Abstract
In the prevention and treatment of cardiovascular disease, pharmacological treatment strategies should have several aims: (i) in individuals without overt cardiovascular disease, but with risk factors such as hypertension and/or diabetes, pharmacotherapy should prevent or delay disease development; (ii) in patients who have already progressed to cardiovascular disease, pharmacotherapy should help either to prevent or regress target organ damage (TOD); and (iii) in patients with TOD, pharmacotherapy should prevent events. Any medication intended for long‐term therapy also should be well tolerated. Inhibiting the renin–angiotensin system has proven a successful therapeutic strategy in cardiovascular and renal medicine. Angiotensin‐converting enzyme (ACE) inhibitors have demonstrated important advantages over conventional agents such as beta‐blockers and thiazide diuretics, and have become a relevant part of treatment for heart failure post‐myocardial infarction, left ventricular dysfunction and renal disease. Tolerability concerns may prevent their use in some patients, however. Angiotensin AT1 receptor blockers (ARBs) provide a different form of blockade of the renin–angiotensin system and a growing body of evidence suggests that this alternative approach may confer additional cardiovascular protection for some patient subgroups. In addition, ARBs generally are better tolerated than ACE inhibitors, enhancing patient compliance and persistence with long‐term therapy. Furthermore, evidence in favour of combining an ACE inhibitor and an ARB in certain circumstances is continuously growing.
Introduction
Essential hypertension is one of the most important contributors to cardiovascular diseases, the leading cause of premature death and associated with considerable morbidity worldwide Citation[1]. Recently three relevant contributions have come to extend the knowledge of the relevance of elevated blood pressure. The first was the Comparative Risk Assessment project Citation[2], which demonstrated that examining 20 different causes of global burden of disease, high blood pressure was the leading cause of death either in developed and underdeveloped countries. The second Citation[3] showed that the relation between blood pressure and cardiovascular risk is a continuous one from values of 115/75 mmHg and the risk doubles for each increase of 20 mmHg in systolic and/or 10 mmHg in diastolic. The third Citation[4] predicts that in the near future more than 1.5 billion hypertensives will require medical attention in our world. Hence, efforts to improve lifestyle in order to reduce cardiovascular risk factors should form part of any preventive cardiovascular health programme.
However, a large proportion of hypertensive patients will also need drug therapy to lower blood pressure. Modern (albeit arbitrary) definitions of hypertension indicate that over one‐third of adults over 16 years of age, more than half of those aged over 55 years and about three‐quarters of those over the age of 65 years are hypertensive. A large proportion of the adult population therefore requires medication to lower blood pressure, according to current guidelines Citation[5–7].
Antihypertensive medications have evolved over the last half‐century, with enormous strides made towards many of these ideal requirements. Inhibiting the renin–angiotensin system has proven a successful therapeutic strategy. Angiotensin‐converting enzyme (ACE) inhibitors are effective drugs and have been shown to be particularly beneficial in a number of cardiovascular disorders Citation[8], Citation[9]. However, many patients require additional therapy to reach current blood pressure targets. On the other hand, ACE inhibitors are not always well tolerated. Consequently, there has been increasing interest in alternative and complementary methods for inhibiting the renin–angiotensin system in patients with cardiovascular disease, particularly angiotensin AT1 receptor blockers (ARBs). A growing body of evidence now indicates that ARBs are well‐tolerated and effective antihypertensive agents, which provide cardiovascular protection Citation[8], Citation[9].
The favourable profile of the ARBs in hypertension and their specific and distinctive differences compared with ACE inhibitors prompted an early inclusion of ARBs among the first‐choice drugs recommended in the 1999 international guidelines for the treatment of hypertension Citation[10]. This recommendation has been reiterated and reinforced in all sets of international guidelines published in 2003 Citation[5–7], Citation[11]. These guidelines also emphasize the evidence‐based indications for ARBs in specific subgroups of hypertensive patients with comorbid illnesses.
This paper reviews ARBs evaluating whether or not they meet the requirements of an ideal antihypertensive medication and whether they possess additional advantages over their antihypertensive effects to prevent or delay the vascular consequences of elevated blood pressure.
Mechanisms of action and potential therapeutic role
Angiotensin II‐mediated vasoconstriction, and renal salt and water retention contribute to arterial hypertension and compromise the pumping ability of the failing heart. AT1 receptor‐mediated growth‐promoting effects participate in vascular and left ventricular hypertrophy, diabetic nephrosclerosis, neointima formation and atherothrombosis, as well as in structural remodelling of the heart following myocardial infarction.
Angiotensin II, acting via the AT1 receptor, also may be instrumental in loss of brain function after brain ischaemia Citation[12], and appears to participate in inflammatory responses and in the production of free oxygen radicals with adverse effects on endothelial function and post‐ischaemic repair () Citation[13].
Figure 1. Schematic representation of the multiple effects of increased tissue production of angiotensin II. ET‐1, endothelin‐1; MCP‐1, monocyte chemoattractant protein–1; MMP, matrix metalloproteinase; NF‐κB, nuclear factor‐κB; NO, nitric oxide; PAI‐1, plasminogen activator type 1; VCAM, vascular cell adhesion molecule.
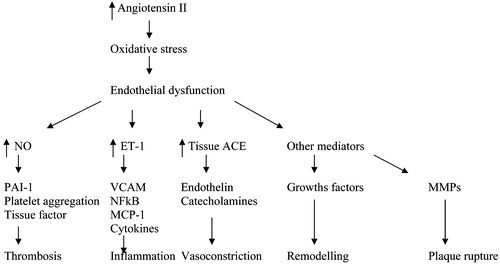
Several of the AT2 receptor‐mediated actions of angiotensin II, on the other hand, seem to antagonize those of the AT1 receptor. In the adult organism, the AT2 receptor is frequently suppressed. Tissue injury dramatically up‐regulates its expression, however, helping to restore endothelial function, for instance by producing nitric oxide Citation[14], and preventing vascular and cardiac cell growth.
Since the advent of the ACE inhibitors, and of the ARBs, many experimental and clinical have demonstrated that inhibition of the renin–angiotensin system at different levels can attenuate several pathophysiological actions of angiotensin II mediated by the AT1 receptor and thus protect vital organs to reduce cardiovascular morbidity and mortality Citation[8].
In contrast to the ACE inhibitors, however, the ARBs do not reduce angiotensin II concentrations. Instead, the ARBs produce a selective, dose‐dependent blockade of the AT1 receptor, independently of the different pathways of angiotensin II generation Citation[13]. Thus, the ARBs prevent the chymase‐mediated angiotensin II production that can occur in the presence of ACE inhibitors. The chymase pathway is known to contribute to the “escape” phenomenon, in which both angiotensin and aldosterone levels, initially lowered by ACE inhibition, eventually increase to pre‐treatment levels Citation[15–17]. In addition, in the presence of AT1 receptor blockade, the binding of angiotensin II to the unopposed AT2 receptor may provide additional beneficial effects Citation[18]. Indeed, stimulation of AT2 receptors following AT1 blockade has already been shown in animal experiments to be involved in tissue repair following myocardial infarction or injury of the nervous system Citation[19–21].
Preclinical and short‐term clinical studies have demonstrated that ARB are effective in lowering BP and offer additional cardiac, cerebral and renal protection () Citation[22].
Table I. Clinical evidence for the angiotensin II receptor blockers (ARB).
Effects of ARBs on BP and ambulatory BP
The extent of blood pressure reduction with ARBs is comparable to that achieved with all other first‐choice classes of antihypertensive drugs Citation[23]. This effect is dose dependent Citation[24].
All ARBs have been shown to reduce 24‐h average blood pressure in hypertensive patients when administrated as monotherapy and their efficacy is greater when combined with a low dose of diuretic or any member of the other classes of antihypertensive drugs Citation[25].
Tolerability, compliance and persistence
Studies performed in many countries around the world consistently show that adequate blood pressure control is achieved and maintained in only a small minority of the hypertensive population Citation[5], Citation[6]. This has negative public health consequences because cardiovascular risk is substantially greater for individuals with a blood pressure of at least 140/90 mmHg compared with those with lower blood pressure levels Citation[26].
The reasons for this poor control of hypertension worldwide continue to be the focus of considerable interest and effort. One reason may be that physicians still often base antihypertensive treatment on monotherapy Citation[27]. A recent meta‐analysis of 119 randomized, double‐blind, placebo‐controlled trials with thiazides, beta‐blockers, ARBs and calcium‐channel blockers has confirmed that combination therapy improves antihypertensive efficacy and tolerability Citation[28]. The first and second drugs, when given separately, lowered blood pressure by an average of 7.0/4.1 and 8.1/4.6 mmHg, respectively, compared with 14.6/8.6 mmHg when the two drugs were combined. In some trials, a major blood pressure reduction (13.3/7.3 mmHg) was achieved when the antihypertensive agents were administered at half‐standard doses.
Achieving the blood pressure targets needed to protect patients effectively is particularly difficult even in trials where expert physicians are treating more highly motivated patients, are using in most cases combination therapy and follow their patients more closely than would be the case in clinical practice Citation[29]. In the general hypertensive population, patients' adherence to the prescribed treatment regimen becomes a key determinant of the success of blood pressure control Citation[30].
Several factors play a role in compliance with the lifestyle changes and antihypertensive drug(s) that may be needed to control hypertension effectively. These include a high acquisition cost, treatment regimen complexity, the information given to the patient about the nature of the disease and the benefit of treatment, and the difficulty and the time involved in consulting the physician and obtaining the prescription.
However, several studies have identified side‐effects as the most frequent reason for stopping medication Citation[31]. Many patients with asymptomatic conditions, such as hypertension, are unwilling to accept treatment‐related problems. The use of drugs without, or with few, side‐effects is clearly crucial to the success of antihypertensive treatment in clinical practice. Progress in the field therefore has been marked by the introduction of agents with better tolerability profiles than their predecessors, rather than a superior antihypertensive effect.
The efficacy of ARBs to lower blood pressure either alone or in combination with other drugs is, as previously stated, similar to that of other classes of antihypertensive agents. Their tolerability, however, is superior. A large database shows that, for each member of this class, the incidence of most side‐effects is not significantly different from placebo Citation[32], Citation[33]. Furthermore, there is evidence that ARBs do not impair quality of life and indeed may even improve it, regardless of the therapeutic dose Citation[34]. Finally, patients are more likely to persist with long‐term treatment when an ARB is prescribed as the initial agent Citation[35].
Strategies intended to reduce the cardiovascular morbidity and mortality of hypertension must not only produce greater reductions in blood pressure but must also treat concomitant risk factors and/or use drugs that directly protect against end‐organ damage. Such strategies, if they are to be successful, also must be applicable to a greater proportion of the hypertensive population. Agents with optimal tolerability, such as ARBs, will be needed to achieve this goal.
Combination of an ACE inhibitor and an ARB
Available evidences recently reviewed show that the combination of an ACE inhibitor and an ARB can be contemplated in clinical practice Citation[36–38]. Symptomatic heart failure patients with left ventricular systolic dysfunction and patients with chronic renal failure and proteinuria above 1 g/day are two examples of this possibility.
Cardiac outcomes in antihypertensive treatment trials
A recent large meta‐analysis of the impact of ACE inhibitors on clinical outcomes in hypertension Citation[39] has shown that, despite their efficacy in lowering blood pressure, ACE inhibitors do not produce cardiovascular outcomes that are significantly superior to those of conventional therapy of hypertension with diuretics and beta‐blockers.
A recent meta‐analysis in hypertensive patients demonstrated that ARBs significantly reduced, by about 12%, compared with conventional antihypertensive therapy, the relative risk of composite primary cardiovascular endpoints, including stroke, non‐fatal myocardial infarction and cardiovascular death Citation[40]. The degree of lowering of blood pressure and especially early control of blood pressure seems to be very important, particularly in high‐risk hypertensive patients, for the cardiovascular outcome as shown by the data of the Valsartan Antihypertensive Long‐term Use Evaluation trial (VALUE) that compared a combination of an ARB/diuretic arm versus a calcium‐channel blocker/diuretic arm Citation[41].
Cerebrovascular outcomes in antihypertensive treatment trials
A recent meta‐analysis of studies using ARBs Citation[42], including IDNT Citation[43], RENAAL Citation[44], LIFE Citation[45] and SCOPE Citation[46], reveals a reduction in stroke risk with ARB‐based, compared with control, regimens. This positive effect is largely due to the findings of the LIFE trial Citation[45]. LIFE demonstrated that, in hypertensive patients with left ventricular hypertrophy, losartan reduced the relative risk of stroke, despite a blood‐pressure‐lowering effect similar to that observed with atenolol. In the SCOPE (Study on Cognition and Prognosis in the Elderly) trial Citation[46], candesartan reduced the relative risk of non‐fatal stroke. Data from the VALUE trial Citation[25] showed that the rate of stroke was lower in the amlodipine arm, although not significantly with an increased risk of 15% for the ARB group. However, the differences in blood pressure control between the two groups (significantly lower in the amlodipine arm) have important implications for the observed results, as the hypothesis of the trial required that similar levels of blood pressure control be achieved.
The efficacy of ACE inhibitors in preventing cerebrovascular disease is similar to that of therapy based either on diuretics and beta‐blockers or on calcium antagonists Citation[39], Citation[42]. The underlying mechanism for this cerebrovascular protection is therefore probably related to blood pressure reduction and ACE inhibitors are unlikely to offer added benefits.
Nevertheless, there is a belief that ACE inhibitors possess a unique ability to prevent stroke that is at least partly independent of blood pressure reduction. Such an interpretation emerged mainly from the results of the HOPE (Heart Outcomes Prevention Evaluation) Citation[47] and PROGRESS (perindopril PROtection aGainst REcurrent Stroke Study) Citation[48] trials. Yet these studies provide, at best, only weak support for this hypothesis. In the HOPE trial, ramipril was associated with a 32% reduction in the relative risk of stroke as compared with placebo. This reduction was associated with a small decease in blood pressure (3.3 mmHg systolic and 1.4 mmHg diastolic), which, at most, could explain only a 13% reduction in stroke. However, in a subgroup of 20 patients undergoing 24‐h ambulatory blood pressure monitoring, most of the antihypertensive effect due to ramipril was exerted during the night. This may indicate that daytime office blood pressure measurements may have underestimated the antihypertensive effect of ramipril administered at night‐time Citation[49]. In the PROGRESS trial, perindopril, which reduced blood pressure by only modestly 5/3 mmHg, did not prevent recurrent stroke. A greater reduction in blood pressure values (by 12/5 mmHg) and in the risk of recurrent stroke was observed only in the group of patients treated with the combination of perindopril and indapamide. It is therefore highly likely that the prevention of cerebrovascular disease associated with ACE inhibition is related to blood pressure reduction per se and not to another, specific but not antihypertensive, drug‐related effect.
Renal outcomes in antihypertensive treatment trials
Diabetic renal disease
Diabetic nephropathy, a common complication in patients with type 2 diabetes, is the leading cause of end‐stage renal disease (ESRD) in the western world Citation[50]. In diabetic patients with nephropathy, two large‐scale randomized placebo‐controlled trials have demonstrated superiority of ARBs over either dihydropyridine calcium antagonists or conventional therapy on renal protection in diabetic patients Citation[43], Citation[44].
ACE inhibitors have been considered agents of choice to protect patients with type 1 diabetes against kidney disease progression Citation[5], Citation[6]. The increasing use of ACE inhibitors to treat the early stage of nephropathy (i.e. microalbuminuria) is a response to the growing emphasis on starting treatment early in the belief that this will prevent future organ damage Citation[5]. Here again, the evidences available on the prevention of development of overt diabetic nephropathy in type 2 diabetic patients correspond to data obtained with an ARB in the IRMA‐2 study Citation[51] and extended with the demonstration at equal BP control that an ARB valsartan differs from a calcium antagonist by its capacity to lower albuminuria in either normo and hypertensive microalbuminuric type 2 diabetic patients Citation[52].
Recently published data have, however enhanced the capacity of ACE inhibitor in the protection of the kidney in type 2 diabetic patients with the demonstration of the capacity of trandolapril alone or in combination with verapamil for the primary prevention of development of microalbuminuria in hypertensive normoalbuminuric type 2 diabetic patients in the BENEDICT study Citation[53] and also by the data from the DETAIL study Citation[54] showing a similar capacity of enalapril and telmisartan for long‐term protection (5 years) of glomerular filtration rate in microalbuminuric type 2 hypertensive diabetic patients. However, no trial with an ACE inhibitor has yet shown positive effects on ESRD or death as single or combined end‐points, or on doubling of serum creatinine coupled with ESRD or death Citation[55].
Non‐diabetic renal disease
In patients with non‐diabetic renal disease, a recent meta‐analysis of 11 studies Citation[56] concluded that ACE inhibitors slow the progression of renal disease via a mechanism that is, in part, independent of their blood pressure lowering effects. Recent data from the AASK trial found no further reduction in the progress of renal dysfunction in African‐American hypertensives with nephrosclerosis by reducing blood pressure to 128/78 mmHg rather than 141/85 mmHg, but ACE inhibitors were shown to be somewhat more effective than beta‐blockers or calcium antagonists Citation[57]. ARBs were shown to have similar efficacy to ACE inhibitors in reducing proteinuria and slowing renal disease progression in the COOPERATE study, although their combined use was superior to monotherapy with either agent Citation[58].
In conclusion, long‐term data on the use of ACE inhibitors for renal protection in type 2 diabetes is limited and conflicting. Although ACE inhibitors have shown positive effects against markers of renal disease in patients with type 2 diabetes, there is insufficient clinical evidence to adequately compare these agents with those of other classes of antihypertensive agents in their effects on time to ESRD. In contrast, ARBs have been shown in type 2 diabetes to be superior to other classes of antihypertensive agent in delaying renal disease progression, and to have better tolerability, although no head‐to‐head comparison with an ACE inhibitor exists. In non‐diabetic disease, the COOPERATE trial shows that ARBs and ACE have similar effects in delaying renal progression.
Effects on heart disease progression
Congestive heart failure
In the developed world, congestive heart failure is the cardiovascular disorder with the greatest impact on public health resources Citation[59]. Optimal therapy, which can slow the progression of this disease, is based on potent inhibitors of the renin–angiotensin–aldosterone system, which is now know to be central to the pathophysiology of congestive heart failure. Effective inhibition of this system requires a combination of agents to block each of its three main components: a beta‐blocker; an aldosterone antagonist; and an ACE inhibitor and/or an ARB to block angiotensin II‐mediated effects.
An ARB combined with an ACE inhibitor
Ex vivo studies of human cardiac tissue Citation[60] have shown that complete inhibition of the effects of angiotensin II requires both ACE inhibition and AT1 receptor blockade. Similar conclusions emerged from the RESOLVD (randomized evaluation of strategies for left ventricular dysfunction) pilot trial Citation[61]. In the Valsartan Heart Failure Trial (Val‐HeFT) Citation[62] a further reduction in cardiovascular morbidity and mortality was achieved with the addition of valsartan, at a daily dose of up to 320 mg, to conventional therapy including an ACE inhibitor. The most marked effect was that of a 27.5% reduction (p<0.001) in hospitalization for heart failure, the most frequent, disabling and costly morbid event associated with the condition. Similar findings were obtained with the addition of candesartan to conventional therapy in the CHARM‐added trial Citation[63]. In this study, 2548 patients received candesartan in addition to standard therapy, including an ACE inhibitor. These results showed a significant 15% reduction in the primary endpoint of combined cardiovascular mortality and heart failure admission. Candesartan also reduced the total number of hospitalizations for heart failure.
These added benefits may be attributable to the greater sustained reductions in blood pressure and in suppression of plasma noradrenalin Citation[64] and aldosterone Citation[65] achieved with an ARB. Heart failure management guidelines therefore need an update to include the possibility of addition of ARBs to conventional treatment in symptomatic patients.
An ARB instead of an ACE inhibitor
In the Val‐HeFT trial, a subgroup analysis, in the heart failure patients intolerant of ACE inhibitors, demonstrated for the first time that the use of valsartan substantially reduced the risk of the combined morbidity and mortality endpoints, including survival and hospitalization for cardiovascular events Citation[66]. These results were fully confirmed by the CHARM‐alternative trial Citation[67]. Both ARBs valsartan and candesartan have the specific indication for heart failure patients.
Various trials have compared the efficacy of ARBs with ACE inhibitors in patients with congestive heart failure. ELITE II Citation[68] and OPTIMAAL Citation[69] both failed to demonstrate non‐inferiority for losartan 50 mg once a day compared with captopril, but this may have been attributable to an insufficiently effective dose of losartan.
Post‐myocardial infarction (MI)
The RAS is activated in patients with acute MI, as it is in subjects with LV dysfunction and heart failure. Plasma renin activity is also predictive of cardiovascular mortality post‐MI. ACE inhibitors reduce mortality, slow progression to heart failure and are of great benefits in high‐risk patients Citation[70]. Trials of non‐selected acute myocardial infarction (AMI) patients, including the Fourth International Study of Infarct Survival (ISIS‐4) (ISIS‐4 (Fourth International Study of Infarct Survival) Citation[71] and the Third Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardio (GISSI‐3) Citation[72], showed a modest benefit with ACE inhibitor treatment, with mortality reduced by 7% to 12%. Greater benefits were seen in several trials that focused on selected patient populations Citation[73].
Two studies evaluated the effects of ARBs in post‐MI patients. In the VALIANT trial, valsartan 160 mg bid was, in the 14 703 patients with AMI complicated by heart failure, left ventricular dysfunction or both, at least as effective in reducing the risk of death and cardiovascular death, non‐fatal myocardial infarction, or heart failure as a proven dose of captopril Citation[74]. The VALIANT findings therefore suggest that valsartan is a clinically effective alternative to an ACE inhibitor. However, in the OPTIMAAL trial (Optimal Trial in Myocardial Infarction with the Angiotensin II Antagonist Losartan), Kenneth Dickstein and colleagues compared losartan and captopril and found a non‐significant difference in total mortality in favour of captopril in high‐risk patients after AMI. This difference seems mainly driven by a significant excess of cardiovascular deaths in the losartan group Citation[69].
Taken together, the results of trials with ARBs indicate as suggested in recently published reviews Citation[36], Citation[37] that these drugs may be used as an alternative to ACE inhibitor therapy in patients following myocardial infarction and in those with heart failure who cannot tolerate ACE inhibitors. They may also be added to ACE inhibitors and other conventional treatments in symptomatic heart failure patients.
Effect on target organ damage and metabolic disorders
Protection against left ventricular hypertrophy
In hypertensive patients, left ventricular hypertrophy (LVH) represents a mechanism of adaptation to abnormal loading. However, LVH is also the first step toward the development of clinical cardiovascular diseases, such as congestive heart failure, ischaemic heart disease, stroke and sudden death Citation[75], Citation[76]. Several mechanisms have been proposed to explain these increased cardiovascular risks. These include cardiac diastolic and systolic dysfunction, predisposition to arrhythmias, alteration of autonomic nervous system activity, and reduced coronary flow reserve.
Blood pressure reduction by pharmacological treatment may reverse LVH. Increasing evidence demonstrates that changes in left ventricular mass during antihypertensive treatment may directly influence the risk of clinical cardiovascular complications Citation[77]. However, different classes of antihypertensive drugs may not be similarly effective in reducing left ventricular mass for the same antihypertensive effect. Certain drugs may interfere differently with several non‐haemodynamic factors, which may contribute to the increase in left ventricular mass. These include the renin–angiotensin system, the sympathetic nervous system and growth factors.
Treatment with agents that interfere with the renin–angiotensin system has produced positive effects on cardiovascular structural changes. A meta‐analysis of studies of LVH regression has indicated that ACE inhibitors and ARBs, together with calcium‐channel blockers, may be more effective than beta‐blockers and diuretics in reducing left ventricular mass, for similar blood pressure reductions Citation[78]. In LIFE, losartan was more effective than atenolol in inducing LVH regression and provided superior cardiovascular protection Citation[45]. In another study in hypertensive patients, valsartan was superior to enalapril in improving diastolic function Citation[79].
In hypertensive patients with cardiovascular hypertrophy, blood pressure reduction by pharmacological treatment may improve and even completely reverse structural changes. Blockade of the renin–angiotensin system may confer an additional benefit beyond blood pressure reduction. To date, as stated in a recent review Citation[80], plenty of echocardiographic studies reported a superiority or non‐inferiority of an ARB vs a comparator regimen, including ACE inhibitors in reducing the extent, and consequences, of cardiovascular remodelling.
Protection against arterial vascular hypertrophy
Structural and functional abnormalities of small and large arteries are also frequently observed in patients with arterial hypertension. In small resistance arteries, increased arterial wall thickness and lumen reduction seem to play an important role in the increase of vascular resistance, and may reduce maximal flow reserve, thus inducing a greater change in resistance for any given degree of smooth muscle shortening. In the large elastic arteries of hypertensive patients, an increased stiffness has been described, leading to higher systolic pressure and to an increased load on the heart.
Long‐term antihypertensive treatment with ACE inhibitors, calcium antagonists and ARBs also can normalize small resistance artery structure, an effect not achieved with beta‐blockers, despite similar blood pressure reductions Citation[81]. Renin–angiotensin system blockade was also highly beneficial in Val‐PREST (Valsartan for Prevention of Restenosis after Stenting of type B2/C lesions), in which valsartan treatment halved the incidence of restenosis in patients undergoing coronary stenting Citation[82].
Protection against endothelial dysfunction and atherosclerosis
Endothelial dysfunction: possible mechanisms
Angiotensin II exerts a negative effect, via AT1 receptor, on endothelial function by releasing endothelin‐1 (ET‐1) Citation[83] and vasoconstrictor prostanoids Citation[84], and by inhibiting nitric oxide synthase activity. Moreover, angiotensin II increases oxygen free‐radical production via membrane‐bound NADH/NADPH oxidases Citation[85]. In the presence of ARBs, which selectively block AT1 receptors, angiotensin II can bind to unblocked AT2 receptors Citation[86], which may stimulate nitric oxide synthesis Citation[87]. ARBs therefore theoretically should be superior to ACE inhibitors in restoring normal endothelial function. Although ACE inhibitors are known to inhibit the degradation, and hence increase the plasma concentration, of bradykinin, an endothelium‐dependent vasodilator, some experimental evidence suggests that AT1 receptor antagonists, too, can activate the bradykinin system Citation[88].
Effect of ACE inhibitors and ARBs on endothelium‐dependent vasodilation in humans: Studies in patients with essential hypertension
Studies in conduit arteries
In the coronary epicardial arteries from patients with essential hypertension but with no overt atherosclerosis, intravenous administration of perindoprilat (1 mg i.v.) restored the normal vascular response to the cold pressor test and flow‐mediated dilation Citation[89]. We are aware of no similar studies with ARBs. In the peripheral circulation of hypertensive patients, 6 months' treatment with perindopril (2–4 mg daily), but not with telmisartan (40–80 mg daily), restored brachial artery flow‐mediated dilation Citation[90].
Studies in the microcirculation
Studies in subcutaneous small vessels demonstrated that 2 years', but not 1 year's, treatment with cilazapril improved, but did not normalize, the blunted response to acetylcholine Citation[91], Citation[92]. Similar results were obtained by 3 years' treatment with lisinopril Citation[93]. In contrast, 1 year's treatment with losartan fully restored the vasodilatory effect of acetylcholine Citation[94].
All studies assessing the effect of ACE inhibitors or ARBs in the forearm microcirculation have been negative. Two months' treatment with captopril or enalapril Citation[95], 5 months' treatment with cilazapril Citation[96] or 1 year's treatment with lisinopril Citation[97] induced no change in the impaired response to methacholine or acetylcholine. On the other hand, in the group of hypertensive patients enrolled in one of these trials, lisinopril increased the vasodilatory response to bradykinin Citation[98]. However, the increased bradykinin‐induced vasodilation was related to an ouabain‐sensitive pathway, possibly hyperpolarization, rather than to restoration of nitric oxide availability Citation[98]. Similarly, treatment with candesartan Citation[98] or valsartan Citation[99] failed to improve acetylcholine‐induced vasodilation in the forearm microcirculation of patients with essential hypertension.
The available evidence indicates that AT1 antagonists and ACE inhibitors are similarly effective in reversing endothelial dysfunction, with the more convincing effect being observed in the conduit artery of patients with coronary artery disease. Although ACE inhibitors seem more effective in the brachial artery of patients with essential hypertension, this finding is confined to one study. Both drug classes are effective in the subcutaneous, but not in the forearm, microcirculation, although ACE inhibitors can at least potentiate the vasodilation to bradykinin, an effect which is not mediated, however, by the restoration of NO availability.
Preserving cognition
Another important aspect of treating hypertensive patients with ACE inhibitors and ARBs is their potential to prevent cognitive decline and even to improve cognitive function. This effect, which has been demonstrated in animal models, is specific to agents that inhibit the renin–angiotensin system and is related to the negative effects of brain angiotensin II and its fragments on learning and memory paradigms Citation[100]. In the SCOPE study, the proportion of patients who had a significant decline or developed dementia was similar in the candesartan and placebo arms Citation[46]. However, in a recent study valsartan, but not enalapril, significantly improved cognitive function in patients with essential hypertension Citation[101].
Metabolic syndrome and diabetes
Prognosis in hypertension is directly related to blood pressure reduction. Even small reductions in blood pressure are associated with large reductions in cardiovascular risk, especially in hypertensive patients with additional cardiovascular risk factors and, in particular, diabetes mellitus Citation[102]. This reinforces the need to determine the optimal blood pressure reduction for patients with type 2 (non‐insulin‐dependent) diabetes.
There is also a need to target people without overt diabetes but in whom hypertension is accompanied by disturbed glucose and insulin metabolism, i.e. the metabolic syndrome Citation[103], Citation[104]. There is much evidence showing that these subjects are at increased risk of cardiovascular disease and premature all‐cause mortality as well. Moreover, the finding that the metabolic syndrome and hypertension often co‐exist has increased interest in avoiding the adverse metabolic effects of antihypertensive agents that could precipitate the development of new‐onset diabetes during long‐term treatment Citation[102]. The cardiovascular risk associated with new‐onset diabetes appears similar to that observed in patients diagnosed with type 2 diabetes at baseline, given a sufficiently long follow‐up Citation[106], Citation[105]. ARBs have been shown to prevent the development of new‐onset diabetes when compared with diuretics and beta‐blockers in long‐term studies in hypertensive patients, including LIFE Citation[45] and SCOPE Citation[46]. Similar results have been obtained during ARB therapy in patients with heart failure Citation[74], Citation[107]. In the recent VALUE trial Citation[41], patients in the valsartan arm had 23% fewer cases of new‐onset diabetes compared with those with amlodipine: 13.1% vs 16.4%, respectively (p<0.0001).
Type 2 diabetes
A recent review of antihypertensive therapy in type 2 diabetic patients found that, in most trials analysing cardiovascular outcome, most patients were on two‐, three‐, or even four‐drug therapy Citation[102]. This is also true in trials comparing ARBs with other therapies, such in the substudy of diabetic patients in LIFE (Losartan Intervention For Endpoint reduction in hypertension study) Citation[108], RENAAL (Reduction of End points in NIDDM (non‐insulin‐dependent diabetes mellitus) with Angiotensin II Antagonist Losartan) Citation[44] and IDNT (Irbesartan Diabetic Nephropathy Trial) Citation[43]. The LIFE study showed a consistently significant reduction of major cardiovascular events, cardiovascular death and total mortality when losartan was compared with a beta‐blocker Citation[108]. Similar positive effects for non‐fatal cardiovascular events were also observed in a meta‐analysis of ARB trials Citation[109] that included data from RENAAL, IDNT and IRMA‐2 (Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria Study II) Citation[51]. In addition, data from the LIFE study indicate that losartan affords better protection than atenolol from cardiac death from arrhythmia Citation[110]. These data suggest a greater protective effect not only on the kidney but also on the cardiovascular system in patients with type 2 diabetes.
Metabolic syndrome
Strict blood pressure control is also necessary in patients with hypertension and the metabolic syndrome and there is a strong rationale for including an ARB in the combination therapy that most of these patients need Citation[112].
Angiotensin receptor blockade improves insulin sensitivity in animal models of insulin resistance Citation[113] and in humans Citation[114], Citation[115]. Furthermore, irbersartan and telmisartan have been shown to enhance PPARγ activity, which may represent new pleiotropic actions of ARBs, providing a potential mechanism for their insulin‐sensitizing/antidiabetic effects Citation[116], Citation[117]. This contrasts with the disturbances in glucose metabolism associated with other antihypertensive agents and which may worsen long‐term outcome Citation[102]. Thus, an improvement in insulin sensitivity with ARB therapy could be the main mechanism impeding or retarding the appearance of new‐onset diabetes.
In conclusion, the cardiovascular protective effects of ARBs appear to be at least as good as those of ACE inhibitors in patients with the metabolic syndrome or type 2 diabetes.
Conclusions
The available data demonstrate that ARBs contribute to improving the prognosis of patients with cardiovascular and/or renal disease and appear to provide clinical benefit across the risk spectrum and regardless of the stage of disease. These benefits are facilitated by the long‐term adherence of the patients to ARBs due to their excellent tolerability similar to that of placebo.
References
- Kannel W. B. Blood pressure as a cardiovascular risk factor: Prevention and treatment. JAMA 1996; 275: 1571–1576
- Ezzati M., Lopez A. D., Rodgers A., Vander Hoorn S., Murray C. J. Selected major risk factors and global and regional burden of disease. Lancet 2002; 360: 1347–1360
- Lewington S., Clarke R., Qizilbash N., Peto R., Collins R. Prospective Studies Collaboration. Age‐specific relevance of usual blood pressure to vascular mortality: A meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360: 1903–1913
- Kearney P. M., Whelton M., Reynolds K., Muntner P., Whelton P. K., He J. Global burden of hypertension. Analysis of worldwide data. Lancet 2005; 365: 217–223
- Chobanian A. V., Bakris G. L., Black H. R., Cushman W. C., Green L. A., Izzo J. L., Jr. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA 2003; 289: 2560–2572
- European Society of Hypertension–European Society of Cardiology Guidelines Committee. 2003 European Society of Hypertension–European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens 2003; 21: 1011–1053
- Williams B., Poulter N. R., Brown M. J., Davis M., McInnes G. T., Potter J. F. British Hypertension Society guidelines for hypertension management 2004 (BHS‐IV): Summary. BMJ 2004; 328: 634–640
- Kjeldsen S. E., Julius S. Hypertension mega‐trials with cardiovascular end points: Effect of angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers. Am Heart J 2004; 148: 747–754
- Abbott K. C., Bakris G. L. What have we learned from the current trials?. Med Clin North Am 2004; 88: 189–207
- Subcommittee. 1999 World Health Organization and International Society of Hypertension Guidelines for the Management of Hypertension. Guidelines Subcommittee. J Hypertension 1999; 17: 151–183
- World Health Organization, International Society of Hypertension Writing Group. 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens 2003; 21: 1983–1992
- Dai W. J., Funk A., Herdegen T., Unger T., Culman J. Blockade of central angiotensin AT(1) receptors improves neurological outcome and reduces expression of AP‐1 transcription factors after focal brain ischemia in rats. Stroke 1999; 30: 2391–2398
- De Gasparo M., Catt K. J., Inagami T., Wright J. W., Unger T. The angiotensin II receptors. Pharmacol Rev 2000; 52: 415–472
- Gohlke P., Pees C., Unger T. AT2 receptor stimulation increases aortic cyclic GMP in SHRSP by a kinin‐dependent mechanism. Hypertension 1998; 31: 349–355
- Biollaz J., Brunner H. R., Gavras I., Waeber B., Gavras H. Antihypertensive therapy with MK 421: Angiotensin II‐‐renin relationships to evaluate efficacy of converting enzyme blockade. J Cardiovasc Pharmacol 1982; 4: 966–972
- Pitt B. “Escape” of aldosterone production in patients with left ventricular dysfunction treated with an angiotensin‐converting enzyme inhibitor: Implications for therapy. Cardiovasc Drugs Ther 1995; 9: 145–149
- Cicoira M., Zanolla L., Franceschini L., Rossi A., Golia G., Zeni P. Relation of aldosterone “escape” despite angiotensin‐converting enzyme inhibitor administration to impaired exercise capacity in chronic congestive heart failure secondary to ischaemic or idiopathic dilated cardiomyopathy. Am J Cardiol 2002; 89: 403–407
- Siragy H. Angiotensin II receptor blockers: Review of the binding characteristics. Am J Cardiol 1999; 84: 3S–8S
- Sandmann S., Minghuan Yu., Kaschina E., Blume A., Bouzinova E., Aalkjaer Ch. Differential effects of angiotensin AT1 and AT2 receptors on the expression, translation and function of the Na+‐H+ exchanger and Na+‐HCO3 cotransporter in the rat heart after myocardial infarction. J Am Coll Cardiol 2001; 37: 2154–2165
- Lucius R., Gallinat S., Rosenstiel P., Herdegen T., Sievers J., Unger T. The angiotensin II type 2 (AT2) receptor promotes axonal regeneration in the optic nerve of adult rats. J Exp Med 1998; 188: 661–670
- Reinecke K., Lucius R., Reinecke A., Rickert U., Herdegen T., Unger T. Angiotensin II accelerates functional recovery in the rat sciatic nerve in vivo: Role of the AT2 receptor nd the transcription factor NF‐κB. FASEB J 2003; 17: 2094–2096, Available from: URL: http://www.fasebj.org/cgi/doi/10.1096//fj.02‐1193fje
- Schmieder R. E. Mechanisms for the clinical benefits of angiotensin II receptor blockers. Am J Hypertension 2005; 18: 720–730
- Conlin P. R., Gerth W. C., Fox J., Roehm J. B., Boccuzzi S. J. Four‐year persistence patterns among patients initiating therapy with the angiotensin II receptor antagonist losartan versus other antihypertensive drug classes. Clin Ther 2001; 23: 1999–2010
- Pool J., Glazer R., Chiang Y. T., Gatlin M. Dose–response efficacy of valsartan, a new angiotensin II receptor blocker. J Hum Hypertens 1999; 13: 275–281
- Neutel J., Smith D. H. Evaluation of angiotensin II receptor blockers for 24‐hour blood pressure control: Meta‐analysis of a clinical database. J Clin Hypertens 2003; 5: 58–63
- Benetos A., Thomas F., Bean K., Gautier S., Smulyan H., Guize L. Prognostic value of systolic and diastolic blood pressure in treated hypertensive men. Arch Intern Med 2002; 162: 577–581
- Colhoun H. M., Dong W., Poulter N. R. Blood pressure screening, management and control in England: Results from the health survey for England 1994. J Hypertens 1998; 16: 747–752
- Elliott W. J. Therapeutic trials comparing angiotensin converting enzyme inhibitors and angiotensin II receptor blockers. Curr Hypertens Rep 2000; 2: 402–411
- Mancia G., Grassi G. Systolic and diastolic blood pressure control in antihypertensive drug trials. J Hypertens 2002; 20: 1461–1464
- Mallion J. M., Poggi L. A population‐based survey of drug treatment efficacy by the French National Committee for the control of arterial hypertension. Am J Hypertens 1998; 11: 903–904
- Rudd P. Clinicians and patients with hypertension: Unsettled issues about compliance. Am Heart J 1995; 130: 572–579
- Mancia G., Seravalle G., Grassi G. tolerability and treatment compliance with angiotensin II receptor antagonists. Am J Hypertens 2003; 16: 1066–1073
- Oparil S., Barr E., Elkins M., Liss C., Vrecenak A., Edelman J. Efficacy, tolerability, and effects on quality of life of losartan, alone or with hydrochlorothiazide, versus amlodipine, alone or with hydrochlorothiazide, in patients with essential hypertension. Clin Ther 1996; 18: 608–625
- Dahlöf B., Lindholm L. H., Carney S., Pentikainen P. J., Ostergren J. Main results of the losartan versus amlodipine (LOA) study on drug tolerability and psychological general well‐being. LOA Study Group. J Hypertens 1997; 15: 1327–1335
- Gerth W. C. Compliance and persistence with newer antihypertensive agents. Curr Hypertens Rep 2002; 4: 424–433
- Lee V. C., Rhew D. C., Dylan M., Badamgarav E., Braunstein G. D. Meta‐analysis: Angiotensin‐receptor blockers in chronic heart failure and high risk myocardial infarction. Ann Intern Med 2004; 141: 693–704
- Mc Murray J. J. V., Pfeffer M. A., Swedberg K., Dzau V. J. Which inhibitor of the rennin‐angiotensin system should be used in chronic heart failure and acute myocardial infarction?. Circulation 2004; 110: 3281–3288
- Wolf G., Ritz E. Combination therapy with ACE inhibitors and angiotensin II receptor blockers to halt progression of chronic renal disease: Pathophysiology and indications. Kidney Int 2005; 67: 799–812
- Blood Pressure Lowering Treatment Trialists' Collaboration. Effect of ACE inhibitors, calcium antagonists, and other blood pressure lowering drugs: Results of prospectively designed overviews of randomized trials. Lancet 2000; 356: 1955–1964
- Turnbull F. Effects of different blood pressure‐lowering regimens on major cardiovascular events: Results of prospectively‐designed overviews of randomised trials. Lancet 2003; 362: 1527–1535
- Julius S., Kjeldsen S. E., Weber M., Brunner H. R., Ekman S., Hansson L., for the VALUE group. Outcome in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: The VALUE randomised trial. Lancet 2004; 363: 2022–2031
- Blood Pressure Lowering Trialists' Collaboration. Effect of different blood‐pressure‐lowering regimens in major cardiovascular events: Results of prospectively‐designed overviews of randomised trials. Lancet 2003; 362: 1257–1235
- Lewis E. J., Hunsicker L. G., Clarke W. R., Berl T., Pohl M. A., Lewis J. B. Renoprotective effects of the angiotensin‐receptor antagonists irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345: 851–860
- Brenner B. M., Cooper M. E., de Zeeuw D., Keane W. F., Mitch W. E., Parving H. H. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345: 861–869
- Dahlöf B., Devereux R. B., Kjeldsen S., Julius S., Beevers G., de Faire U. Cardiovascular morbidity and mortality in the Losartan Intervention For End Point reduction in hypertension study (LIFE): A randomised trial against atenolol. Lancet 2002; 359: 955–1003
- Lithell H., Hansson L., Skoog I., Elmfeldt D., Hofman A., Olofsson B. The Study on Cognition and Prognosis in the Elderly (SCOPE): Principal results of a randomized double‐blind intervention trial. J Hypertens 2003; 21: 875–886
- Yusuf S., Sleight P., Pogue J., Bosch J., Davies R., Dagenais G. Effects of an angiotensin‐converting‐enzyme inhibitor, ramipril, on cardiovascular events in high‐risk patients. The Heart Outcomes Prevention Evaluation Study investigators. N Engl J Med 2000; 342: 145–153
- PROGRESS Collaborative Group. Randomised trial of a perindopril‐based blood‐pressure‐lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 2001; 358: 1033–1041
- Svensson P., de Faire U., Sleight P., Yusuf S., Ostergren J. Comparative effects of ramipril on ambulatory and office blood pressures. A HOPE substudy. Hypertension 2001; 38: E28–32
- Zimmet P., Alberti K. G., Shaw J. Global and societal implications of the diabetes epidemic. Nature 2001; 414: 782–787
- Parving H. H., Lehnert H., Brochner‐Mortensen J., Gomis R., Andersen S., Arner P., Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria Study Group. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 2001; 345: 870–878
- Viberti G. C., Wheeldon M. N. Microalbuminuria reduction with valsartan in patients with type 2 diabetes mellitus: A blood pressure‐independent effect. Circulation 2002; 106: 672–678
- Ruggenenti P., Fassi A., Ilieva A. P., Bruno S., Iliev I. P., Brusegan V., Rubis N. Preventing microalbuminuria in type 2 diabetes. N Eng J Med 2004; 351: 1941–1951
- Barnett A. H., Bain S. C., Bouter P., Karlberg B., Madsbad S., Jervell J. Angiotensin‐receptor blockade versus converting‐enzyme inhibition in type 2 diabetes and nephropathy. N Eng J Med 2004; 351: 1952–1961
- Bakris G. L., Weir M. ACE inhibitors and protection against kidney disease progression in patients with type 2 diabetes: What's the evidence. J Clin Hypertens (Greenwich) 2002; 4: 420–423
- Jafar T. H., Stark P. C., Schmid C. H., Landa M., Maschio G., de Jong P. E. Progression of chronic kidney disease: The role of blood pressure control, proteinuria, and angiotensin‐converting enzyme inhibition: A patient‐level meta‐analysis. Ann Intern Med 2003; 139: 244–252
- Agodoa L. Y., Appel L., Bakris G. L., Beck G., Bourgoignie J., Briggs J. P., African American Study of Kidney Disease and Hypertension (AASK) Study Group. Effect of ramipril vs. amlodipine on renal outcomes in hypertensive nephrosclerosis: A randomized controlled trial. JAMA 2001; 285: 2719–2728
- Nakao N., Yoshimura A., Morita H., Takada M., Kayano T., Ideura T. Combination treatment of angiotensin‐II receptor blocker and angiotensin‐converting‐enzyme inhibitor in non‐diabetic renal disease (COOPERATE): A randomised controlled trial. Lancet 2003; 361: 117–124
- Cowie M. R., Mosterd A., Wood D. A., Deckers J. W., Poole‐Wilson P. A., Sutton G. C. The epidemiology of heart failure. Eur Heart J 1997; 18: 208–225
- Wolney A., Clozel J., Rein J., Mory P., Vogt P., Turino M. Functional and biochemical analysis of angiotensin II forming pathways in the human heart. Circ Res 1997; 80: 219–227
- McKelvie R. S., Yusuf S., Pericak D., Avezum A., Burns R. J., Probstfield J. Comparison of candesartan, enalapril, and their combination in congestive heart failure: Randomized evaluation of strategies for left ventricular dysfunction (RESOLVD) pilot study. The RESOLVD Pilot Study Investigators. Circulation 1999; 100: 1056–1064
- Cohn J. N., Tognoni G. A randomized trial of the angiotensin‐receptor blocker valsartan in chronic heart failure. N Engl J Med 2001; 345: 1667–1675
- McMurray J. J., Ostergren J., Swedberg K., Granger C. B., Held P., Michelson E. L. Effects of candesartan in patients with chronic heart failure and reduced left‐ventricular systolic function taking angiotensin‐converting‐enzyme inhibitors: The CHARM‐Added trial. Lancet 2003; 362: 767–771
- Latini R., Masson S., Anand I., Judd D., Maggioni A. P., Chiang Y. T. Effects of valsartan on circulating brain natriuretic peptide and norepinephrine in symptomatic chronic heart failure: The Valsartan Heart Failure Trial (Val‐HeFT). Circulation 2002; 106: 2454–2458
- Anand I., Latini R., Masson S. Valsartan caused a sustained decrease in plasma aldosterone in Val‐HeFT independent of concomitant ACEI inhibitor and/or Beta‐Blocker therapy. Circulation 2002; 106((19)Suppl:Abs)1763
- Maggioni A. P., Anand I., Gottlieb S. O., Latini R., Tognoni G., Cohn J. N. Effects of valsartan on morbidity and mortality in patients with heart failure not receiving angiotensin‐converting enzyme inhibitors. J Am Coll Cardiol 2002; 40: 1414–1421
- Granger C. B., McMurray J. J., Yusuf S., Held P., Michelson E. L., Olofsson B. Effects of candesartan in patients with chronic heart failure and reduced left‐ventricular systolic function intolerant to angiotensin‐converting‐enzyme inhibitors: The CHARM‐Alternative trial. Lancet 2003; 362: 772–776
- Pitt B., Poole‐Wilson P. A., Segal R., Martinez F. A., Dickstein K., Camm A. J. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: Randomised trial – the Losartan Heart Failure Survival Study ELITE II. Lancet 2000; 355: 1582–1587
- Dickstein K., Kjekshus J., OPTIMAAL Steering Committee of the OPTIMAAL Study Group. Effects of losartan and captopril on mortality and morbidity in high‐risk patients after acute myocardial infarction: The OPTIMAAL randomised trial. Lancet 2002; 360: 752–760
- Rouleau J. L., Packer M., Moye L., de Champlain J., Bichet D., Klein M. Prognostic value of neurohumoral activation in patients with an acute myocardial infarction: Effect of captopril. J Am Coll Cardiol 1994; 24: 583–591
- Collaborative Group. ISIS‐4: A randomised factorial trial assessing early oral captopril, oral mononitrate, and intravenous magnesium sulphate in 58 050 patients with suspected acute myocardial infarction. Lancet 1995; 345: 669–685
- Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico. GISSI‐3: Effects of lisinopril and transdermal glyceryl trinitrate singly and together on 6‐week mortality and ventricular function after acute myocardial infarction. Lancet 1994; 343: 1115–1122
- Pfeffer M. A., Braunwald E., Moyé L. A., Basta L., Brown EJ J. r., Cuddy T. E. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the Survival and Ventricular Enlargement trial. N Engl J Med 1992; 327: 669–677
- Pfeffer M. A., McMurray J. J., Velazquez E. J., Rouleau J. L., Kober L., Maggioni A. P. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 2003a; 349: 1893–1906
- Kannel W. B., Gordon T., Offut T. Left ventricular hypertrophy by electrocardiogram. Prevalence, incidence and mortality in the Framingham study. Ann Int Med 1969; 71: 89–95
- Muiesan M. L., Salvetti M., Rizzoni D., Castellano M., Donato F., Agabiti‐Rosei E. Association of change in left ventricular mass with prognosis during long term antihypertensive treatment. J Hypertens 1995; 13: 1091–1097
- Devereux R. B., Wachtell K., Gerdts E., Boman K., Nieminen M. S., Papademetriu V. Prognostic significance of left ventricular mass change during treatment of hypertension. JAMA 2004; 292: 2350–2356
- Klingbeil A. U., John S., Schneider M. P., Jacobi J., Handrock R., Schmieder R. E. Effect of AT1 receptor blockade on endothelial function in essential hypertension. Am J Hypertens 2003; 16: 123–128
- Cuocolo A., Storto G., Izzo R., Iovino G. L., Damiano M., Bertocchi F. Effects of valsartan on left ventricular diastolic function in patients with mild or moderate essential hypertension: Comparison with enalapril. J Hypertens 1999; 17: 1759–1766
- Cuspidi C., Leonetti G., Zanchetti A. Left ventricular hypertrophy regression with antihypertensive treatment: Focus on candesartan. Blood Press Suppl 2003; 12 Suppl 2: 5–15
- Schiffrin E. L. Remodeling of resistance arteries in essential hypertension and effects of antihypertensive treatment. Am J Hypertens 2004; 17: 1192–2000
- Peters S., Gotting B., Trummel M., Rust H., Brattstrom A. Valsartan for prevention of restenosis after stenting of type B2/C lesions: The VAL‐PREST trial. J Invasive Cardiol 2001; 13: 93–97
- Hahn A. W., Resink T. J., Scott‐Burden T., Powell J., Dohi Y., Buhler F. R. Stimulation of endothelin mRNA and secretion in rat vascular smooth muscle cells: A novel autocrine function. Cell Regul 1990; 1: 649–659
- Lin L., Mistry M., Stier CT J. r., Nasjletti A. Role of prostanoids in renin‐dependent and renin‐independent hypertension. Hypertension 1991; 17: 517–525
- Rajagopalan S., Kurz S., Munzel T., Tarpey M., Freeman B., Griendling K. Angiotensin II mediated hypertension in rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation: Contribution to alteration of vasomotor tone. J Clin Invest 1996; 97: 1916–1923
- Maeso R., Navarro‐Cid J., Munoz‐Garcìa R., Rodrigo E., Ruilope L. M., Lahera V. Losartan reduces phenylephrine constrictor response in aortic rings from spontaneously hypertensive rats. Hypertension 1996; 28: 967–972
- Wiemer G., Scholkens B. A., Busse R., Wagner A., Heitsch H., Linz W. The functional role of angiotensin II subtype AT2‐receptors in endothelial cells and isolated ischemic rat hearts. Pharm Pharmacol Lett 1993; 3: 24–27
- De Moura R. S., Resende A. C., Emiliano A. F., Tano T., Mendes‐Ribeiro A. C., Correia M. L. The role of bradykinin, AT2 and angiotensin 1–7 receptors in the EDRF‐dependent vasodilator effect of angiotensin II on the isolated mesenteric vascular bed of the rat. Br J Pharmacol, 2004 (in press)
- Antony I., Lerebours G., Nitenberg A. Angiotensin‐converting enzyme inhibition restores flow‐dependent and cold pressor test‐induced dilations in coronary arteries of hypertensive patients. Circulation 1996; 94: 3115–3122
- Ghiadoni L., Magagna A., Versari D., Kardasz I., Huang Y., Taddei S. Different effect of antihypertensive drugs on conduit artery endothelial function. Hypertension 2003; 41: 1281–1286
- Schiffrin E. L., Deng Li Y. Comparison of effects of angiotensin I‐converting enzyme inhibition and beta‐blockade for two years on function of small arteries from hypertensive patients. Hypertension 1995; 25: 699–703
- Schiffrin E. L. Correction of remodelling and function of small arteries in human hypertension by cilazapril, an angiotensin i‐converting enzyme inhibitor. J Cardiovasc Pharmacol 1996; 27 Suppl 2: S13–S18
- Rizzoni D., Muiesan M. L., Porteri E., Castelano M., Zulli G., Bettoni G. Effect of long‐term antihypertensive treatment with lisinopril on resistance arteries in hypertensive patients with left ventricular hypertrophy. J Hypertens 1997; 15: 197–204
- Schiffrin E. L., Park J. B., Integan H. D., Toyuz R. M. Correction of arterial structure and endotelial dysfunction in human essential hipertensión by the angiotensin receptor antagonist losartan. Circulation 2000; 101: 1653–1659
- Creager M. A., Roddy M. A. Effect of captopril and enalapril on endothelial function in hypertensive patients. Hypertension 1994; 24: 499–505
- Kiowski W., Linder L., Nuesch R., Martina B. Effect of cilazapril on vascular structure and function in essential hypertension. Hypertension 1996; 27: 371–376
- Taddei S., Virdis A., Mattei P., Ghiadoni L., Salvetti A. Effect of lisinopril on endothelial function. J Hypertens 1998; 16: 371–376
- Taddei S., Ghiadoni L., Virdis A., Buralli S., Salvetti A. Vasodilation to bradykinin is mediated by an ouabain‐sensitive pathway as a compensatory mechanism for impaired NO availability in essential hypertensive patients. Circulation 1999; 100: 1400–1405
- Ghiadoni L., Virdis A., Magagna A., Taddei S., Salvetti A. Effect of the angiotensin II type 1 receptor blocker candesartan on endothelial function in patients with essential hypertension. Hypertension 2000; 35: 501–506
- Baranowska D., Braszko J. J., Wisniewski K. Effect of angiotensin II and vasopressin on acquisition and extinction of conditioned avoidance in rats. Psychopharmacology (Berlin) 1983; 81: 247–251
- Fogari R., Mugellini A., Zoppi A., Marasi G., Pasotti C., Poletti L. Effects of valsartan compared with enalapril on blood pressure and cognitive function in elderly patients with essential hypertension. Eur J Clin Pharmacol 2004; 59: 863–868
- Zanchetti A., Ruilope L. M. Antihypertensive treatment in patients with type‐2 diabetes mellitus: What guidance from recent controlled randomized trials?. J Hypertens 2002; 20: 2099–2110
- Lakka H. M., Laaksonen D. E., Lakka T. A., Niskanen L. K., Kumpusalo E., Tuomilehto J. The metabolic syndrome and total and cardiovascular disease mortality in middle‐aged men. JAMA 2002; 288: 2709–2716
- Narayan K. M. V., Imperatore S. M., Benjamin S. M., Engelgau M. M. Targeting people with prediabetes. Lifestyle interventions should also be aimed at people with prediabetes. BMJ 2002; 325: 403–404
- Alderman M. H., Cohen H., Madhavan S. Diabetes and cardiovascular events in hypertensive patients. Hypertension 1999; 33: 1130–1134
- Aguilar D., Solomon S. D., Kober L., Rouleau J. L., Skali H., MacMurray J. J. Newly diagnosed and previously known diabetes mellitus and 1‐year outcomes of acute myocardial infarction: The VALsartan In Acute myocardial iNfarcTion (VALIANT) trial. Circulation 2004; 110: 1572–1578
- Pfeffer M. A., Swedberg K., Granger C. B., Held P., McMurray J. J. V., Michelson E. L. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: The CHARM‐Overall programme. Lancet 2003b; 362: 759–766
- Lindholm L. H., Ibsen H., Dahlöf B., Devereux R. B., Beevers G. H., de Faire U. Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): A randomised trial against atenolol. Lancet 2002; 359: 1004–1010
- Pourdjabbar A., Lapointe N., Rouleau J. L. Angiotensin receptor blockers: Powerful evidence with cardiovascular outcomes?. Can J Cardiol 2002; 18 Suppl A: 7A–14A
- Lindholm L. H., Dahlöf B., Edelman J. M., Ibsen H., Borch‐Johnsen K., Olsen M. H. Effect of losartan on sudden cardiac death in people with diabetes: Data from the LIFE study. Lancet 2003a; 362: 619–620
- Izzo JL J. r. Hypertension in the metabolic syndrome and diabetes: Pathogenesis, clinical studies and treatment. J Clin Hypertens 2003; 5((6 Suppl 4))3–10
- Henriksen E. J., Jacob S., Kinnick T. R., Teachey M. K., Krekler M. Selective angiotensin II receptor antagonism reduces insulin resistance in obese Zucker rats. Hypertension 2001; 38: 884–890
- Lindholm L. H., Persson M., Alaupovic P., Carlberg B., Svensson A., Samuelsson O. Metabolic outcome during 1 year in newly detected hypertensives: Results of the Antihypertensive and Lipid Profile in a North of Sweden Efficacy Evaluation (ALPINE study). J Hypertens 2003; 21: 1563–1574
- Grassi G., Seravalle G., DelĺOro R., Trevano F. Q., Bombelli M., Scopelliti F. Comparative effects of candesartan and hydrochlorothiazide on blood pressure, insulin sensitivity, and sympathetic drive in obese hypertensive individuals: Results of the CROSS study. J Hypertens 2003; 21: 1761–1769
- Top C., Cingozbay B. Y., Terekeci H., Kucukardali Y., Onde M. E., Danaci M. The effects of valsartan on insulin sensitivity in patients with primary hypertension. J Int Med Res 2002; 30: 15–20
- Schupp M., Janke J., Clasen R., Unger T., Kintscher U. Angiotensin type 1 receptor blockers induce peroxisome proliferator‐activated receptor‐gamma activity. Circulation 2004; 109: 2054–2057
- Benson S. C., Pershadsigh H. A., Ho C. I., Chittiboyina A., Desai P., Pravenec M. Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPAR gamma‐modulating activity. Hypertension 2004; 43: 993–1002