Abstract
Treatment of hypertension remains a difficult task despite the availability of different types of medications lowering blood pressure by different mechanisms. In order to reach the target blood pressures recommended today combination therapy is required in most patients. The co‐administration of two drugs with different impacts on the cardiovascular system markedly increases the antihypertensive effectiveness without altering adversely tolerability. Fixed low‐dose combinations are becoming a valuable option not only as second‐line, but also as first‐line therapy. In this respect the co‐administration of thiazide diuretic with an AT1‐receptor blocker is particularly appealing. The diuretic‐induced decrease in total body sodium activates the renin–angiotensin system, thus rendering blood pressure maintenance angiotensin II‐dependent. During blockade of the renin–angiotensin system low doses of thiazides generally suffice, allowing the prevention of undesirable metabolic effects. Also, blockade of the AT1‐receptor, particularly when angiotensin II production is enhanced in response to diuretic therapy, is expected to be beneficial, since angiotensin II seems to contribute importantly to the pathogenesis of cardiovascular and renal complications of hypertension.
Introduction
The rationale for combination therapy in the management of hypertension is well established. Co‐administering two drugs having different impacts on the cardiovascular system enhances markedly the antihypertensive efficacy. Actually, an increased blood pressure lowering effect can be expected from the co‐administration of all classes of agents Citation[1], Citation[2]. A meta‐analysis of 119 randomized double‐blind placebo‐controlled trials involving thiazides, beta‐blockers, angiotensin‐converting enzyme (ACE) inhibitors, AT1‐receptor antagonists and calcium‐channel blockers, given alone or in combination, has been published recently Citation[3]. The “first” and the “second” drug administered separately lowered blood pressure by an average of 7.0/4.1 and 8.1/4.6 mmHg, respectively, compared with 14.6/8.6 mmHg when the two agents were co‐administered. In some trials, the antihypertensive agents were co‐administered at half standard doses, which also resulted in a major blood pressure reduction (13.3/7.3 mmHg).
Some combinations appear especially rational, for instance those associating a diuretic and a blocker of the renin–angiotensin system Citation[4]. This is because inhibition of this pressor system renders ineffective the hyperreninemia triggered by salt‐depletion, allowing maximum benefit to be derived from diuretic therapy. The present paper is aimed to review the potential advantages of combining an AT1‐receptor blocker and a thiazide diuretic. This is an important issue considering the growing number of fixed‐dose preparations containing such agents. Focus will be given on the various reasons why it appears today highly desirable to prevent the actions of angiotensin II when stimulating the renin–angiotensin system using a diuretic.
Why should the renin–angiotensin system be blocked during diuretic therapy?
The adverse impact of angiotensin II on the arterial wall
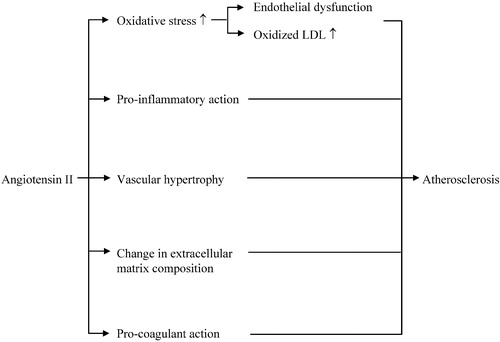
The potential for angiotensin II to contribute to the pathogenesis of vascular diseases in humans has been first suggested by the observation that a high renin state is predictive for myocardial infarction and stroke Citation[5], Citation[6]. Angiotensin II appears to play a critical role in a variety of non‐hemodynamic mechanisms implicated in the development of the vascular remodeling known to occur in the arterial wall of hypertensive patients and, ultimately, of atherosclerosis (Figure ; Citation[7–10]): (i) it activates membrane NADH/NADPH oxidase in endothelial and vascular smooth muscle cells, enhancing thereby the generation of superoxide anion; (ii) it causes vascular hypertrophy and favors collagen deposition, mainly by triggering the production by vascular smooth muscle cells of growth factors such as platelet‐derived growth factor, insulin‐like growth factor, basic fibroblast growth factor and transforming growth factor‐β1; (iii) it activates the expression of chemokines (monocyte chemotactic protein‐1, MCP‐1), leukocyte adhesion molecules (VCAM), intracellular adhesion molecule (ICAM) and P‐selectin; (iv) it activates macrophages, leading to the release of pro‐inflammatory cytokines [tumor necrosis factor (TNF‐α), interleukin 6 (IL‐6)]; (v) it upregulates the receptor for oxidized low‐density lipoprotein (LDL), resulting in an increased cellular uptake of this lipoprotein by macrophages and an activation of these cells; (vi) it alters extracellular matrix composition, for example by stimulating the synthesis by monocytes/macrophages of metalloproteinases, i.e. proteases involved in the degradation of connective tissue matrix proteins; (vii) it induces a procoagulant state by stimulating the formation of plasminogen‐activator inhibitor (PAI‐I) by endothelial cells. Notably, the increased oxidative stress caused by angiotensin II, while playing a pivotal role in most of the effects described above, has another detrimental consequence. NO generated in the endothelium is converted, in the presence of reactive oxygen species, in the biologically inactive peroxynitrite, so that the contractile effect of angiotensin II, unopposed by NO, becomes predominant. The deleterious effects of angiotensin II may even be amplified by an upregulation of AT1‐receptors in the arterial wall, as suggested by the fact that oxidized LDL increase AT1‐receptor expression on endothelial and vascular smooth muscle cells Citation[11], Citation[12].
Figure 1 Schematic representation of the main processes that implicate angiotensin II in the pathogenesis of atherosclerosis.
Angiotensin II continues to exert adverse effects when the atherosclerotic plaque has developed. Large amounts of angiotensin II can be produced locally by inflammatory cells present in human vascular lesions, for instance by monocytes/macrophages which overexpress ACE Citation[13]. An increased chymase‐dependent angiotensin II formation has also been demonstrated in human atherosclerotic aorta Citation[14]. In addition, overexpression of cathepsin G, an angiotensin II‐generating enzyme, has been observed in human carotid arteries with atherosclerotic lesions Citation[15]. Angiotensin II represents therefore the starter of a vicious circle: it activates inflammatory cells in the arterial wall, which in turn promote the local generation of angiotensin II, with an ensuing perpetuation of inflammation. Finally angiotensin II, by increasing metalloproteinase activity, might weaken the fibrous cap of a vulnerable atherosclerotic plaque, resulting in an increased risk of rupture and, thereby, of clinical event Citation[16].
Beneficial vascular effects of AT1‐receptor blockade
Does blockade of AT1‐receptors have beneficial effects on the arterial wall in humans? Angiotensin II has been shown to increase superoxide production in internal mammary arteries obtained from patients during elective coronary artery revascularization surgery. This effect of angiotensin II could be completely prevented by co‐incubation of the arterial fragment with the AT1‐receptor blocker losartan Citation[17]. Blockade of the renin–angiotensin reduces the oxidative stress not only in vascular preparations, but also in patients. In one study, 102 hypertensive patients were divided into five groups to receive for 3 months one of the following monotherapies: (i) the AT1‐receptor blocker valsartan, 80 mg/day; the ACE inhibitor ramipril, 5 mg/day; the alpha1‐blocker doxazosin, 4 mg/day; the beta‐blocker metoprolol, 100 mg/day; the dihydropyridine calcium antagonist amlodipine, 10 mg/day Citation[18]. Plasma levels of malondialdehyde, a marker of lipid peroxidation, were measured at baseline and again at the end of the trial. Plasma malondialdehyde levels were significantly higher in the hypertensive patients than in 51 normal control subjects. Losartan was the most effective in decreasing the formation of malondialdehyde (−67.2%), followed by ramipril (−57.6%), doxazosin (−15.9%), metoprolol (−2.7%) and amlodipine (−2.4%).
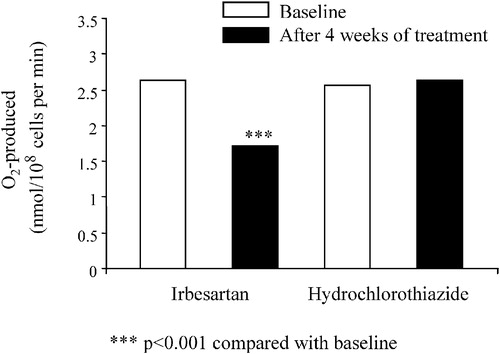
The diuretic‐induced salt depletion activates the renin–angiotensin system, which conceivably might have a negative impact on the oxidative and inflammatory state of the vasculature. This issue has been addressed in a trial in which 38 hypertensive patients were randomly assigned to a 4‐week treatment with either the AT1‐receptor blocker eprosartan, 600 mg/day, or hydrochlorothiazide (HCTZ), 50 mg/day Citation[19]. The two medications lowered blood pressure similarly. Eprosartan had a number of potentially favorable effects since it reduced significantly the neutrophil superoxide generating capacity (−28%), the serum levels of soluble monocyte chemotactic protein‐1 (−34%) as well as those of the soluble vascular cell adhesion molecule (−35%), whereas HCTZ had no effect on the above parameters. In another trial Citation[20], 40 hypertensive patients were randomly allocated to receive either irbesartan, 150 mg/day, or HCTZ, 25 mg/day. The collagen‐induced production of superoxide anion by platelets was determined in each subject before and after 4 weeks of treatment. The blood pressure response to the two medications was of similar magnitude. Notably, irbesartan significantly decreased superoxide anion production by platelets, whereas no change was seen in patients on HCTZ (Figure ).
Figure 2 Collagen‐stimulated production of superoxide anion (O2−) in hypertensive patients treated for 4 weeks with either irbesartan or hydrochlorothiazide (modified from ref. 20).
Also very interesting are the observations made in normotensive patients with stable coronary artery disease (n = 33) who were treated for 6 months with irbesartan (75–150 mg/day) Citation[21]. These patients were compared with subjects having no known coronary atherosclerosis (n = 24). The patients with atherosclerosis showed increased plasma levels of inflammatory markers, such as solubilized TNF‐α and VCAM‐1. Irbesartan treatment brought these levels to normal values, despite the fact that it did not lower blood pressure. Serum levels of superoxide anion were also increased in patients with coronary artery disease, and this abnormality was corrected by AT1‐receptor blockade. The same authors extended their studies to 47 patients with documented coronary artery disease Citation[22]. A significant decrease in the susceptibility to LDL oxidation as well as in the production of superoxide by neutrophils was seen in these patients. Other investigators found that AT1‐receptor blockade with losartan, 50 mg/day for 4 weeks, reduces the cellular uptake of oxidized LDL by monocyte/macrophages obtained from normotensive patients with hypercholesterolemia Citation[23]. Another trial was conducted in patients with coronary heart disease maintained on aspirin and hypocholesterolemic therapy with atorvastatin Citation[24]. These patients were randomized to a 24‐week treatment with the ACE inhibitor quinapril, 20 mg/day, irbesartan, 150 mg/day, or placebo. The addition of placebo to atorvastatin and aspirin had no effect on serum levels of interleukin‐6 (IL‐6), whereas both quinapril and irbesartan induced a significant decrease in this inflammatory cytokine, suggesting that blockade of the renin–angiotensin system has a powerful additive anti‐inflammatory effect compared with that obtained by aspirin and cholesterol lowering.
Angiotensin II blockade has also been shown to stabilize atherosclerotic plaques in humans Citation[25]. Seventy patients with symptomatic carotid artery stenosis were randomized to receive for 4 months before endarterectomy either irbesartan (300 mg/day) or chlortalidone (50 mg/day). Interestingly, plaques obtained from the irbesartan group exhibited significantly fewer macrophages, decreased immuno‐reactivity for metalloproteinase and increased collagen content, which might contribute to plaque stabilization.
AT1‐receptor antagonists improve endothelial‐dependent vasodilation in the forearm vasculature of patients with essential hypertension Citation[26], Citation[27]. In one trial, 60 hypertensive patients were randomized to 6 weeks of therapy with the AT1‐receptor antagonist valsartan, 80 mg/day, HCTZ, 25 mg/day, or placebo Citation[28]. Blood pressure was similarly decreased by the two active treatments. A major finding was that only valsartan enhanced in the forearm circulation the vasoconstriction induced by L‐NMMA, an inhibitor of NO‐synthase, suggesting that basal production of NO was increased by the AT1‐receptor antagonist, but not by the diuretic. This is a key observation considering the fact that valsartan and HCTZ have opposite effects on the renin–angiotensin system. AT1‐receptor blockers have also been shown to ameliorate endothelial vasomotion during stress in the coronary microcirculation of patients with atherosclerosis Citation[29] and to improve endothelium‐dependent dilation in the forearm circulation of patients with type 2 diabetes Citation[30].
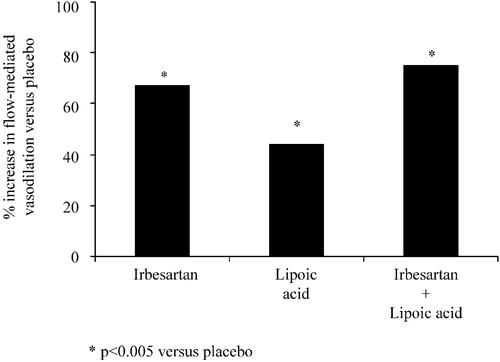
The metabolic syndrome represents a high risk constellation linked with abdominal (central) obesity, increased likelihood of developing abnormalities such as type 2 diabetes, hypertension and characteristic dyslipidemia [high plasma triglyceride and low high‐density lipoprotein (HDL)‐cholesterol] Citation[31], Citation[32]. Very recently, a trial was performed in 58 subjects with the metabolic syndrome to compare in a double‐blind manner, the effects of a 4‐week treatment with irbesartan (150 mg/day), lipoic acid (300 mg/day), both irbesartan and lipoic acid, or matching placebo, on endothelium‐dependent flow‐mediated vasodilation in the brachial artery as well as various markers of inflammation Citation[33]. Lipoic acid was used as at is known to have strong antioxidant properties. The flow‐mediated vasodilations induced by irbesartan and/or lipoic acid were significantly greater than that of placebo (Figure ). The active treatments also significantly reduced plasma IL‐6 and PAI‐I levels, in the absence of any change in systemic blood pressure.
Figure 3 Percentage increase in flow‐mediated vasodilation of the brachial artery induced in subjects with the metabolic syndrome by irbesartan, lipoic acid or irbesartan+lipoic acid compared with placebo (modified from ref. 33).
AT1‐receptor blockade decreases arterial stiffness of large vessels. For example, in one trial involving only a small number of patients (n = 11), losartan, 50 mg/day, was compared with HCTZ, 12.5 mg/day, administered for 4‐week periods according to a crossover design Citation[34]. The two medications lowered blood pressure to a similar extent, but only the AT1‐receptor blocker slowed pulse wave velocity, implying a decrease in aortic stiffness and arterial wave reflection. In addition, AT1‐receptor blockade has been reported to correct the structural abnormalities (lumen narrowing and media thickening) and the endothelial dysfunction of resistance arteries dissected from gluteal subcutaneous biopsies taken from hypertensive patients Citation[35]. Such beneficial effects could not be obtained with a beta‐blocker, even if the blood pressure lowering was equivalent.
AT1‐receptor blockade and the coagulant state
Another point to consider is the detrimental impact of AT1‐receptor stimulation on the coagulant state. There is strong evidence that angiotensin II creates an imbalance between the levels of tissue plasminogen activator (t‐PA) and plasminogen activator inhibitor type‐1 (PAI‐I) favouring the development of adverse thrombo‐embolic cardiovascular events Citation[36]. Both t‐PA and PAI‐I are synthesized by the vascular endothelium. Angiotensin II infusion in humans induces a dose‐dependent increase in plasma PAI‐I levels Citation[37]. Plasma levels of PAI‐I antigen and plasma PAI‐I activity are higher in hypertensive patients compared with normotensive individuals Citation[38]. Treatment of hypertensive patients for 6 months with either the ACE inhibitor perindopril, 2–8 mg/day (n = 15), or losartan, 50–100 mg/day (n = 15) caused a significant decrease in PAI‐I production. The effects of AT1‐receptor blockade on plasma fibrinolytic activity was also explored in 54 hypertensive patients who were randomized to receive for 6 months either irbesartan, 75–300 mg/day, or atenolol, 25–150 mg/day. The two compounds equally lowered blood pressure, but only irbesartan decreased plasma levels of PAI‐I Citation[39].
AT1‐receptor blockade and the heart
Left ventricular hypertrophy has been recognized as an independent cardiovascular risk factor Citation[40] and left ventricular mass reduction during antihypertensive therapy is associated with a reduced rate of subsequent cardiovascular events Citation[41]. Angiotensin II is thought to modulate cardiac growth and function Citation[42]. By stimulating AT1‐receptors, it contributes to the development of cardiac hypertrophy and might have in addition a positive chronotropic effect. Some angiotensin II can be generated in cardiac tissue independently of ACE, for instance by chymase, a serine protease which cannot be blocked by ACE inhibitors Citation[43]. Some trials have suggested that AT1‐receptor blockade is more effective than beta‐adrenoceptor blockade or calcium entry blockade in regressing cardiac hypertrophy Citation[44], Citation[45]. This was confirmed in a recent meta‐analysis (Figure ; Citation[46]). AT1‐receptor antagonists lowered left ventricular mass index by 13%, as compared with 11%, 10%, 8% and 6% with calcium antagonists, ACE inhibitors, diuretics and beta‐blockers, respectively. AT1‐receptor blockade may even improve left ventricular function in hypertensive patients with no evidence of cardiac hypertrophy at echocardiography Citation[47].
Figure 4 Mean reductions in left ventricular mass index(expressed as percentage from baseline, with 95% confidence intervals) with different classes of antihypertensive agents. *p<0.05; **p<0.01 vs beta‐blockers (modified from ref. 46).
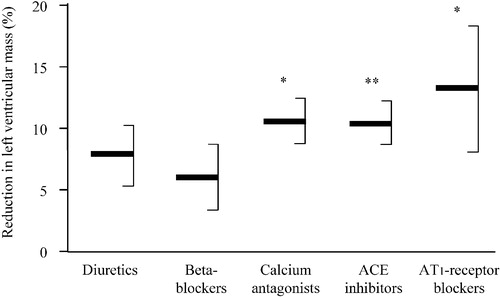
The lesser effectiveness of beta‐blockers, as compared with AT1‐receptor blockers, in regressing left ventricular hypertrophy is in accordance with the results of the LIFE study Citation[48]. In that trial, the losartan‐ and the atenolol‐based treatments equally lowered blood pressure, but the AT1‐receptor blocker conferred a significantly better regression of electrocardiographically determined cardiac hypertrophy. HCTZ was the second line medication in both treatment groups and had to be added in most patients during the follow‐up. The LIFE trial should therefore be regarded as a comparison between AT1‐receptor blocker–HCTZ and beta‐adrenoceptor blocker–HCTZ drug regimens rather than a comparison between monotherapies.
QT dispersion, as defined by the difference between maximal and minimal QT intervals within a 12‐lead surface electrocardiogram, is increased in hypertensive patients Citation[49], especially in those with left ventricular hypertrophy Citation[50]. This abnormality is regarded as an early indicator of cardiac damage in hypertension and might be associated with an enhanced risk of malignant arrhythmias and sudden death. The effects of irbesartan, 75–150 mg once a day, and amlodipine, 5–10 mg once a day, were studied in a double‐blind trial including 104 elderly patients with essential hypertension Citation[51]. Equivalent blood pressure control rates were obtained in the two treatment arms. A significant reduction in QT dispersion was seen in the irbesartan‐treated patients but not in those allocated to the calcium antagonist.
AT1‐receptor blockade and the kidney
Angiotensin II plays a key role in the regulation of intraglomerular pressure by inducing a contraction of both the afferent and the efferent arterioles. In hypertensive patients AT1‐receptor blockade for 2 weeks with candesartan has been shown to lower systemic blood pressure, to increase renal blood flow, but to leave unchanged glomerular filtration rate Citation[52]. As a consequence of these renal responses to AT1‐receptor blockade, filtration fraction was reduced, which suggested a predominant dilation of the efferent arterioles. Another well‐documented action of AT1‐receptor is the stimulation of the Na+/H+ exchanger in the renal proximal tubule, leading to sodium reabsorption Citation[53]. It now appears that angiotensin II can accelerate the progression of renal injury by inducing cell growth and matrix accumulation in glomerular cells Citation[54].
The major controlled trials aimed to assess whether chronic AT1‐receptor blockade is renoprotective have been performed in patients with type 2 diabetes, i.e. in patients prone to developing nephropathy Citation[55–58]. In such patients, an increased intrarenal production of angiotensin II and/or an exaggerated renal susceptibility to the actions of angiotensin II seem to play a pivotal role in the pathogenesis of nephropathy Citation[59], Citation[60]. Classically the progression of nephropathy goes in patients with type 2 diabetes through a stage of microalbuminuria to a stage of overt proteinuria and, ultimately, to terminal renal failure. The frequent co‐existence of hypertension accelerates the progression of this renal injury, which depends critically on glomerular capillary pressure Citation[61].
One study investigated the renoprotective effect of irbesartan in 590 hypertensive patients with type 2 diabetes and microalbuminuria Citation[57]. The patients were randomly allocated to irbesartan (150 mg once daily), irbesartan (300 mg once daily) or matching placebo. A target blood pressure was set in the three groups at less than 135/85 mmHg. To reach this goal, other antihypertensive medications could be used, including diuretics, beta‐blockers, calcium antagonists (except dihydropyridines) and alpha‐blockers, but ACE inhibitors were not allowed. Blood pressure was equally lowered in the three treatment arms during the 2‐year follow‐up. At the end of the study 56% of the patients received antihypertensive agents on top of placebo, while 45 and 43% of patients were given blood pressure lowering medications in addition to irbesartan 150 and 300 mg, respectively. Conversion of microalbuminuria to albuminuria occurred in 5.2% and 9.7% of the patients in the irbesartan 300 and 150 mg groups, respectively, and 14.9% in the placebo group (p<0.001, irbesartan 300 mg vs placebo). Restoration of normoalbuminuria during the study was also statistically more frequent in the irbesartan 300 mg‐treated patients than in patients on placebo (34 vs 21% of patients, p<0.006). These beneficial effects of AT1‐receptor blockade in microalbuminuric hypertensive patients with type 2 diabetes could be confirmed by comparing a valsartan‐ with an amlodipine‐based treatment Citation[58].
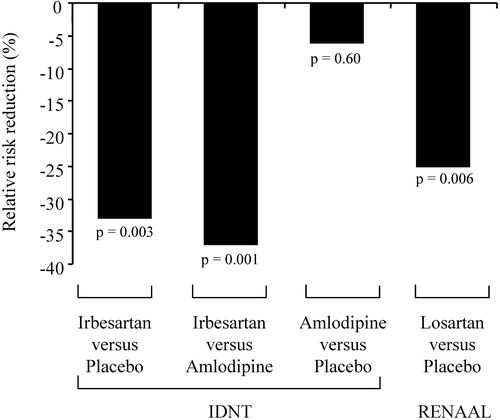
Two interventional trials examined the potential of AT1‐receptor blockade in slowing the progression of diabetic nephropathy. The first one, abbreviated as IDNT (Irbesartan Diabetic Nephropathy Trial), included 1715 type 2 diabetic hypertensive patients with albuminuria Citation[55]. These patients were randomized to a treatment with irbesartan (75–300 mg once daily), amlodipine (2.5–10 mg once daily) or placebo. In order to reach a target blood pressure of 135/85 mmHg or less, other types of antihypertensive agents could be added, at the exclusion of ACE inhibitors, AT1‐receptor blockers and calcium antagonists. Notably, patients in the irbesartan and amlodipine groups received an average of three non‐study drugs, compared with 3.3 in the placebo group. Blood pressure levels achieved at the end of a mean 2.6‐year follow‐up were similar in the three arms. Irbesartan reduced the relative risk of doubling serum creatinine concentration (−33%, p = 0.003 vs placebo and −37%, p = 0.001 vs amlodipine (Figure ). AT1‐receptor blockade also lowered the relative risk of end‐stage renal disease (−23% vs both placebo and amlodipine, p = 0.07). The second trial randomized for a mean follow‐up of 3.4 years 1513 hypertensive patients with type 2 diabetes and albuminuria to either the AT1‐receptor blocker losartan (50–100 mg daily) or placebo, both taken as needed with other antihypertensive medications to achieve a target blood pressure of less than 140/90 mmHg Citation[56]. Most patients required combination therapy. For example, diuretics were given in addition to losartan or placebo in 83.8 and 84% of patients, respectively. The quality of blood pressure control was comparable in the two groups. The losartan‐based regimen significantly reduced the relative risk of doubling serum creatinine concentration (−25%, p = 0.006) as well as that of developing end‐stage renal disease (−28%, p = 0.002; Figure ).
AT1‐receptor blockade and the brain
One recent trial assessed in elderly hypertensive patients whether an AT1‐receptor blocker‐based treatment confers benefits compared with a conventional drug regimen in the prevention of cardiovascular events and dementia Citation[62]. To this end, 4964 patients were randomized to either candesartan, 8 mg once a day, or matching placebo. Patients were followed for a mean treatment period of 3.7 years. To reach the target blood pressure (<160/90 mmHg) there was the possibility first to double the dose of the blinded drug, and then to add open‐label active antihypertensive medications (preferentially HCTZ 12.5 mg once a day), except AT1‐receptor blockers and ACE inhibitors. Cognitive function was evaluated during the trial using the Mini Mental State Examination (MMSE) test. A minority of patients remained on monotherapy during the course of the study (25% in the irbesartan group and 16% in the placebo group). The treatment‐induced blood pressure reduction was significantly more pronounced, with a difference of 3.2/1.6 mmHg (p<0.001), in the patients receiving candesartan than in those on placebo. The main finding was a significant reduction of non‐fatal stroke (−27.8%, p = 0.04). No consistent difference between the two groups was observed with regard to cognitive function, even if angiotensin II is believed to play an important role in modulating cognitive processes.
The long‐term responses of cognitive function to AT1‐receptor blockade and diuretic therapy has been investigated in a double‐blind trial involving 69 patients (age range: 30–73 years) with mild to moderate hypertension Citation[63]. These patients were randomly allocated to a 26‐week once‐daily treatment with either 50 mg losartan or 25 mg HCTZ. Cognitive function was evaluated using the MMSE test. The MMSE score significantly improved (p<0.001) in the losartan group (from 23±3 to 27±3, means±SD), whereas it did not change in the HCTZ group (from 24±3 to 25±3). The losartan‐induced blood pressure decrease, as assessed by 24‐h ambulatory monitoring, was however more pronounced in the losartan (from 151/94±9/5 to 130/82±10/4 mmHg, p<0.001/p<0.001) than in the HCTZ group (from 150/93±11/6 to 142/87±12/9 mmHg, p<0.02/p<0.01).
Finally it worth mentioning the results of a prospective randomized controlled trial performed recently to compare an AT1‐receptor blocker – with a dihydropyridine – based regimen in the secondary prevention of stroke Citation[64]. A total of 1405 hypertensive patients with cerebral event within the last 24 h were randomized for a mean follow‐up of 2.5 years to either 600 mg eprosartan or 10 mg nitrendipine once daily, with the possibility to add, when required to reach a target blood pressure of 140/90 mmHg or below, a beta‐blocker, and alpha1‐blocker or a centrally acting drug. Blood pressure was lowered to a similar extent in the two treatment groups, but the AT1‐receptor blocker significantly reduced both cerebrovascular and cardiovascular events.
Antihypertensive efficacy of combinations containing an AT1‐receptor antagonist and a thiazide diuretic
Antihypertensive efficacy of AT1‐receptor blockade alone or combined with HCTZ in randomized, placebo‐controlled trials
There exists a close interplay between the activity of the renin–angiotensin system and sodium balance. Thus, renin secretion can be stimulated by reducing a total body sodium, enhancing thereby the contribution of angiotensin II to blood pressure maintenance Citation[4]. This could be confirmed in “factorial design” trials involving the co‐administration of an AT1‐receptor blocker and HCTZ Citation[65–71]. In such trials, hypertensive patients are randomized to one or several doses of the two components, allowing the definition of the most suitable doses to be combined in a fixed‐dose product. The results of all these trials showed a better antihypertensive efficacy of AT1‐receptor blocker combinations than the individual monotherapies. For instance, a study was carried out to assess the antihypertensive efficacy of olmesartan monotherapy, HCTZ monotherapy, and the combination of both Citation[65]. A total of 502 hypertensive patients with a diastolic blood pressure ranging from 100 to 115 mmHg received for 8 weeks once‐daily doses of olmesartan (10, 20 or 40 mg), HCTZ (12.5 or 25 mg), a combination of both (10, 20 or 40 mg/12.5 or 25 mg) or placebo. Table shows the blood pressure control rate (<90 mmHg for diastolic and <140 mmHg for systolic) observed in the different groups at the end of the trial. The blood pressure lowering effect of olmesartan was dose‐dependent and the co‐administration of HCTZ markedly improved systolic and diastolic blood pressure control, the best results being achieved with the 25‐mg dose.
Table I. Blood pressure control rate obtained with olmesartan monotherapy, HCTZ monotherapy and the olmesartan/HCTZ combination (from ref. 65).
Antihypertensive efficacy of AT1‐receptor blocker/HCTZ combinations in hypertensive patients inadequately controlled on AT1‐receptor blocker or HCTZ monotherapy
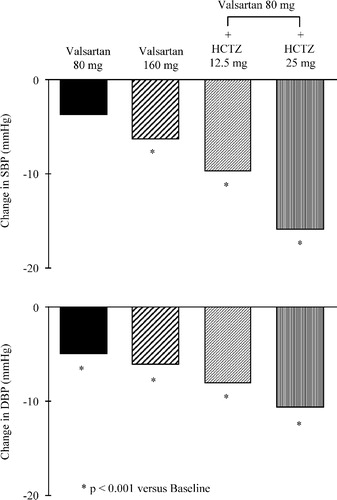
Using monotherapies, it is possible to normalize blood pressure in only a fraction of hypertensive patients. This is also true for AT1‐receptor blockers and thiazide diuretics. Several trials were aimed to assess the blood pressure response to the addition of an AT1‐receptor blocker or HCTZ in patients inadequately controlled with HCTZ or AT1‐receptor blocker therapy, respectively Citation[72–75]. In all of them, the addition of the second drug induced a further decrease in blood pressure. As an example, a trial was performed in 708 hypertensive patients with diastolic blood pressure inadequately controlled (seated diastolic blood pressure between 95 and 115 mmHg) by a 4‐week treatment with valsartan, 80 mg once daily Citation[74]. The patients were randomly allocated to receive for 8 additional weeks 80 mg valsartan, 160 mg valsartan, 80 mg valsartan+12. 5 mg HCTZ or 80 mg valsartan+25 mg HCTZ. All medications were administered once daily. Figure illustrates the mean changes from baseline in sitting systolic and diastolic blood pressure. The blood pressure reductions were significantly more pronounced in the valsartan+HCTZ groups as compared with valsartan monotherapies. There was no significant difference however between the 80‐ and the 160‐mg doses of valsartan given alone. The best blood pressure results were obtained with the association of the largest dose of both valsartan and HCTZ. Thus, these data indicate that adding a small dose of a thiazide diuretic is more effective in the treatment of hypertension resistant to angiotensin II blockade than doubling the dose of the Ang II antagonist used as monotherapy.
Figure 6 Mean changes in sitting systolic(SBP) and diastolic (DBP) blood pressure induced by a 8‐week treatment with valsartan, 80 or 160 mg, or valsartan 80 mg combined with hydrochlorothiazide (HCTZ), either 12.5 or 25 mg. At randomization, all patients had their diastolic blood pressure still inadequately controlled despite a 4‐week treatment with valsartan, 80 mg per day. The medications were administered once daily (from ref. 74).
Safety and tolerability of combinations containing an AT1‐receptor antagonist and a thiazide diuretic
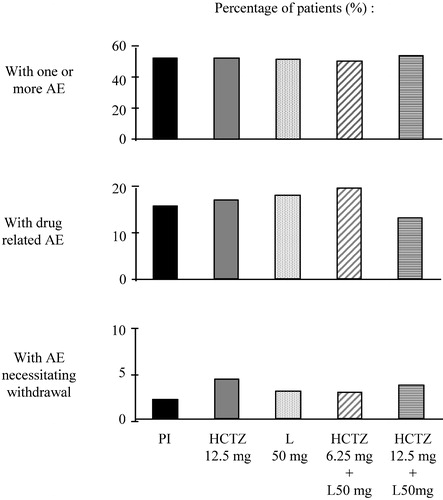
Fixed‐dose combinations containing an AT1‐receptor blocker and a low dose of a thiazide, have a placebo‐like tolerability, like AT1‐receptor blockers given alone Citation[66–70]. This is illustrated in Figure , which depicts the adverse clinical experiences reported in patients having be treated once daily for 12 weeks by placebo, 12.5 mg HCTZ, 50 mg losartan, 50 mg losartan+6.25 mg HCTZ or 50 mg losartan+12.5 mg HCTZ. No difference was observed between the treatment groups regarding the percentage of patients who reported adverse effects, or had to interrupt therapy because of the occurrence of an adverse event Citation[71].
Figure 7 Percentage of patients having reported adverse clinical experience(AEs) during 12‐week treatment with placebo (Pl), hydrochlorothiazide 12.5 mg (HCTZ 12.5 mg), losartan 50 mg (L 50 mg) or L 50 mg+HCTZ 12.5 mg (modified from ref. 71).
AT1‐receptor antagonists, owing to their flat tolerability dose–response curve, can be co‐administered with a diuretic at a dose large enough to provide optimal blockade of the renin–angiotensin system whilst renin secretion is stimulated by the diuretic‐induced negation of sodium balance.
The metabolic unwanted effects of thiazides, including hypokalemia, hyperglycemia, hyperuricemia and hypercholesterolemia, are known to be dose‐dependent Citation[76]. Notably, metabolic disturbances are practically not seen anymore when thiazides are given together with an AT1‐receptor antagonist, partly because low doses of diuretics are usually sufficient during blockade of the renin–angiotensin system Citation[65–67], Citation[77–79]. Also, the diuretic‐induced secondary aldosteronism is blunted during AT1‐receptor blockade, which contributes to the maintenance of a normal potassium balance. To be also pointed out is the uricosuric effect of the AT1‐receptor blocker losartan, which offsets the thiazide‐induced hyperuricemia Citation[80].
Increasing attention is paid nowadays on type 2 diabetes, a high‐risk condition whose prevalence is increasing dramatically Citation[81]. A key factor predicting the development of type 2 diabetes is the existence of insulin resistance (defined as a decreased responsiveness to insulin of skeletal muscle), which represents the hallmark of the metabolic syndrome associating characteristically central obesity, hypertension and dyslipidemia (high plasma triglyceride and low HDL; Citation[82]). Antihypertensive agents may exert differential effects on the incidence of type 2 diabetes. In particular, thiazide diuretics might have an adverse impact, at the contrary of AT1‐receptor blockers Citation[83]. In this context, it is worth mentioning two trials. In the first one, 127 obese hypertensive patients were randomly allocated to a 12‐week treatment with either the AT1‐receptor blocker candesartan (8–16 mg once daily) or HCTZ (25–50 mg once daily; Citation[84]). Candesartan significantly improved insulin resistance index, calculated as the ratio of the area under the curve for glucose to that for insulin during a glucose load test performed at the beginning and at the end of the study, whereas HCTZ tended to worsen this index. In the second trial 392 hypertensive patients were randomly assigned to a 1‐year treatment with either candesartan (16 mg once daily) or HCTZ (25 mg once daily), with the possibility to add the calcium antagonist felodipine (slow‐release formulation, 2.5–5 mg once daily) or the beta‐blocker atenolol (50–100 mg once daily) in the candesartan and HCTZ groups, respectively Citation[85]. Compared with the HCTZ‐based treatment, the candesartan‐based treatment significantly lowered blood insulin, glucose, triglyceride and LDL‐cholesterol levels, while significantly increasing blood HDL‐cholesterol levels. Notably, diabetes mellitus was diagnosed during the follow‐up in eight patients in the HCTZ group, but in only one patient in the candesartan group (p<0.05). Also, 18 patients receiving the diuretic developed a metabolic syndrome, vs five in the AT1‐receptor blocker group (p<0.01). The beneficial effects of AT1‐receptor blockade on the incidence of type 2 diabetes in hypertensive patients were confirmed in large interventional trials Citation[48]. The mechanisms responsible for the reduction in insulin resistance may depend in part on muscle vasodilatation, a sympathoinhibition linked to AT1‐receptor blockade and the maintenance of body potassium when an AT1‐receptor is co‐administered with a diuretic Citation[76], Citation[84], Citation[86], Citation[87]. Also, AT1‐receptor blockade increases plasma adiponectin concentrations in patients with essential hypertension Citation[88]. This is noteworthy considering the fact that this cytokine produced by adipocytes might have antidiabetic, anti‐inflammatory and antiatherosclerotic functions Citation[89].
The position of fixed‐dose combinations in the treatment of hypertension
All major hypertension guidelines released in 2003 point out the rationale of fixed‐dose combinations to treat patients with high blood pressure Citation[90–94]. They recall that monotherapies are insufficient to control blood pressure in most patients, and that the co‐administration of two agents with different mechanisms of action increases considerably the antihypertensive effectiveness, but not at the expense of a deterioration in tolerability. Another well recognized advantage of fixed‐dose combinations is their simplicity of use, which is expected to facilitate long‐term compliance.
Should fixed‐dose combination be considered as a valuable option to initiate antihypertensive therapy? For some recommendations, the answer is clearly yes (Table ). The most innovative guidelines are proposed by the ESH and the ESC: according to those societies, fixed low‐dose combinations can be used as first‐line treatment in all patients, regardless of the severity of hypertension Citation[92]. The American view summarized in the JNC 7 document is only slightly different: the use of fixed drug combinations as starting medications should be restricted to patients with blood pressures ⩾160 mmHg for systolic or ⩾100 mmHg for diastolic Citation[91]. The ISHIB guidelines also consider fixed‐dose combinations as useful to start therapy, but recommend taking into account severity criteria of hypertension (degree of blood pressure elevation, presence or absence of complication; Citation[90]). The WHO/ISH guidelines recommend the use of thiazide diuretics as initial therapy in all categories of patients and do not specifically state when fixed‐dose combinations may be prescribed, which probably implies that fixed‐dose drug preparations are indicated as second‐line therapy Citation[93]. According to the IFHA guidelines fixed‐dose combinations come into consideration when blood pressure results obtained with a single drug, mainly with diuretic therapy, are unsatisfactory Citation[94]. Notably, when combination therapy is required to achieve blood pressure goals, all guidelines recognize the strong rationale of co‐administering a blocker of the renin–angiotensin system, for instance an AT1‐receptor blocker, with a thiazide diuretic.
Table II. Position of fixed‐dose combinations in hypertension guidelines.
References
- Brunner H. R., Menard J., Waeber B., Burnier M., Biollaz J., Nussberger J., et al. Treating the individual hypertensive patient: Considerations on dose, sequential monotherapy and drug combinations. J Hypertens 1990; 8: 3–11
- Epstein M., Bakris G. Newer approaches to antihypertensive therapy. Use of fixed‐dose combination therapy. Arch Intern Med 1996; 156: 1969–1978
- Law M. R., Moris J. K., Jordan R. E. Value of low dose combination treatment with blood pressure lowering drugs: Analysis of 354 randomized trials. BMJ 2003; 326: 1–8
- Brunner H. R., Waeber B., Nussberger J. Renin secretion responsiveness: Understanding the efficacy of renin–angiotensin inhibition. Kidney Int 1988; 26: S80–S85
- Brunner H. R., Laragh J. H., Baer L., Newton M. A., Goodwin F. T., Krakoff L. R., et al. Essential hypertension: Renin and aldosterone, heart attack and stroke. N Engl J Med 1972; 286: 441–449
- Alderman M. H., Madhavan S., Ooi W. L., Cohen H., Sealey J. E., Laragh J. H. Association of the renin‐sodium profile with the risk of myocardial infarction in patients with hypertension. N Engl J Med 1991; 324: 1098–1104
- Weiss D., Sorescu D., Taylor W. R. Angiotensin II and atherosclerosis. Am J Cardiol 2001; 87: 25C–32C
- Dzau V. J. Theodore Cooper Lecture: Tissue angiotensin and pathobiology of vascular disease: A unifying hypothesis. Hypertension 2001; 37: 1047–1052
- Ruiz‐Ortega M., Lorenzo O., Suzuki Y., Ruperez M., Egido J. Proinflammatory actions of angiotensins. Curr Opin Nephrol Hypertens 2001; 10: 321–329
- Touyz R. M. The role of angiotensin II in regulating vascular structural and functional changes in hypertension. Current Hypertens Rep 2003; 5: 155–164
- Li D., Saldeen T., Romeo F., Mehta J. L. Oxidized LDL upregulates angiotensin II type 1 receptor expression in cultured human coronary artery endothelial cells: The potential role of transcription factor NF‐kappaB. Circulation 2000; 102: 1970–1976
- Nickenig G., Bohm M. Regulation of the angiotensin AT1 receptor expression by hypercholesterolemia. Eur J Med Res 1997; 2: 285–289
- Diet F., Pratt R. E., Berry G. J., Momose N., Gibbons G. H., Dzau V. J. Increased accumulation of tissue ACE in human atherosclerotic coronary artery disease. Circulation 1996; 94: 2756–2767
- Ihara M., Urata H., Kinoshita A., Suzumiya J., Sasaguri M., Kikuchi M., et al. Increased chymase‐dependent angiotensin II formation in human atherosclerotic aorta. Hypertension 1999; 33: 1399–1405
- Legedz L., Randon J., Sessa C., Baguet J., Feugier P., Cerutti C., et al. Cathepsin G is associated with atheroma formation in human carotid artery. J Hypertens 2004; 22: 157–166
- Lee R. T., Schoen F. J., Loree H. M., Lark M. W., Libby P. Circumferential stress and matrix metalloproteinase 1 in human coronary atherosclerosis. Implications for plaque rupture. Arteriosclerosis Thromb Vasc Biol 1996; 16: 1070–1073
- Berry C., Hamilton C. A., Brosnan M. J., Magill F. G., Berg G. A., McMurray J. J., et al. Investigation into the sources of superoxide in human blood vessels: Angiotensin II increases superoxide production in human internal mammary arteries. Circulation 2000; 101: 2206–2212
- Baykal Y., Yilmaz M. I., Celik T., Gok F., Rehber H., Akay C., Kocar I. H. Effects of antihypertensive agents, alpha receptor blockers, beta blockers, angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers and calcium channel blockers, on oxidative stress. J Hypertens 2003; 21: 1207–1211
- Rahman S. T., Lauten W. B., Khan Q. A., Navalkar S., Parthasarathy S., Khan B. V. Effects of eprosartan versus hydrochlorothiazide on markers of vascular oxidation and inflammation and blood pressure (renin–angiotensin system antagonists, oxidation, and inflammation). Am J Cardiol 2002; 89: 686–690
- Germano G., Sanguignib V., Pignatellia P., Cacceseb D., Lentia L., Ragazzoa M., et al. Enhanced platelet release of superoxide anion in systemic hypertension: Role of AT1 receptors. J Hypertens 2004; 22: 1151–1156
- Navalkar S., Parthasarathy S., Santanam N., Khan B. V. Irbesartan, an angiotensin type 1 receptor inhibitor, regulates markers of inflammation in patients with premature atherosclerosis. J Am Coll Cardiol 2001; 37: 440–444
- Khan B. V., Navalkar S., Khan Q. A., Rahman S. T., Parthasarathy S. Irbesartan, an angiotensin type 1 receptor inhibitor, regulates the vascular oxidative state in patients with coronary artery disease. J Am Coll Cardiol 2001; 38: 1662–1667
- Hayek T., Aviram M., Heinrich R., Sakhnini E., Keidar S. Losartan inhibits cellular uptake of oxidized LDL by monocyte‐macrophages from hypercholesterolemic patients. Biochem Biophys Res Commun 2000; 273: 417–420
- Lauten W. B., Khan Q. A., Rajagopalan S., Lerakis S., Rahman S. T., Parthasarathy S., Khan B. V. Usefulness of quinapril and irbesartan to improve the anti‐inflammatory response of atorvastatin and aspirin in patients with coronary heart disease. Am J Cardiol 2003; 91: 1116–1119
- Cipollone F., Fazia M., Iezzi A., Pini B., Cuccurullo C., Zucchelli M., et al. Blockade of the angiotensin II type 1 receptor stabilizes atherosclerotic plaques in humans by inhibiting prostaglandin E2‐dependent matrix metalloproteinase activity. Circulation 2004; 109: 1482–1488
- von zur Muhlen B., Kahan T., Hagg A., Millgard J., Lind L. Treatment with irbesartan or atenolol improves endothelial function in essential hypertension. J Hypertens 2001; 19: 1813–1818
- Ghiadoni L., Virdis A., Magagna A., Taddei S., Salvetti A. Effect of the angiotensin II type 1 receptor blocker candesartan on endothelial function in patients with essential hypertension. Hypertension 2000; 35: 501–506
- Klingbeil A. U., John S., Schneider M. P., Jacobi J., Handrock R., Schmieder R. E. Effect of AT1 receptor blockade on endothelial function in essential hypertension. Am J Hypertens 2003; 16: 123–128
- Prasad A., Halcox J. P., Waclawiw M. A., Quyyumi A. A. Angiotensin type 1 receptor antagonism reverses abnormal coronary vasomotion in atherosclerosis. J Am Coll Cardiol 2001; 38: 1089–1095
- Cheetham C., Collis J., O'Driscoll G., Stanton K., Taylor R., Green D. Losartan, an angiotensin type 1 receptor antagonist, improves endothelial function in non‐insulin‐dependent diabetes. J Am Coll Cardiol 2000; 36: 1461–1466
- Abate N. Obesity and cardiovascular disease. Pathogenetic role of the metabolic syndrome and therapeutic implications. J Diab Complic 2000; 14: 154–174
- Haffner S. M. Pre‐diabetes, insulin resistance, inflammation and CVD risk. Diab Res Clin Pract 2003; 61(Suppl 1)S9–S18
- Sola S., Mir M. Q., Cheema F. A., Khan‐Merchant N., Menon R. G., Parthasarathy S., et al. Irbesartan and lipoic acid improve endothelial function and reduce markers of inflammation in the metabolic syndrome: Results of the Irbesartan and Lipoic Acid in Endothelial Dysfunction (ISLAND) study. Circulation 2005; 111: 343–348
- Mahmud A., Feely J. Effect of angiotensin II receptor blockade on arterial stiffness: Beyond blood pressure reduction. Am J Hypertens 2002; 15: 1092–1095
- Schiffrin E. L., Park J. B., Intengan H. D., Touyz R. M. Correction of arterial structure and endothelial dysfunction in human essential hypertension by the angiotensin receptor antagonist losartan. Circulation 2000; 101: 1653–1659
- Vaughan D. E. Angiotensin, fibrinolysis, and vascular homeostasis. Am J Cardiol 2001; 87: 18C–24C
- Ridker P. M., Gaboury C. L., Conlin P. R., Seely E. W., Williams G. H., Vaughan D. E. Stimulation of plasminogen activator inhibitor in vivo by infusion of angiotensin II. Evidence of a potential interaction between the renin–angiotensin system and fibrinolytic function. Circulation 1993; 87: 1969–1973
- Erdem Y., Usalan C., Haznedaroglu I. C., Altun B., Arici M., Yasavul U., et al. Effects of angiotensin converting enzyme and angiotensin II receptor inhibition on impaired fibrinolysis in systemic hypertension. Am J Hypertens 1999; 12: 1071–1076
- Makris T. K., Stavroulakis G. A., Krespi P. G., Hatzizacharias A. N., Triposkiadis F. K., Tsoukala C. G., et al. Fibrinolytic/hemostatic variables in arterial hypertension: Response to treatment with irbesartan or atenolol. Am J Hypertens 2000; 13: 783–788
- Levy D., Garrison R. J., Savage D. D., Kannel W. B., Castelli W. P. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 1990; 322: 1561–1566
- Verdecchia P., Schillaci G., Borgioni C., Ciucci A., Gattobigio R., Zampi I., et al. Prognostic significance of serial changes in left ventricular mass in essential hypertension. Circulation 1998; 97: 48–54
- Schuijt M. P., Danser A. H. Cardiac angiotensin II: An intracrine hormone?. Am J Hypertens 2002; 15: 1109–1116
- Urata H., Nishimura H., Ganten D., Arakawa K. Angiotensin‐converting enzyme‐independent pathways of angiotensin II formation in human tissues and cardiovascular diseases. Blood Press 1996; 5(Suppl 2)22–28
- Malmqvist K., Kahan T., Edner M., Held C., Hagg A., Lind L., et al. Regression of left ventricular hypertrophy in human hypertension with irbesartan. J Hypertens 2001; 19: 1167–1176
- Gaudio C., Ferri F. M., Giovannini M., Pannarale G., Puddu P. E., Vittore A., et al. Comparative effects of irbesartan versus amlodipine on left ventricular mass index in hypertensive patients with left ventricular hypertrophy. J Cardiovasc Pharmacol 2003; 42: 622–628
- Klingbeil A. U., Schneider M., Martus P., Messerli F. H., Schmieder R. E. A meta‐analysis of the effects of treatment on left ventricular mass in essential hypertension. Am J Med 2003; 115: 41–46
- Cuocolo A., Storto G., Izzo R., Iovino G. L., Damiano M., Bertocchi F., et al. Effects of valsartan on left ventricular diastolic function in patients with mild or moderate essential hypertension: Comparison with enalapril. J Hypertens 1999; 17: 1759–1766
- Dahlöf B., Devereux R. B., Kjeldsen S. E., Julius S., Beevers G., de Faire U., et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): A randomised trial against atenolol. Lancet 2002; 359: 995–1003
- Clarkson P. B., Naas A. A., McMahon A., MacLeod C., Struthers A. D., MacDonald T. M. QT dispersion in essential hypertension. QJM 1995; 88: 327–332
- Davey P. P., Bateman J., Mulligan I. P., Forfar C., Barlow C., Hart G. QT interval dispersion in chronic heart failure and left ventricular hypertrophy: Relation to autonomic nervous system and Holter tape abnormalities. Br Heart J 1994; 71: 268–273
- Lim P. O., Nys M., Naas A. A., Struthers A. D., Osbakken M., MacDonald T. M. Irbesartan reduces QT dispersion in hypertensive individuals. Hypertension 1999; 33: 713–718
- Kawabata M., Takabatake T., Ohta H., Nakamura S., Hara H., Ohta K., et al. Effects of an angiotensin II receptor antagonist, TCV‐116, on renal haemodynamics in essential hypertension. Blood Press 1994; 5(Suppl)117–121
- Navar L. G., Harrison‐Bernard L. M., Imig J. D., Cervenka L., Mitchell K. D. Renal responses to AT1 receptor blockade. Am J Hypertens 2000; 13(Suppl)45S–54S
- Taal M. W., Brenner B. M. Renoprotective benefits of RAS inhibition: From ACEI to angiotensin II antagonists. Kidney Int 2000; 57: 1803–1817
- Lewis E. J., Hunsicker L. G., Clarke W. R., Berl T., Pohl M. A., Lewis J. B., et al. Renoprotective effect of the angiotensin‐receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345: 851–860
- Brenner B. M., Cooper M. E., de Zeeuw D., Keane W. F., Mitch W. E., Parving H. H., et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345: 861–869
- Parving H. H., Lehnert H., Brochner‐Mortensen J., Gomis R., Andersen S., Arner P. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 2001; 345: 870–878
- Viberti G., Wheeldon N. M. Microalbuminuria reduction with valsartan in patients with type 2 diabetes mellitus: A blood pressure‐independent effect. Circulation 2002; 106: 672–678
- Price D. A., Porter L. E., Gordon M., Fisher N. D., De'Oliveira J. M., Laffel L. M., et al. The paradox of the low‐renin state in diabetic nephropathy. J Am Soc Nephrol 1999; 10: 2382–2391
- Fliser D., Keller C., Bahrmann P., Franek E., Schreckling H., Bergis K., et al. Altered action of angiotensin II in patients with type 2 diabetes mellitus of recent onset. J Hypertens 1997; 15: 293–299
- Waeber B., Feihl F., Ruilope L. Diabetes and hypertension. Blood Press 2001; 10: 311–321
- Lithell H., Hansson L., Skoog I., Elmfeldt D., Hofman A., Olofsson B., et al. The Study on Cognition and Prognosis in the Elderly (SCOPE): Principal results of a randomized double‐blind intervention trial. J Hypertens 2003; 21: 875–886
- Tedesco M. A., Ratti G., Mennella S., Manzo G., Grieco M., Rainone A. C., et al. Comparison of losartan and hydrochlorothiazide on cognitive function and quality of life in hypertensive patients. Am J Hypertens 1999; 12: 1130–1134
- Schrader J., Luders S., Kulschewski A., Hammersen F., Plate K., Berger J., et al. Morbidity and mortality after stroke, eprosartan compared with nitrendipine for secondary prevention: Principal results of a prospective randomized controlled study (MOSES). Stroke 2005; 36: 1218–1226
- Chrysant S. G., Weber M. A., Wang A. C., Hinman D. J. Evaluation of antihypertensive therapy with the combination of olmesartan medoxomil and hydrochlorothiazide. Am J Hypertens 2004; 17: 252–259
- Kochar M., Guthrie R., Triscari J., Kassler‐Taub K., Reeves R. A. Matrix study of irbesartan with hydrochlorothiazide in mild‐to‐moderate hypertension. Am J Hypertens 1999; 12: 797–805
- MacKay J. H., Arcuri K. E., Goldberg A. I., Snapinn S. M., Sweet C. S. Losartan and low‐dose hydrochlorothiazide in patients with essential hypertension. A double‐blind, placebo‐controlled trial of concomitant administration compared with individual components. Arch Intern Med 1996; 156: 278–285
- Philipp T., Letzel H., Arens H. J. Dose‐finding study of candesartan cilexetil plus hydrochlorothiazide in patients with mild to moderate hypertension. J Hum Hypertens 1997; 11(Suppl 2)67–68
- Benz J. R., Black H. R., Graff A., Reed A., Fitzsimmons S., Shi Y. Valsartan and hydrochlorothiazide in patients with essential hypertension. A multiple dose, double‐blind, placebo controlled trial comparing combination therapy with monotherapy. J Hum Hypertens 1998; 12: 861–866
- McGill J. B., Reilly P. A. Telmisartan plus hydrochlorothiazide versus telmisartan or hydrochlorothiazide monotherapy in patients with mild to moderate hypertension: A multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group trial. Clin Ther 2001; 23: 833–850
- Schoenberger J. A. Losartan with hydrochlorothiazide in patients with mild to moderate hypertension. J Hypertens 1995; 13(Suppl 1)S43–S47
- Campbell M., Sonkodi S., Soucek M., Wiecek A. A candesartan cilexetil/hydrochlorothiazide combination tablet provides effective blood pressure control in hypertensive patients inadequately controlled on monotherapy. Clin Exp Hypertens 2001; 23: 345–355
- Plouin P. F. Combination therapy with candesartan cilexetil plus hydrochlorothiazide in patients unresponsive to low‐dose hydrochlorothiazide. J Hum Hypertens 1997; 11(Suppl 2)65–66
- Hall D., Motoro R., Littlejohn T., Jain A., Feliciano N., Zheng H. Efficacy and tolerability of valsartan in combination with hydrochlorothiazide in essential hypertension. Clinical Drug Investigations 1998; 16: 203–210
- Rosenstock J., Rossi L., Lin C. S., MacNeil D., Osbakken M. The effects of irbesartan added to hydrochlorothiazide for the treatment of hypertension in patients non‐responsive to hydrochlorothiazide alone. J Clin Pharmacol Ther 1998; 23: 433–440
- Perez‐Stable E., Caralis P. V. Thiazide‐induced disturbances in carbohydrate, lipid, and potassium metabolism. Am Heart J 1983; 106: 245–251
- McGill J. B., Reilly P. A. Combination treatment with telmisartan and hydrochlorothiazide in black patients with mild to moderate hypertension. Clin Cardiol 2001; 24: 66–72
- Wellington K., Faulds D. M. Valsartan/hydrochlorothiazide: A review of its pharmacology, therapeutic efficacy and place in the management of hypertension. Drugs 2002; 62: 1983–2005
- Melian E. B., Jarvis B. Candesartan cilexetil plus hydrochlorothiazide combination: A review of its use in hypertension. Drugs 2002; 62: 787–816
- Soffer B. A., Wright J. T, Jr., Pratt J. H., Wiens B., Goldberg A. I., Sweet C. S. Effects of losartan on a background of hydrochlorothiazide in patients with hypertension. Hypertension 1995; 26: 112–117
- Amos A. F., McCarty D. J., Zimmet P. The rising global burden of diabetes and its complications: Estimates and projections to the year 2010. Diab Med 1997; 14(Suppl 5)S1–S85
- Reaven G. M., Lithell H., Landsberg L. Hypertension and associated metabolic abnormalities–the role of insulin resistance and the sympathoadrenal system. N Engl J Med 1996; 334: 374–381
- Padwal R., Laupacis A. Antihypertensive therapy and incidence of type 2 diabetes: A systematic review. Diabetes Care 2004; 247–255
- Grassi G., Seravalle G., Dell'Oro R., Trevano F. Q., Bombelli M., Scopelliti F., et al. Comparative effects of candesartan and hydrochlorothiazide on blood pressure, insulin sensitivity, and sympathetic drive in obese hypertensive individuals: Results of the CROSS study. J Hypertens 2003; 21: 1761–1769
- Lindholm L. H., Persson M., Alaupovic P., Carlberg B., Svensson A., Samuelsson O. Metabolic outcome during 1 year in newly detected hypertensives: Results of the Antihypertensive Treatment and Lipid Profile in a North of Sweden Efficacy Evaluation (ALPINE study). J Hypertens 2003; 21: 1563–1574
- Pepine C. J., Handberg E. M., Cooper‐DeHoff R. M., Marks R. G., Kowey P., Messerli F. H., et al. A calcium antagonist vs a non‐calcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil‐Trandolapril Study (INVEST): A randomized controlled trial. JAMA 2003; 290: 2805–2816
- Baron A. D., Brechtel‐Hook G., Johnson A., Hardin D. Skeletal muscle blood flow. A possible link between insulin resistance and blood pressure. Hypertension 1993; 21: 129–135
- Furuhashi M., Ura N., Higashiura K., Murakami H., Tanaka M., Moniwa N., et al. Blockade of the renin–angiotensin system increases adiponectin concentrations in patients with essential hypertension. Hypertension 2003; 42: 76–81
- Matsuzawa Y., Funahashi T., Kihara S., Shimomura I. Adiponectin and metabolic syndrome. Arteriosclerosis Thromb Vasc Biol 2004; 24: 29–33
- Douglas J. G., Bakris G. L., Epstein M., Ferdinand K. C., Ferrario C., Flack J. M., et al. Management of high blood pressure in African Americans: Consensus statement of the Hypertension in African Americans Working Group of the International Society on Hypertension in Blacks. Arch Intern Med 2003; 163: 525–541
- Chobanian A. V., Bakris G. L., Black H. R., Cushman W. C., Green L. A., Izzo J. L, Jr., et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA 2003; 289: 2560–2572
- 2003 European Society of Hypertension–European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens 2003; 21: 1011–1053
- 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens 2003; 21: 1983–1992
- Lemogoum D., Seedat Y. K., Mabadeje A. F., Mendis S., Bovet P., Onwubere B., et al. Recommendations for prevention, diagnosis and management of hypertension and cardiovascular risk factors in sub‐Saharan Africa. J Hypertens 2003; 21: 1993–2000