Abstract
Background. The clinical effects of percutaneous transluminal renal artery angioplasty (PTRA) in patients with renal vascular stenosis and hypertension is controversial. Methods. We consecutively recruited all 23 patients referred for evaluation of renovascular hypertension that eventually underwent unilateral PTRA, to be investigated with captopril MAG3 renography (CR), both before and after the endovascular procedure. Data were evaluated on an intention‐to‐treat basis. Results. We found that the relative MAG3 clearance of the stenotic kidney increased (from 29.9±14% to 35.1±14%, p = 0.01) and that the creatinine levels fell following the intervention (from 110±19 to 99±17 µmol/l, p = 0.0003). Blood pressure levels were also lowered (from 173±32/93±17 to 158±31/86±15 mmHg, p<0.006) while the mean number of anti‐hypertensive drugs was unchanged following PTRA (2.9±1.4 before and 2.8±1.3 drugs after the intervention, respectively, p = 0.6). Conclusion. This prospective trial showed statistically significant improvements of individual kidney function as measured by CR and blood pressure in subjects with suspected renovascular hypertension treated with PTRA. Although the endovascular procedure was found to be safe, the magnitude of the absolute improvements was rather modest.
Introduction
The clinical effects of percutaneous transluminal renal artery angioplasty (PTRA) on kidney function in patients with renal vascular stenosis and hypertension is controversial Citation[1–3]. Although the endovascular techniques have improved, in particular with the introduction of stenting, it is still not clear whether such intervention actually improves the overall renal function. Earlier studies have shown favourable effects on blood pressure (BP) control following PTRA, but very few of these were prospective and analysed on an intention‐to‐treat basis Citation[2]. At the University Hospital of Linkoping, captopril renography has been the preferred technique for screening for renovascular hypertension for more than a decade Citation[4]. In 1996, 99mTc‐mercapto‐acetyl‐triglycin (MAG3) was introduced as renographic agent in Linkoping. Since then, all patients referred for evaluation of renovascular hypertension were investigated with a standardized MAG3 renography. In order to evaluate the prognostic information using this technique, and in particular to evaluate the clinic effects of PTRA, all subjects undergoing PTRA were re‐evaluated with MAG3 renography after the PTRA procedure. Both the initial and the follow‐up renographic examinations were performed after the patient had ingested 25 mg captopril, on top of existing medication, to increase the sensitivity to detect signs of renal artery stenosis, a study design that to our knowledge has not been used previously. Herein we present the results of unilateral PTRA on renographic MAG3 clearance and BP in 23 subjects with renal artery stenosis analysed prospectively on an intention‐to‐treat basis.
Methods and patients
Patients
All subjects that were referred to the university hospital for evaluation of renovascular hypertension and who subsequently underwent unilateral PTRA were consecutively recruited and followed prospectively. The patients all had renographic findings indicative of renovascular hypertensions in addition to an elevated BP Citation[4] and subsequently underwent renal arterial angiography. When the renal angiography confirmed the suspicion of significant (>50% diameter) stenosis, there was an immediate attempt to correct this by PTRA, with or without stenting.
Captopril renography
All patients were investigated with captopril stimulated renographic examinations (captopril renography, CR). Hydration was performed the hour before the CR by drinking 10 ml/kg body weight water. One hour before the CR test the patient received a crushed 25 mg captopril pill (Capoten®, Bristol‐Myers Squibb AB), which was swallowed with water. There were no particular restrictions regarding medication before the CR, i.e. patients taking angiotensin‐converting enzyme (ACE) inhibitors or angiotensin II receptor antagonists were allowed to continue with this medication. BP was measured in the supine position prior to the captopril intake after 15 min of rest (in three cases, however, the BP record was lost for technical reasons and the BP recorded at the doctors office was used in the analysis instead). In each patient, the urine flow‐rate during acquisition of the renographic examination was calculated from voiding immediately before and after the performance of the test. The patient was in the supine position over the gamma camera with the kidneys, heart and bladder in the field of view. Starting immediately after the intravenous injection of 70 MBq 99mTc‐MAG3 (Mallinckrodt Ltd.), serial 10‐second/frame images were obtained for 16–20 min. A single‐headed large field of view gamma camera (GE XRT, Starcam 2000) equipped with a general‐purpose parallel‐hole collimator was used and the images were collected in a 128×128 matrix. Subsequent analyses were made using in‐house software for renal scintigraphy. Regions of interest (ROI) were manually drawn around the whole kidney and automatically to get perirenal background ROI. The renographic curves of the right and left kidneys were analysed separately, and the relative function of each kidney (in per cent of total function) as well as the absolute function (camera‐based MAG3‐clearance in ml/min) were calculated using a Patlak plot according to the algorithm proposed by Granerus & Moonen Citation[5]. Interpretation was made with strict adherence to the criteria agreed on at the consensus conference in Santa Fe, 1995 Citation[6]. The patient was considered to have a positive CR if one of the kidneys had 45% or less of total kidney function. In two cases (patients 7 and 11 in Table ), there was a marked difference in the time to maximal uptake (10 min in both cases), which can also be indicative of renal artery stenosis, although this is an unspecific finding. In those two cases, another renographic examination was performed without pre‐treatment with captopril. This second renography did not show a difference in time to maximal MAG3 uptake, which increased the suspicion of renovascular hypertension enough to allow a renal angiography and confirmed presence of a renal artery stenosis that was immediately treated with PTRA.
Table I. Characteristics of patients before and after percutaneous dilatation of unilateral renal arterial stenosis. Kidney function is the individual function (expressed as % of total kidney function) of the kidney with stenotic artery subjected to PTRA.
PTRA
The patients fasted for at least 4 h prior to the PTRA. Thirty minutes before the angiography, the patient received a rectal application of 5–10 mg of diazepam and a subcutaneous injection of 0.25–0.5 mg of atropine. Renal aortography was performed with a pigtail catheter placed in the abdominal aorta, slightly above the level of the renal arteries. The finding of a >50% reduction of the arterial diameter was followed by an attempt to correct this: A 6 F or 7 F introducer sheath was placed reaching up from the femoral artery to the aorta; 5000 IE of Heparin was injected intra‐arterially through the sheath. The lesion was traversed with standard 4 F catheters and teflon‐coated or hydrophilic 0.032 guide‐wires. Next, the guide‐wire was switched to a Rosen, or similar, stiff wire with short tip. A balloon catheter approximately 20% bigger than true non‐stenotic arterial diameter was chosen for the dilatation. The result of the dilatation was evaluated visually by an immediate angiography. If the result was unsatisfactory, a matching stainless steel balloon expandable stent was placed at the site of the lesion. In case of an osteal lesion, the stent was placed protruding a couple of millimetres into the aortic lumen.
The patient was kept in bed for 10 h following the PTRA and 5000 IE of low molecular weight heparin (Fragmin®) was administered subcutaneously on the same and the 2 following days. The patients' antihypertensive drugs were kept unchanged during the stay in hospital unless very significant hypotension occurred. The great majority of patients (19/23) underwent renographic examination on the day after the PTRA as a safety precaution for assessment of potential complications or acute failure of the endovascular procedure. None of these 19 examinations displayed occurrence of any such problems. All patients underwent a follow‐up clinic examination after 3 months and a second CR after 3 months or more (there could be delay due to availability of the examination). In several cases, the patient underwent CR after 3 months as well as after 12 months. In these cases, the latest examination was that used in Table and in calculations of outcome. The creatinine following the PTRA was that measured at the clinical follow‐up at 3 months.
Ethics
The study was approved by the Ethics Committee of Linköping University and performed in accordance with the Declaration of Helsinki.
Statistics and correction for blood pressure medication
Statistical calculations were made using StatView 4.5 (Abacus Concepts Inc., Berkeley, CA, USA) software. Comparisons within and between groups were made with Student's paired and unpaired two‐tailed t‐test and linear correlations with Pearson's test or as stated in the text. Mean±standard deviation is given unless otherwise stated. Statistical significance was considered at the 5% level (p≤0.05).
Many of the patients underwent considerable changes in the antihypertensive medication during the follow‐up. To allow a reasonably accurate appreciation of the effects of the PTRA on blood pressure, despite changes in concomitant medication, we assumed that the antihypertensive effect of any antihypertensive drug was −12/8 mmHg Citation[7]. Thus, “corrected systolic BP” was the measured systolic blood pressure level plus the number of antihypertensive drugs×12. The diastolic corrected blood pressure was calculated in the corresponding fashion.
Results
We recruited 29 patients from 1996 to 2003. Five of these had bilateral renovascular disease and underwent bilateral dilatation. Since the aim of this trial was to investigate the potential improvement in the percentage of MAG3 clearance in the stenotic kidney, these patients were excluded from the study. In one patient, there was deterioration of blood pressure control shortly after dilatation of the left renal artery, which turned out to be caused by the occurrence of right‐sided renal arterial stenosis. This patient was also excluded from the investigation. Table shows characteristics and outcome of the 23 patients that were investigated. Two of the included subjects had diabetes.
As can be seen in Table and Figure the relative MAG3 clearance of the stenotic kidney expressed as a percentage of total function increased at the subsequent CR (performed on average 8 months later) following PTRA of the stenosis (from 29.9±14% to 35.1±14%, p = 0.01). Furthermore, creatinine levels fell following the intervention (from 110±19 to 99±17 µmol/l, p = 0.0003, see also Table ). Blood pressure levels were also lowered by the intervention (uncorrected blood pressures fell from 173±32/93±17 to 158±31/86±15 mmHg, p = 0.0009 and 0.006 for systolic and diastolic BP, respectively). The mean number of anti‐hypertensive drugs was unchanged following intervention (2.9±1.4 prescribed antihypertensive drugs before and 2.8±1.3 prescribed drugs after the intervention, respectively, p = 0.6, see also Table ), although there were large individual variations (Table ). No major complications were seen following the PTRA procedure in any of the 23 patients. However, one patient, number 6 in the table, had an initial misplacement of the stent, which was subsequently removed and placed in the femoral artery. She recovered uneventfully.
Figure 1 Graphic illustration of the effect of percutaneous dilatation of the renal arterial stenosis on the individual kidney function(expressed as % of total kidney function).
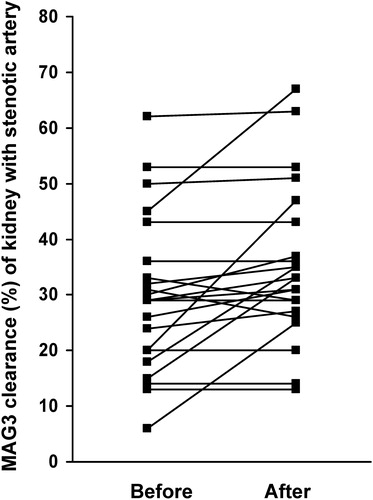
Patients who responded with an improvement of the renographic function of the kidney with the stenotic artery (defined as an increase of 5% or more, n = 7), compared with those that had no or very modest improvement (eight patients had no change, one experienced 4% and another patient 5% deterioration, total number of n = 10), were similar with regard to age, body mass index (BMI), number of antihypertensive drugs, percentage of kidney function at stenotic side, creatinine levels and blood pressure at baseline. On the other hand, patients who had an improvement of systolic blood pressure corrected for the amount of antihypertensive medication (improvement ranging from 9 to 62 mmHg, n = 16) had higher initial corrected systolic and diastolic blood pressures (221±39/124±20 mmHg) than those that did not experience a reduction of corrected systolic blood pressure (183±31/98±15 mmHg, p = 0.04 and 0.01 for systolic and diastolic blood pressures, respectively) following intervention (an increase ranging from 5 to 34 mmHg, n = 6). In a linear multivariate analysis of age, BMI, creatinine, corrected systolic BP, amount of antihypertensive drugs and kidney function of the stenotic side at baseline as independent variables and the improvement of blood pressure or not as dependent variable, there was a positive relation between the blood pressure (p = 0.03) and creatinine (p = 0.01) levels to improvement. BMI, on the other hand, was negatively related to improvement (p = 0.03). Among the six patients that did not improve corrected systolic BP, five patients did improve the relative function of the kidney with the stenotic artery (range 2–22%). Similarly, there were no statistically significant relationships between the change in corrected blood pressures to changes in either improvement of renographic function of the kidney with the stenotic kidney or change in creatinine following the PTRA.
There was no difference with regard to effects of PTRA on blood pressure, change in renographic function of kidney with stenotic artery or creatinine when the four subjects that did not receive a stent was compared with the remaining 19 subjects. In one case (patient 1 in Table ), the kidney that was subjected to PTRA had better renographic function then the non‐treated kidney/artery. This was a consequence of advanced general arteriosclerosis and judgement by the interventional radiologist to primarily dilate the renal artery that potentially could result in the most benefit to the patient.
The result of the CR performed after both 3 and 12 months was available in 13 subjects. The renographic result was very similar in both these investigations, the r‐value for the correlation being 0.99 and r2 = 0.98 (p<0.0001).
Discussion
In this prospective study of patients with suspected renovascular hypertension, PTRA was followed by an increase in captopril–renographic MAG3 clearance of the kidney with stenotic artery that was statistically significant. The absolute increase was rather modest, however, being only 5% on average. Similarly, we found statistically significant improvements of blood pressure and decrease of serum creatinine, with moderate absolute reductions of −15/7 mmHg and −11 µmol/l, respectively. On a more individual basis, 16 individuals had an improved corrected systolic blood pressure, and a minority, seven patients, experienced an unchanged or worsened blood pressure control. One single patient, 38 years old, was cured with regard to blood pressure levels, by the intervention, and judged not to need antihypertensive medication any more. In general, the PTRA was quite safe and no major complications were caused by the intervention. Since recruitment was performed on a consecutive basis, the results should apply to patients meeting the inclusion criteria.
We prospectively recruited all patients that were assumed to have renovascular hypertension during 8 years, and the final cohort consisted of 29 subjects. In a unique American epidemiological study, the prevalence of renal arterial stenosis was estimated to be 6.8% according to ultrasonography of renal arteries in elderly subjects Citation[8]. Thus the rather modest number of patients referred for evaluation of renovascular hypertension reflects reluctance to search for this condition among physicians that care for the 1,000,000 subjects that live in the catchment area of the University Hospital of Linkoping. However, it is highly likely that many patients with renovascular hypertension are not easily identified due to treatment with ACE inhibitors or angiotensin II receptor antagonists, which particularly effective mask the symptoms related to the disease Citation[9].
The blood pressure decrease following the intervention, 15/7 mmHg, was of a similar range as can be achieved by addition of a single antihypertensive drug Citation[10]. Bearing this comparison in mind, the risks and in the particular the costs of the PTRA procedure might be hard to motivate. However, it is quite possible that the blood pressure might have increased further if the stenotic arteries had been left untreated, as arteriosclerosis and degree of stenosis increases with time Citation[11]. On the other hand, it is also possible that dilatation of a stenotic renal artery prevents deterioration that might have clinical significance if the patient is followed up for a longer time Citation[12], although the speed of deterioration of kidney function in renal artery stenosis is debated Citation[9], Citation[13].
Since restenosis after PTRA is rather uncommon, we did not find it clinically and ethically justified to perform a follow‐up angiography in the patients Citation[14], Citation[15]. Furthermore, to our knowledge, our study is unique with respect to use of captopril also in adjunction with the follow‐up renography, which significantly increases the chance of detection of renal artery stenosis and renovascular hypertension Citation[6], Citation[16–18]. We cannot completely exclude the possibility of poor correction of the stenosis as the explanation of the fact that seven patients did not achieve a better BP control following the PTRA. However, the majority of these patients did indeed improve renal function assessed by the renographic examination. In the multivariate analysis, BMI was negatively related to the outcome, which suggests that several patients had other reasons for the increased blood pressure than those related to a decrease in renal artery perfusion. Insulin resistance and the metabolic syndrome are highly related to BMI and are causes of high blood pressure that are not expected to be improved by PTRA Citation[19]. The fact that improvement of blood pressure following PTRA was positively related to blood pressure and creatinine levels at baseline in the multivariate analysis in our study is a confirmation of earlier trials Citation[20], Citation[21]. The great majority of our patients received a stent (19/23 = 83%), which might relate to the relatively good results on creatinine levels compared with some other trials Citation[22–24]. Our study only included four subjects below 50 years of age, which limits the statistical power of calculation with age as a variable for outcome. It is reasonable to assume that long‐standing hypertension, being renovascular or of other origin, and high age of the majority of patients in our study negatively effected the outcome.
In conclusion, this prospective trial showed statistically significant improvements of blood pressure control and measures of kidney function in subjects with suspected renovascular hypertension treated with PTRA when analysed on an intention‐to‐treat basis. The PTRA procedure was quite safe but the magnitude of the absolute improvement was rather modest.
References
- Ives N. J., Wheatley K., Stowe R. L., Krijnen P., Plouin P. F., van Jaarsveld B. C., et al. Continuing uncertainty about the value of percutaneous revascularization in atherosclerotic renovascular disease: A meta‐analysis of randomized trials. Nephrol Dial Transplant 2003; 18: 298–304
- Ramos F., Kotliar C., Alvarez D., Baglivo H., Rafaelle P., Londero H., et al. Renal function and outcome of PTRA and stenting for atherosclerotic renal artery stenosis. Kidney Int 2003; 63: 276–282
- Textor S. C. Ischemic nephropathy: Where are we now?. J Am Soc Nephrol 2004; 15: 1974–1982
- Granerus G., Aurell M., Delin K., Karlberg B. E., Lorelius L. E. A Swedish view on the diagnosis of renovascular hypertension. J Intern Med 1992; 232: 15–24
- Granerus G., Moonen M. Effects of extra‐renal background subtraction and kidney depth correction in the measurement of GFR by gamma camera renography. Nucl Med Commun 1991; 12: 519–527
- Taylor A., Nally J., Aurell M., Blaufox D., Dondi M., Dubovsky E., et al. Consensus report on ACE inhibitor renography for detecting renovascular hypertension. Radionuclides in Nephrourology Group. Consensus Group on ACEI Renography. J Nucl Med 1996; 37: 1876–1882
- 2003 European Society of Hypertension–European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens 2003; 21: 1011–1053
- Hansen K. J., Edwards M. S., Craven T. E., Cherr G. S., Jackson S. A., Appel R. G., et al. Prevalence of renovascular disease in the elderly: A population‐based study. J Vasc Surg 2002; 36: 443–451
- Plouin P. F. Stable patients with atherosclerotic renal artery stenosis should be treated first with medical management. Am J Kidney Dis 2003; 42: 851–857
- Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002; 288: 2981–2997
- Rimmer J. M., Gennari F. J. Atherosclerotic renovascular disease and progressive renal failure. Ann Intern Med 1993; 118: 712–719
- Caps M. T., Perissinotto C., Zierler R. E., Polissar N. L., Bergelin R. O., Tullis M. J., et al. Prospective study of atherosclerotic disease progression in the renal artery. Circulation 1998; 98: 2866–2872
- Webster J., Marshall F., Abdalla M., Dominiczak A., Edwards R., Isles C. G., et al. Randomised comparison of percutaneous angioplasty vs continued medical therapy for hypertensive patients with atheromatous renal artery stenosis. Scottish and Newcastle Renal Artery Stenosis Collaborative Group. J Hum Hypertens 1998; 12: 329–335
- Bakker J., Goffette P. P., Henry M., Mali W. P., Melki J. P., Moss J. G., et al. The Erasme study: A multicenter study on the safety and technical results of the Palmaz stent used for the treatment of atherosclerotic ostial renal artery stenosis. Cardiovasc Intervent Radiol 1999; 22: 468–474
- Morganti A., Bencini C., Del Vecchio C., Strata M. Treatment of atherosclerotic renal artery stenosis. J Am Soc Nephrol 2002; 13(Suppl 3)S187–S189
- Taylor A. T, Jr., Fletcher J. W., Nally J. V, Jr., Blaufox M. D., Dubovsky E. V., Fine E. J., et al. Procedure guideline for diagnosis of renovascular hypertension. Society of Nuclear Medicine. J Nucl Med 1998; 39: 1297–1302
- Muller‐Suur R., Tidgren B., Fehrm A., Lundberg H. J. Captopril‐induced changes in MAG3 clearance in patients with renal arterial stenosis and the effect of renal angioplasty. J Nucl Med 2000; 41: 1203–1208
- Nally J. V., Barton D. P. Contemporary approach to diagnosis and evaluation of renovascular hypertension. Urol Clin North Am 2001; 28: 781–791
- Malmqvist K., Ohman K. P., Lind L., Nystrom F., Kahan T. Relationships between left ventricular mass and the renin‐angiotensin system, catecholamines, insulin and leptin. J Intern Med 2002; 252: 430–439
- Geddes C. C., Jardine A. G. Diagnosis and treatment of atherosclerotic renal artery stenosis (ARAS). Minerva Urol Nefrol 2002; 54: 29–36
- Marshall F. I., Hagen S., Mahaffy R. G., Petrie J. C., Roy‐Chaudhury P., Russell I. T., et al. Percutaneous transluminal angioplasty for atheromatous renal artery stenosis – blood pressure response and discriminant analysis of outcome predictors. Q J Med 1990; 75: 483–489
- van Jaarsveld B. C., Krijnen P., Pieterman H., Derkx F. H., Deinum J., Postma C. T., et al. The effect of balloon angioplasty on hypertension in atherosclerotic renal‐artery stenosis. Dutch Renal Artery Stenosis Intervention Cooperative Study Group. N Engl J Med 2000; 342: 1007–1014
- Plouin P. F., Guery B., La Batide Alanore A. Atherosclerotic renal artery stenosis: Surgery, percutaneous transluminal angioplasty, or medical therapy?. Curr Hypertens Rep 2000; 2: 482–489
- Plouin P. F., Chatellier G., Darne B., Raynaud A. Blood pressure outcome of angioplasty in atherosclerotic renal artery stenosis: A randomized trial. Essai Multicentrique Medicaments vs Angioplastie (EMMA) Study Group. Hypertension 1998; 31: 823–829