Abstract
The study aimed to evaluate, over a 3‐year period, the progression towards sustained hypertension and left ventricular (LV) changes in patients with isolated office (IO) hypertension (office BP>140 and/or 90 mmHg, daytime BP<130/80 mmHg). After 3 years from the basal evaluation, 38 subjects with basal normal BP and 42 subjects with basal IO hypertension underwent a second 24‐h BP monitoring and echocardiography; 19 patients of the basal IO hypertension group were not revaluated because they had already developed ambulatory hypertension and were on antihypertensive treatment. At the second evaluation, the 38 normotensive subjects had unchanged BP and LV parameters; 25 IO hypertensives have developed sustained hypertension. Considering them together with the 19 patients already treated, 72% of 61 IO hypertensives developed ambulatory hypertension over a 3‐year period. The patients who subsequently developed hypertension differed from the group who did not only for lower basal values of LV diastolic parameters; all the patients with basal LV hypertrophy and/or preclinical diastolic impairment subsequently developed sustained hypertension. In conclusion, IO hypertensive patients show a high rate of progression towards sustained hypertension. Basal LV hypertrophy and/or preclinical diastolic dysfunction were the only markers of a greater risk of becoming hypertensives.
Introduction
Isolated office (IO) hypertension, also defined as “white‐coat” hypertension, is characterized by persistently elevated office blood pressure (BP) and normal daytime ambulatory BP Citation[1]. The correct characterization of IO hypertension has several clinical implications, the most important being to avoid the prescription of unnecessary antihypertensive therapy and to identify subjects who are probably at an increased cardiovascular risk compared with normotensive population. In fact, whereas some authors regard IO hypertension as a benign condition Citation[2–4], others have found it associated with a prevalence of target organ damage lower than sustained hypertension, but significantly higher than in normotensive subjects Citation[5–9]. Moreover, an increased incidence of cardiovascular events has been demonstrated in patients with IO hypertension compared with normotensive subjects Citation[10–12]. The few studies that evaluated if IO hypertension represents a transient state towards sustained hypertension, obtained conflicting results: a high rate of progression to sustained hypertension (74%) Citation[13], a lower rate (37%) Citation[14], or a rate of progression similar to that found in normotensive controls (11%) Citation[15]. Moreover it is still not known if some characteristics, such as BP values or presence of target organ damage, could be a prognostic indicator of the development of sustained hypertension.
Therefore, we have considered it of interest to evaluate, over a 3‐year period, the rate of progression towards sustained hypertension and the changes in left ventricular (LV) morpho‐functional characteristics in a group of patients with never‐treated IO hypertension, looking also for basal demographic, BP or LV characteristics that could possibly predict the development of sustained hypertension. We also evaluated over the same 3‐year period a group of normotensive subjects.
Material and methods
For a previous study Citation[8], we selected subjects with normal BP (office BP<140/90 mmHg and daytime BP<130/80 mmHg) and subjects with IO hypertension, defined as office BP repeatedly>140 and/or 90 mmHg (four or more measurements in 4 months) and daytime BP<130/80 mmHg. Other selection criteria were as follows: no previous antihypertensive treatment, LV M‐mode echocardiogram of good quality; no clinical, electrocardiographic or echocardiographic evidence of heart failure, coronary artery disease or valvular heart diseases; no systemic diseases, such as diabetes mellitus or connective tissue disorders, which could influence per se LV structure and function.
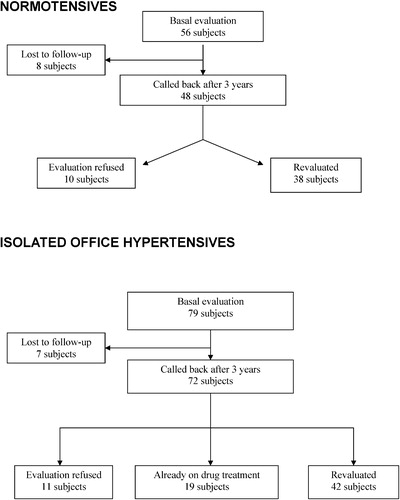
After at least 3 years from the basal evaluation, we called back the IO hypertensive patients and the normotensive subjects. Of the normotensive group (basal evaluation: 56 subjects) it was possible to contact 48 subjects and 38 agreed to be revaluated (). Among the IO hypertensive patients (basal evaluation: 79 subjects) it was possible to contact 72 subjects: 11 refused to undergo the second evaluation, 19 patients have already developed ambulatory hypertension (diagnosed by means of 24‐h BP monitoring) and were on chronic antihypertensive treatment; therefore we revaluated 42 subjects, all still not treated with antihypertensive drugs ().
Each patient underwent a 24‐h ambulatory BP monitoring and an echocardiographic examination. Mean clinic BP was obtained by averaging BP values taken during two visits, 1 week apart; during each visit, the same operator, using a mercury sphygmomanometer, measured BP three times at 10‐min intervals with the patient in the sitting position, after a 20‐min rest. The study was approved by the Ethical Committee of the Department of Clinical Medicine and all the subjects gave their informed consent.
Non‐invasive 24‐h ambulatory BP monitoring
Non‐invasive 24‐h ambulatory BP monitoring was performed with a portable automated Takeda TM 2421 and simultaneous 24‐h heart rate monitoring was obtained. The unit was set to take readings every 15 min throughout the 24 h. The following parameters were evaluated: mean 24‐h, daytime (from 07.00 to 22.00 h) and night‐time (from 22.00 to 07.00 h) systolic and diastolic BP and heart rate.
Echocardiographic examination
The echocardiographic examination was performed by the same operator who did the basal evaluation, using a Hewlett‐ Packard Sonos 5500 with a 2.0/2.5 MHz transducer. LV M‐mode echocardiograms were recorded under two‐dimensional control, at a paper speed of 100 mm/s, with a simultaneous electrocardiogram. A single operator, unaware of the clinical characteristics of the patients, evaluated the M‐mode tracings, digitizing four consecutive cardiac cycles of each echocardiogram, as originally described by Upton & Gibson Citation[16], using a Numonics 2205 graphic tablet. An IBM personal computer processed digitized data, averaging the four cardiac cycles. We evaluated LV end‐diastolic diameter, end‐diastolic thickness of interventricular septum and posterior wall, LV mass Citation[17], peak shortening rate of LV diameter, peak lengthening rate of LV diameter and peak thinning rate of LV posterior wall. LV mass was normalized for body surface area. The normal limits of the parameters in our laboratory have been derived from the evaluation of 200 normal adults. The reproducibility of the echocardiographic measurements has been tested on 20 normal subjects (each examined three times by the same ultrasonic technique); the same operator digitized four consecutive cardiac cycles of each echocardiogram. The coefficients of variation were as follows: LV end‐diastolic diameter 0.4%, septal thickness 3.2%, posterior wall thickness 3.4%, peak shortening rate 1.1%, peak lengthening rate 4.7%, peak thinning rate 7.3%.
Mitral inflow velocities were evaluated by pulsed‐wave Doppler with the sample volume placed at the tips of the mitral leaflets, from the apical four‐chamber view. Using the average of 5 beats for the analysis, we measured the ratio between peak early transmitral flow velocity (E) and peak late transmitral flow velocity (A) (E/A ratio), and the deceleration time of E velocity (DT, time from peak E velocity to the time when E wave descent intercepted the zero line).
Statistical analysis
The results have been evaluated using analysis of variance (ANOVA), paired and unpaired Student's t‐tests as needed. The data are presented as mean±standard deviation; a p‐value <0.05 was considered statistically significant.
Results
At the basal evaluation IO hypertensive subjects were not significantly different from the normotensive control group with regard to gender (men/women normotensive group 15/23, IO hypertensives 18/24, ns) age, body mass index and 24‐h BP values (Tables and ).
Table I. Clinical and left ventricular (LV) morpho‐functional characteristics of the 38 normotensive subjects.
Table II. Clinical characteristics of the IO hypertensive patients who progressed to sustained hypertension (Group 1) and of those who did not (Group 2).
At the second evaluation, at least 3 years after the basal study, all the 38 subjects with basal normal BP were still normotensive, with clinic and 24‐h BP values unchanged compared with basal values ().
Out of the 42 IO hypertensive patients who underwent the second evaluation, 17 subjects still had IO hypertension (office BP>140 and/or 90 mmHg and daytime BP<130/80 mmHg), whereas 25 subjects had clinic BP>140 and/or 90 mmHg and daytime BP>135 and/or 85 mmHg. Considering these 25 patients together with the 19 patients not revaluated because they have already developed sustained hypertension and were on antihypertensive treatment (see Methods section; ), 44/61 patients with IO hypertension (72%) developed ambulatory hypertension over a 3‐year period.
On the basis of these results, we divided the 42 patients evaluated into two groups: 25 patients who developed sustained hypertension (Group 1) and 17 patients with IO hypertension (Group 2).
Looking for possible markers of the subsequent development of sustained hypertension, we compared basal BP and LV characteristics of Groups 1 and 2. The two groups did not differ with regard to gender (men/women Group1 11/14, Group 2 7/10, ns), age, body mass index and 24‐h heart rate (). Office systolic BP was higher in Group 2, whereas office diastolic BP and ambulatory systolic and diastolic BP throughout the 24 h were not significantly different between the two groups (). Considering basal LV morpho‐functional characteristics (), LV end‐diastolic diameter was normal (<56 mm) in all the patients and similar between the two groups; mean values of LV mass index (LVMI) were also similar between the two groups, whereas LV hypertrophy (LVMI⩾130 g/m2 men, ⩾110 g/m2 women) was found in six patients, all in Group 1. LV systolic function was normal (peak shortening rate of LV diameter>1.9 s−1) in all the patients and similar between the two groups. With regard to LV diastolic function, mean values of peak lengthening rate, peak thinning rate of LV posterior wall and E/A ratio were significantly lower and deceleration time of E wave longer in Group 1 than in Group 2; a preclinical LV diastolic impairment (two or more of the following: peak lengthening rate of LV diameter<3.6 s−1, peak thinning rate of LV posterior wall<8.4 cm/s, E/A ratio<1) was found in 15 patients, all in Group 1. Therefore all the 16 patients with basal myocardial hypertrophy and/or preclinical diastolic dysfunction belonged to Group 1. Comparing the two groups of IO hypertensive patients with the normotensive group, mean values of all LV morpho‐functional characteristics were similar between normotensives and Group 2, whereas Group 1 showed significantly higher LV mass index and lower diastolic indices (Tables and ).
Table III. Left ventricular (LV) morpho‐functional characteristics of IO hypertensive patients who progressed to sustained hypertension (Group 1) and of those who did not (Group 2).
The mean follow‐up period was not significantly different between the groups (Group 1 3.4±0.2 years, Group 2 3.4±0.1 years, Normotensive group 3.4±0.2 years, ANOVA ns).
Considering longitudinal changes within each group, after 3 years, body mass index was unchanged in the normotensive group, as well as in Group 1 and Group 2 (Tables and ). All the 38 normotensive subjects had LV morpho‐functional parameters within the normal limits and unchanged compared with basal values (). In Group 1, office diastolic BP and 24‐h BP were significantly increased, peak thinning rate of LV posterior wall and E/A ratio were significantly decreased and deceleration time of E wave increased (Tables and ); LV hypertrophy was found in eight patients and preclinical diastolic dysfunction in 17 patients, raising the number of subjects with preclinical cardiac damage to 18 subjects.
In Group 2, compared with basal values, office systolic BP was significantly lower, whereas the other BP parameters were unchanged, as well as all LV parameters (Tables and ); moreover all the patients had LV mass index and LV diastolic parameters within the normal limits, as at the basal evaluation.
Discussion
The prevalence of IO hypertension, as well as its association with target organ damage and incidence of cardiovascular events, differ markedly among the studies, not only because of differences in age, sex and body mass index of the subjects evaluated, but also depending on the threshold used to define normal daytime ambulatory BP Citation[18], Citation[19]. Obviously, choosing a high value (e.g. 140/90 mmHg) as upper normal limit of daytime BP can lead to classify as IO hypertensive subjects who actually have sustained hypertension Citation[18], Citation[20], Citation[21]. The most widely used criterion to define IO hypertension refers to a daytime BP<135/85 mmHg Citation[1], Citation[22]. Aiming to evaluate long‐term changes of BP and LV characteristics in patients with IO hypertension, we chose as upper normal limit for daytime BP the more restrictive value of 130/80 mmHg; as BP values below this limit are to be regarded as definitely normotensive Citation[23], using this cut‐off value we aimed to avoid the recruitment of sustained hypertensives as IO hypertensive subjects.
Growing interest has emerged in recent years about IO hypertensive subjects: they share a number of hemodynamic and metabolic characteristics with sustained hypertensives. Several studies Citation[24], Citation[25] have shown that IO hypertension is associated with sympathetic hyperactivity, which not only could play a role in the evolution towards sustained hypertension, but could also contribute to the development of target organ damage. In fact, we can speculate, according to Smith et al. Citation[25], that transient BP increases caused by exaggerated responses to mild stress, such as during medical evaluation, may influence myocellular growth, leading to cardiac hypertrophy and arterial wall thickening.
Today the natural history of patients with IO hypertension is still unclear. Bidlingmeyer and coworkers Citation[13] found that, over a 5–6‐year period, 60 IO hypertensives of 81 subjects (74%) developed sustained hypertension, but the results could have been influenced by the high daytime BP value considered as upper normal limit (140/90 mmHg) for the definition of IO hypertension. A lower rate of progression (37%) to sustained hypertension was found by Verdecchia and coworkers Citation[14] over a period of between 6 months and 6.5 years (mean follow‐up 2.5 years), using as upper normal limit of daytime BP 131/86 mmHg in women and 136/87 mmHg in men. Using a cut‐off value of 132/84 mmHg for the daytime diastolic BP, during a 3.5‐year follow‐up, Polonia and coworkers Citation[15] found a similar rate of progression to ambulatory hypertension in IO hypertensives (22%) and in normotensive subjects (15%). In our study, during a 3‐year period, 72% of IO hypertensives developed sustained hypertension, a rate of progression higher than that found by Verdecchia et al. Citation[14] and by Polonia et al. Citation[15]. Taking into account the lower cut‐off value we used as upper normal limit of daytime BP (<130/80 mmHg) and the shorter follow‐up period, the incidence of ambulatory hypertension in our IO hypertensives was also higher than Bidlingmeyer et al. 's Citation[13]. As an increase in body weight can induce per se an increase in BP Citation[26], we have to underline that during the follow‐up, body mass index did not change in all the patients. Over the same 3‐year period, we evaluated a control group of normotensive subjects. At the basal evaluation, they were similar to IO hypertensive subjects with regard not only to age, gender and body mass index, but also to 24‐h BP values; none of the 38 normotensive subjects developed sustained hypertension during the follow‐up. Our control group was small, therefore we considered it useful to compare our results also with those from the Framingham study Citation[27], which evaluated the progression to hypertension in a large cohort of normotensive subjects: in the age group 35–64 years, during a 3‐year period, 13.5% of subjects with normal BP and 29.6% with high‐normal BP progressed to hypertension. Therefore the incidence of sustained hypertension is significantly higher in our group of IO hypertensives (72%) than in normotensive subjects, in all the baseline BP categories. We do not have an explanation for the lack of progression to hypertension in our normotensive group; we can only underline two major differences in comparison with the Framingham study: the smaller number of subjects we evaluated and the normal BP that we established not only on the basis of office measurements, but also by means of 24‐h ambulatory monitoring, a way to make more likely the recruitment of truly normotensive subjects.
The other aim of our study was to look for possible BP or LV characteristics at the basal evaluation that could predict the progression to sustained hypertension. At baseline, the group who became hypertensive and the group who did not were not significantly different, considering age, gender, body mass index, all ambulatory BP parameters and diastolic clinic BP; only clinic systolic BP was significantly higher, but in the group that did not become hypertensive. Therefore, the subsequent progression to sustained hypertension could not be predicted on the basis of clinic or ambulatory BP values, gender or body mass index.
Considering LV characteristics, at the basal evaluation preclinical cardiac damage (myocardial hypertrophy and/or diastolic dysfunction) was found in 16 patients with IO hypertension, whereas all the LV parameters were within the normal limits in the normotensive control group: this finding is in agreement with many Citation[6], Citation[8], Citation[9], but not all the previous studies Citation[2–4] that evaluated cardiac morphology and function in IO hypertension. Comparing the two IO hypertensive groups, only mean values of diastolic parameters were significantly different with a pattern of preclinical diastolic impairment in the group who subsequently became hypertensive. Moreover, all 16 patients with myocardial hypertrophy and/or preclinical diastolic dysfunction belonged to the group that progressed to sustained hypertension.
The subjects who have not became sustained hypertensive had all LV parameters within the normal limits and similar to normotensive subjects, at the basal as well as at the 3‐year evaluation, whereas the group that progressed to sustained hypertension showed at the 3‐year evaluation a significant worsening of LV diastolic indices.
LV diastolic function is a complex phenomenon that can not be characterized by a single non‐invasive parameter, therefore, in order to better evaluate the diastole, we used two methods (digitized M‐mode echo and Doppler evaluation of transmitral flow) that explore different aspects of LV diastolic function Citation[28], Citation[29]: Doppler indices measure peak velocities of flow, whereas the parameter derived from digitized M‐mode echo measures changes in dimension. These diastolic parameters, less popular than Doppler‐derived indices, have been proved very sensitive in discriminating between normal and abnormal diastole and are far less influenced by age and heart rate than Doppler indices Citation[29].
In conclusion, our results indicate that subjects with IO hypertension are at increased risk of developing sustained hypertension, also when IO hypertension is defined by means of a daytime BP threshold more restrictive than those employed in previous studies. Moreover, beside confirming the presence of cardiac damage in IO hypertension, this study shows that the progression towards sustained hypertension cannot be predicted on the basis of gender, body mass index, clinic or 24‐h ambulatory BP values, whereas the presence of a preclinical cardiac damage seems to be the only characteristic that predicts the subsequent development of sustained hypertension. In fact, all subjects with basal myocardial hypertrophy and/or preclinical diastolic dysfunction became sustained hypertensive. Therefore, all the subjects with IO hypertension should be controlled over time and also counseled about lifestyle modifications in order to reduce the risk of progression towards hypertension; the lifestyle counseling, together with an adequate drug treatment, are mandatory for patients with IO hypertension and preclinical cardiac damage, this latter defining a condition of high cardiovascular risk.
References
- Verdecchia P., O'Brien E., Pickering T., Staessen J. A., Parati G., Myers M., et al. European Society of Hypertension Working Group on Blood Pressure Monitoring. When can the practicing physician suspect white‐coat hypertension? Statement from the Working Group on Blood Pressure Monitoring of the European Society of Hypertension. Am J Hypertension 2003; 16: 87–91
- Gosse P., Promax H., Durandet P., Clementy J. “White coat” hypertension: No harm for the heart. Hypertension 1993; 22: 766–770
- Verdecchia P., Schillaci G., Borgioni C., Ciucci A., Zampi I., Gattobigio R., et al. White coat hypertension and white coat effect. Similarities and differences. Am J Hypertens 1995; 8: 790–798
- Cavallini M. C., Roman M. J., Pickering T. G., Schwartz J. E., Pini R., Devereux R. B. Is white coat hypertension associated with arterial disease or left ventricular hypertrophy?. Hypertension 1995; 26: 413–419
- Glen S. K., Elliott H. L., Curzio J. L., Lees K. R., Reid J. L. White‐coat hypertension as a cause of cardiovascular dysfunction. Lancet 1996; 348: 654–657
- Muscholl M. W., Hense H. W., Brockel U., Doring A., Riegger G. A., Schunkert H. Changes in left ventricular structure and function in patients with white‐coat hypertension: Cross sectional survey. BMJ 1998; 317: 565–570
- Muldoon M. F., Nazzaro P., Sutton‐Tyrrel K., Manuck S. B. White‐coat hypertension and carotid artery atherosclerosis: A matching study. Arch Intern Med 2000; 160: 1507–1512
- Grandi A. M., Broggi R., Colombo S., Santillo R., Imperiale D., Bertolini A., et al. Left ventricular changes in isolated office hypertension: A blood pressure matched comparison with normotension and sustained hypertension. Arch Intern Med 2001; 161: 2677–2681
- Karter Y., Curgunlu A., Altinisik S., Erturk N., Vehid S., Mihmanli I., et al. Target organ damage and changes in arterial compliance in white coat hypertension. Is white coat innocent?. Blood Press 2003; 12: 307–313
- Strandberg T. E., Salomaa V. White coat effect, blood pressure and mortality in men: Prospective cohort study. Eur Heart J 2000; 21: 1714–1718
- Gustavsen P. H., Hoegholm A., Bang L. E., Kristensen K. S. White coat hypertension is a cardiovascular risk factor: A 10‐year follow‐up study. J Hum Hypertens 2003; 17: 811–817
- Verdecchia P., Reboldi G. P., Angeli F., Schillaci G., Schwartz J. E., Pickering T. G., et al. Short‐and long‐term incidence of stroke in white‐coat hypertension. Hypertension 2005; 45: 1–6
- Bidlingmeyer I., Burnier M., Bidlingmeyer M., Waeber B., Brunner H. R. Isolated office hypertension: A prehypertensive state?. J Hypertens 1996; 14: 327–332
- Verdecchia P., Schillaci G., Borgioni C., Ciucci A., Gattobigio R., Sacchi N., et al. Identification of subjects with white‐coat hypertension and persistently normal ambulatory blood pressure. Blood Press Monit 1996; 1: 217–222
- Polonia J. J., Santos A. R., Gama G. M., Basto F., Bettencourt P. M., Martins L. R. Follow‐up clinic and ambulatory blood pressure in untreated white‐coat hypertensive patients (evaluation after 2–5 years). Blood Press Monit 1997; 2: 289–295
- Upton M. T., Gibson D. G. The study of left ventricular function from digitized echocardiograms. Prog Cardiovasc Dis 1978; 20: 359–384
- Devereux R. B., Reichek N. Echocardiographic determination of left ventricular mass in man: Validation of the method. Circulation 1977; 55: 613–618
- Verdecchia P., Schillaci G., Boldrini F., Zampi I., Porcellati C. Variability between current definitions of “normal” ambulatory blood pressure: Implications in the assessment of white coat hypertension. Hypertension 1992; 20: 555–562
- Khoury S., Yarows S. A., O'Brien T. K., Sowers J. R. Ambulatory blood pressure monitoring in a nonacademic setting: Effect of age and sex. Am J Hypertens 1992; 5: 616–623
- Mancia G., Sega R., Bravi C., De Vito G., Valagussa F., Cesana G., et al. Ambulatory blood pressure normality: Results from the PAMELA study. J Hypertens 1995; 13: 1377–1390
- Verdecchia P., Schillaci G., Borgioni C., Ciucci A., Porcellati C. White coat hypertension: Not guilty when correctly defined. Blood Press Monit 1998; 3: 147–152
- Verdecchia P., Staessen J. A., White W. B., Imai Y., O'Brien E. T. Properly defining white coat hypertension. Eur Heart J 2002; 23: 106–109
- Pickering T. G., Coats A., Mallion J. M., Mancia G., Verdecchia P. Blood pressure monitoring. Task force V: White‐coat hypertension. Blood Pressure Monit 1999; 4: 333–341
- Middeke M., Lemmer B. Office hypertension: Abnormal blood pressure regulation and increased sympathetic activity compared with normotension. Blood Press Monit 1996; 1: 403–407
- Smith P. A., Graham L. N., Mackintosh A. F., Stoker J. B., Mary D. A. Sympathetic neural mechanisms in white‐coat hypertension. J Am Coll Cardiol 2002; 40: 126–132
- Lee J. S., Kawakubo K., Kashihara H., Mori K. Effect of long‐term body weight change on the incidence of hypertension in Japanese men and women. Int J Obes Relat Metab Disord 2004; 28: 391–395
- Vasan R. S., Larson M. G., Leip E. P., Kannel W. B., Levy D. Assessment of frequency of progression to hypertension in non hypertensives participants in the Framingham Heart Study: A cohort study. Lancet 2001; 358: 1682–1686
- Rakowski H., Appleton C., Chan K. L., Dumesnil J. G., Honos G., Jue J., et al. Canadian consensus recommendations for the measurement and reporting of diastolic dysfunction by echocardiography. J Am Soc Echocardiogr 1996; 9: 736–760
- Lee C. H., Hogan J. C., Gibson D. G. Diastolic disease in left ventricular hypertrophy: Comparison of M‐mode and Doppler echocardiography for the assessment of rapid ventricular filling. Br Heart J 1991; 65: 194–200