterior end of the cocoon, this portion passed through both ends and came to be located posterior to the cocoon. During this movement, the anterior end of the cocoon got closer to the posterior end, which remained near the centre of the cocoon. The cocoon now assumed a doughnut-like shape ()). Within a second or so, the remaining anterior nine free segments began to withdraw backwards, out of the cocoon ()); Movie S6). In contrast to the posterior segments mentioned above, these anterior segments slipped out the cocoon. The oviposition in Tubifex was complete when the prostomium of the worm was finally freed from the cocoon (); Movie S6).
Figure 8. The withdrawal movement of the anterior segments through the cocoon. Anterior is to the left. Numerals shown in the upper right of each panel indicate the time (sec) after the onset of the movement. (a-e) Withdrawal of segments 9 and 10. (f-j) Withdrawal of segments 1–8. Asterisks, anterior margin of segment 9; open circles, anterior margin of segment 8; open squares, anterior margin of segment 7; solid squares, posterior margin of segment 10. Arrowheads indicate the anteriormost part of the worm. Scale bar = 1 mm.
The oviposition programme in Tubifex
Based on his observations on living worms of Tubifex hattai (synonym of T. tubifex), Hirao (Citation1965) suggested that the oviposition behaviour is divided into two successive phases, i.e. cocoon formation around the clitellum and the release (or deposition) of the cocoon from the worm. In the present study, it has been demonstrated that the cocoon formation is followed by the movement of ovisac eggs to the ovarian coelom of segment 11 and that the cocoon deposition is preceded by the discharge of coelomic eggs from the female gonopores into the cocoon. Thus, I suggest that oviposition in Tubifex is a process consisting of four steps, i.e. cocoon formation, the movement of ovisac eggs to segment 11 ()), the deposition of eggs into the cocoon (); Movies S3 and S4) and cocoon deposition by the backward withdrawal of the body (); Movie S6). It should be mentioned that the movement of ovisac eggs to the ovarian segment is accompanied by the back and forth movements of ovisac eggs that are repeated several times before the eggs settle in the ovarian coelom (Movie S1). It is also interesting to note that the egg deposition at the third step is preceded by slight and quick changes in the contour of the clitellar region or the tapping on the coelomic wall of the clitellar region (Movie S2). These movements appear to be oviposition-specific activities because neither the back and forth movement of ovisac eggs, nor the tapping-like movement are likely observed in ‘usual’ worms, i.e. those out of the reproductive activity. In invertebrates, especially in molluscs such as Aplysia and Limax, the major aspects of female reproduction (i.e. vitellogenesis, ovulation and oviposition) are under the control of neuroendocrines (Kupfermann Citation1967; Arch Citation1976; Takeda Citation1977). It is possible that some of the aforementioned oviposition-specific activities are controlled by similar neurohormones in Tubifex. As for the neurohormones in oligochaetes, it has also been demonstrated that in Eisenia foetida, a hormone that originates from the cerebral ganglia stimulates the secretion of androgens from testicular tissues (Lattaud Citation1980).
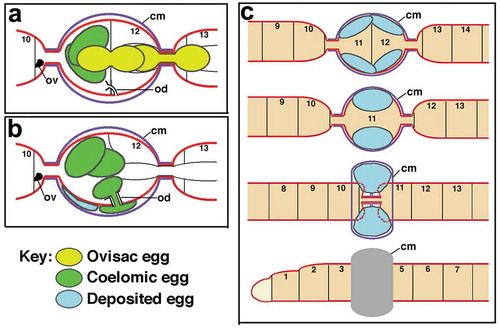
Figure 9. Diagrammatic summary of the behaviour of eggs during oviposition in Tubifex. (a) Ovisac eggs move to the ovarian coelom. (b) Coelomic eggs pass through the oviduct. (c) Deposited eggs stay within the cocoon during the three-step withdrawal of the anterior segments through the cocoon. cm, cocoon membrane; od, oviduct; ov, ovary. Not to scale.
The behaviour of eggs during oviposition: comparisons with the previous observations
Hirao (Citation1965) described the behaviour of eggs during oviposition in Tubifex: ‘the mature eggs located in the coelom of segment 11 and ovisac are released through a pair of ventrally opened female pores to the formed cocoon’. As mentioned before, the present study has revealed that the eggs stored in the ovisac are all transferred to the ovarian coelom of segment 11, which is followed by their discharge from the female gonopores into the cocoon. The present study also provides several pieces of novel information about the behaviour of eggs in the ovisac and ovarian coelom, prior to egg deposition during oviposition.
Ovisac eggs. When the ovisac eggs repeat the back and forth movement, individual eggs separate from the clump and enter the ovarian coelom one at a time. During this process, ovisac eggs do not appear to change their positions in the ovisac (or clump of eggs) and eggs directly facing the ovarian coelom are the first to be released from the clump. It is unlikely that the separation of individual eggs from the ovisac occurs continuously, it is more of an intermittent process.
As to the ovisac eggs, Hirao (Citation1965) described that these eggs are found rotating without direction, but did not mention the back and forth movement of ovisac eggs as was observed in this study. Nor did he report on the movement of ovisac eggs into the ovarian coelom. Instead, Hirao (Citation1965) reported that ‘the flows of coelomic fluid are also observable.’ Given that the cocoon formation and egg deposition proceeded normally during Hirao’s observation, there must have been a moment when the ovisac in segments 13 and 14 became empty as a result of the movement of ovisac eggs to the ovarian coelom. It seems likely that Hirao (Citation1965) interpreted such a movement of eggs along the anteroposterior body axis as a coelomic fluid flow.
Coelomic eggs. When the eggs undergoing deposition are viewed either laterally or on end, it looks as if their contents spring out and spread over the body surface. In other words, such eggs must be able to change their shape freely. It seems likely that coelomic eggs behave like a fluid during deposition. During the observations on sections of the clitellar part of worms undergoing oviposition, Hirao (Citation1965) noticed that eggs that appear to be passing through the oviduct have a dumbbell shape. In the present observations, it has been demonstrated that there is a time when a single egg appears to be divided into two equal-sized spheres, one is located within the coelom and the other outside the body ()); this egg must have had a dumbbell shape. If so, it is likely that the oviducts of Tubifex still remain as narrow tubules when coelomic eggs are passing through them. Conversely, eggs that pass through the oviduct would adapt their shape to the narrow tubules.
Then, how do coelomic eggs gain access to the entrance of the narrow oviduct and pass through it to come out of the gonopore? At present, almost nothing is known about the cellular source of the forces by which coelomic eggs pass through the oviduct. Traditionally, it has been thought that a ciliated funnel in which the internal opening of the oviduct occurs, carries eggs to the oviduct and eventually to the gonopore (Brusca and Brusca Citation2003). It is not difficult to imagine the ciliated funnel conducting coelomic eggs to the internal opening of the oviduct. By contrast, it seems unlikely that the ciliary movement of the funnel alone could squeeze the eggs into a narrow tubule. However, it is possible that at least in the initial stage of entering the tubule, eggs themselves undergo active amoeboid movement to generate forces that can dig into the tubule. Interestingly, in the mollusc Helix aspersa, isolated oocytes show amoeboid movement when they are treated with an extract of the cerebral ganglia in vitro (Saleuddin et al. Citation1983). These authors speculated that this form of motility in oocytes is used for ovulation in Helix. If a similar amoeboid movement occurred in coelomic eggs in Tubifex, it would be able to commence the initial stage of egg deposition. At present, however, whether this form of motility occurs in Tubifex eggs remains to be elucidated.
The constricted ends of the cocoon. Soon after the coelomic eggs are discharged from the female gonopores, worms begin cocoon deposition. During this, segments located in the cocoon and those located anterior to it are withdrawn backwards through the posterior end of the cocoon. In the present study, there were no cases where the deposited eggs were withdrawn through the posterior end of the cocoon with the body being withdrawn backwards. In other words, the deposited eggs are kept within the cocoon throughout the process of cocoon deposition. As shown in this study, nascent cocoons, which still surround the clitellum, take a lemon shape with constricted ends. The diameter of the constricted end of the cocoon measures approximately a half of that of the worm’s body trunk. It is apparent that the cocoon membrane strangles the worm’s body so tightly at the margin of the cocoon that its contents such as small particles and eggs are unable to pass through it. This suggests the possibility that the constricted ends play a role in keeping the deposited eggs within the cocoon. However, if the deposited eggs adhere to the inner surface of the cocoon membrane, it would be possible to keep the eggs within the cocoon, independent of the constriction of its ends. Nonetheless, this is not the case for Tubifex, because deposited eggs appear to freely change their positions relative to the cocoon membrane as well as to the body surface. It is apparent that only when the cocoon ends are constricted, can deposited eggs be prevented from escaping. In summary, I suggest that the constricted form of the cocoon ends plays an essential role in keeping deposited eggs within the cocoon during its deposition.
Comparison with other oligochaetes
To my knowledge, this is the first report with video recordings of the oviposition behaviour in living oligochaetes. It should be mentioned, however, that there are precedents for ‘direct’ observations on cocoon formation and deposition in oligochaetes other than Tubifex. Nearly a century ago, Foot and Strobell (Citation1902) and Grove and Cowley (Citation1926) made ‘direct’ observations on oviposition in living lumbricids Allolobophora foetida and Eisenia foetida, respectively, and demonstrated that ‘when the worm is not disturbed, it will withdraw backward, through the slime-tube and cocoon, finally pulling the head through the smaller end of the cocoon’. As to the behaviour of the worms undergoing cocoon deposition, Grove and Cowley (Citation1926) made a detailed report as follows: at the commencement of cocoon deposition in Eisenia, some of the segments of the clitellum (which covers the segments 26–32) are withdrawn from the posterior margin of the cocoon and swell up immediately to force the cocoon forwards. This method of withdrawal continues until the worm is free, apart from the first four or five segments. During the time that these anterior segments are being liberated, there are four to five visible characteristic jerks and the worm moves backwards, out of the cocoon. Taking these pioneer observations on Eisenia and the present observations on Tubifex into consideration, it is safe to say that aquatic oligochaete species such as Tubifex and terrestrial species such as Eisenia undergo cocoon deposition in an almost identical way.
As described before, the oviduct/female gonopore system in Tubifex is located within the clitellum, i.e. at the position of the septum 11/12 (); Dixon Citation1915). By contrast, the female gonopore and clitellum in earthworms that belong to the four oligochaete families (Almidae, Kynotidae, Glossoscolecidae and Lumbricidae) are separate from each other (Jamieson Citation2006). For instance, the female gonopores of Eisenia foetida open to the outside near the septum 13/14 while the clitellum covers the segments 26–32 (Grove and Cowley Citation1926). These differences in the relative position of the female gonopores and clitellum help to explain how the process and/or mode of egg deposition in the cocoons are different between Tubifex and Eisenia. Traditionally, egg deposition in earthworms has been thought to occur during cocoon deposition; ‘the cocoon receives the ova and spermatozoa during its passage over the apertures of the oviducts and spermathecae, respectively’ (Grove and Cowley Citation1926; Tembe and Dubash Citation1961; Brusca and Brusca Citation2003). If this traditional view is correct, egg deposition would occur only after cocoon deposition commences and there would not be any eggs in the cocoon that still surrounds the clitellum. However, as has already been known, in the lumbricid earthworms Allolobophora and Eisenia, the eggs are present in the cocoon that is located in the position of the clitellum (Foot and Strobell Citation1902; Grove and Cowley Citation1926). These important observations, which have been ignored by or have escaped the notice of more recent investigators, suggest that in these earthworms, the eggs that have been discharged from the female gonopore somehow enter the cocoon before it leaves the clitellum (Barnes Citation1968). It is highly possible that just like in Tubifex, egg deposition in the lumbricids is completed before cocoon deposition commences. To verify this, there is a need to re-examine egg deposition by directly observing living earthworms in combination with the use of such video recordings as introduced in this study.
It is well known that deposited cocoons of oligochaete clitellates assume an oval shape (Foot Citation1898; Penners Citation1933). In the lumbricids Allolobophora and Eisenia, the anterior and posterior ends of the cocoon are constricted even before the onset of cocoon deposition (Foot and Strobell Citation1902; Grove and Cowley Citation1926). As demonstrated in this study, the cocoon of Tubifex assumes a lemon shape even before the onset of the movement of ovisac eggs to the ovarian coelom. It is evident that the constriction of the cocoon ends before the withdrawal of the body through the cocoon begins is not unique to aquatic oligochaetes, but is widespread among oligochaete clitellates, even in terrestrial species. As discussed before, the constricted form of the cocoon ends is essential to keep the deposited eggs in the lumen of the cocoon during cocoon deposition. Hence, it is tempting to consider that the constriction of the cocoon ends before the onset of cocoon deposition, is a universal phenomenon in the oligochaete clitellates. In the megascolecid earthworm Pheretima posthuma, neither the anterior nor the posterior end of the cocoon appear constricted, even during the time of cocoon deposition. Only after it leaves from the worm, does the cylindrical cocoon assume an oval shape (see Text- in Tembe and Dubash Citation1961; also see Fig. 13.36 in Brusca and Brusca Citation2003). (Here, it should be pointed out that in the 1961 paper these authors presented a diagrammatic figure depicting the process of cocoon formation and deposition but did not mention the shape of the cocoon prior to the completion of the process.) If the cylindrical shape of the cocoon during its deposition is the case for Pheretima, it is apparent that egg deposition and cocoon deposition in Pheretima would proceed in a way distinct from that in other oligochaetes such as Tubifex and Eisenia. To verify this, examining these processes by ‘direct’ observations on living worms of Pheretima is required in future research.
MovieS6.mov
Download QuickTime Video (4.5 MB)MovieS5.mov
Download QuickTime Video (3.4 MB)MovieS4.mov
Download QuickTime Video (3.6 MB)MovieS3.mov
Download QuickTime Video (3.3 MB)MovieS2.mov
Download QuickTime Video (3.6 MB)MovieS1.mov
Download QuickTime Video (3.6 MB)Acknowledgments
I would like to thank Dr. Ayaki Nakamoto (Tohoku University) for his help in my access to key references. I am also grateful to Dr. Asuna Arai (Hokkaido University) for her everlasting interest in this study.
Disclosure statement
No potential conflict of interest was reported by the author.
Supplementary material
Supplemental data for this article can be accessed here.
References
- Arch S. 1976. Neuroendocrine regulation of egg laying in Aplysia californica. Am Zool. 16(2):167–175. doi:10.1093/icb/16.2.167.
- Barnes RD. 1968. Invertebrate zoology. 2nd ed. Philadelphia: Saunders.
- Brusca RC, Brusca GJ. 2003. Invertebrates. 2nd ed. Sunderland: Sinauer Associates.
- Dixon GC. 1915. Tubifex. In: Herdman WA, editor. L.M.B.C. Memoirs on typical british marine plants and animals. London: Williams and Norgate; p. 2–99.
- Foot K. 1898. The cocoons and eggs of Allolobophora foetida. J Morphol. 14(3):481–505. doi:10.1002/jmor.1050140305.
- Foot K, Strobell EC. 1902. Further notes on the cocoons of Allolobophora foetida. The Biological Bulletin. 3(5):206–213. doi:10.2307/1535874.
- Grove AJ, Cowley LF. 1926. On the reproductive processes of the brandling worm, Eisenia foetida. Q J Microsc Sci. 70:559–581.
- Hirao Y. 1964. Reproductive system and oogenesis in the freshwater oligochaete, Tubifex hattai. J Faculty Sci. 15:439–448.
- Hirao Y. 1965. Cocoon formation inTubifex, with its relation to the activity of the clitellar epithelium. J Faculty Sci. 15:625–632.
- Jamieson BGM. 2006. Non-leech Clitellata. In: Rouse G, Pleijel F, editors. Reproductive biology and phylogeny of annelida. Enfield: Science Publishers; p. 235–392.
- Kupfermann I. 1967. Stimulation of egg laying: possible neuroendocrine function of bag cells of abdominal ganglion of Aplysia californica. Nature. 216(5117):814–815. doi:10.1038/216814a0.
- Lattaud C. 1980. Demonstration by organ culture of a cerebral hormone stimulating the secretion of testicular androgen in the oligochaete annelid, Eisenia foetida f. typica Sav. Int J Invertebrate Reprod. 2(1):23–36. doi:10.1080/01651269.1980.10553339.
- Penners A. 1933. Über Unterschiede der Kokons einiger Tubificiden. Zool Anz. 103:93–95.
- Romdhane Y, Ahmed RB, Tekaya S. 2017. Sexual behavior, insemination and development of the freshwater leech Helobdella stagnalis (Annelida, hirudinea, glossiphoniidae). Invertebrate Reprod Dev. 61(4):246–253. doi:10.1080/07924259.2017.1340354.
- Saidel WM, Saglam N, Cruz DS, Saunders R, Shain DH. 2017. Elaborate ultrastructure of the Hirudo (Annelida: hirudinae) cocoon surface. J Morphol. 279(4):545–553. doi:10.1002/jmor.v279.4.
- Saleuddin ASM, Farrell CL, Gomot L. 1983. Brain extract causes amoeboid movement in vitro in oocytes in Helix aspersa (Mollusca). Int J Invertebrate Reprod. 6(1):31–34. doi:10.1080/01651269.1983.10510021.
- Shimizu T. 1982. Development in the freshwater oligochaete Tubifex. In: Harrison FW, Cowden RR, editors. Developmental biology of freshwater invertebrates. New York: Alan R Liss; p. 283–316.
- Suzutani C. 1977. Light and electron microscopical observations on the clitellar epithelium of Tubifex. J Faculty Sci. 21:1–11.
- Suzutani-Shiota C. 1980. Ultrastructural study on cocoon formation in the freshwater oligochaete, Tubifex hattai. J Morphol. 164(1):25–38. doi:10.1002/jmor.1051640103.
- Takeda N. 1977. Stimulation of egg-laying by nerve extracts in slugs. Nature. 267(5611):513–514. doi:10.1038/267513a0.
- Tembe VB, Dubash PJ. 1961. The earthworms: A review. J Bombay Nat Hist Soc. 51:171–201.
